Key Points
-
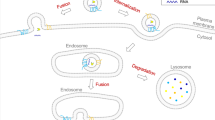
The role of extracellular vesicles (EVs) in the nervous system is just beginning to be understood, but has been substantiated both in cell culture and in vivo. Major challenges in this young field include the establishment of a unified nomenclature for EVs and unified methods for their isolation, as well as the transition from studies in cell culture to those documenting their function in healthy and diseased organisms, a feat that is beginning to be achieved in invertebrate models.
-
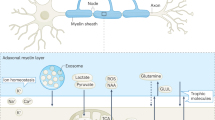
The release and uptake of EVs in the nervous system by neurons and glial cells provide a novel mechanism of transcellular communication. Indeed, EVs are utilized for the transcellular transport of proteins, enzymes, lipids and RNA, thus influencing the physiology of the receiving cell. Neurons and glia also use EVs as a mechanism to regulate intracellular protein and RNA levels and for protein quality control.
-
Neurons can release EVs both in vivo and in cell culture, and this release is often regulated by depolarization or by agents that increase neuronal excitability. In turn, the release of EVs by glial subpopulations sometimes requires neuronal excitation.
-
Glial cells utilize EVs to regulate differentiation, myelin sheath formation and repair after injury. EVs also serve to propagate inflammatory signals in response to tissue damage and disease.
-
A role for EVs during communication in the nervous system of intact organisms has been demonstrated at the Drosophila melanogaster neuromuscular junction (NMJ) and in Caenorhabditis elegans sensory neurons. At the D. melanogaster NMJ, EVs are used to convey Wnt signals from neurons to muscles, and in C. elegans, such vesicles are used to transmit behaviourally relevant signals between organisms.
-
EVs are emerging as potent participants in the progression of disease, serving as vehicles to spread misfolded proteins in a prion-like fashion, transmitting tumorigenic activity and communicating neuroinflammation. Concomitantly, because of their unique properties, EVs are being explored as shuttles to target therapies.
Abstract
Functional neural competence and integrity require interactive exchanges among sensory and motor neurons, interneurons and glial cells. Recent studies have attributed some of the tasks needed for these exchanges to extracellular vesicles (such as exosomes and microvesicles), which are most prominently involved in shuttling reciprocal signals between myelinating glia and neurons, thus promoting neuronal survival, the immune response mediated by microglia, and synapse assembly and plasticity. Such vesicles have also been identified as important factors in the spread of neurodegenerative disorders and brain cancer. These extracellular vesicle functions add a previously unrecognized level of complexity to transcellular interactions within the nervous system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Colombo, M., Raposo, G. & Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289 (2014). An important review of the current understanding of EV biogenesis and secretion, including protocols for EV isolation and current challenges for the field.
Crescitelli, R. et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J. Extracell. Vesicles. http://dx.doi.org/10.3402/jev.v3402i3400.20677 (2013).
Hurley, J. H. ESCRTs are everywhere. EMBO J. 34, 2398–2407 (2015).
Babst, M. MVB vesicle formation: ESCRT-dependent, ESCRT-independent and everything in between. Curr. Opin. Cell Biol. 23, 452–457 (2011). An excellent discussion on the role of the ESCRT versus lipids in forming MVB ILVs.
Lorent, J. H. & Levental, I. Structural determinants of protein partitioning into ordered membrane domains and lipid rafts. Chem. Phys. Lipids 192, 23–32 (2015).
Raposo, G. & Stoorvogel, W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383 (2013).
Friend, D. S. Cytochemical staining of multivesicular body and Golgi vesicles. J. Cell Biol. 41, 269–279 (1969).
Nilsson, P. et al. Autophagy-related protein 7 deficiency in amyloid β (Aβ) precursor protein transgenic mice decreases Aβ in the multivesicular bodies and induces Aβ accumulation in the Golgi. Am. J. Pathol. 185, 305–313 (2015).
Kolesnikova, L., Berghofer, B., Bamberg, S. & Becker, S. Multivesicular bodies as a platform for formation of the Marburg virus envelope. J. Virol. 78, 12277–12287 (2004).
Gould, S. J. & Raposo, G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J. Extracell. Vesicles http://dx.doi.org/10.3402/jev.v2i0.20389 (2013).
Andreu, Z. & Yanez-Mo, M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 5, 442 (2014).
Batrakova, E. V. & Kim, M. S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release 219, 396–405 (2015).
Guescini, M., Genedani, S., Stocchi, V. & Agnati, L. F. Astrocytes and glioblastoma cells release exosomes carrying mtDNA. J. Neural Transm. 117, 1–4 (2010).
Miranda, K. C. et al. Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int. 78, 191–199 (2010).
Cai, J. et al. Extracellular vesicle-mediated transfer of donor genomic DNA to recipient cells is a novel mechanism for genetic influence between cells. J. Mol. Cell. Biol. 5, 227–238 (2013).
Lotvall, J. et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 3, 26913 (2014).
Gangoda, L., Boukouris, S., Liem, M., Kalra, H. & Mathivanan, S. Extracellular vesicles including exosomes are mediators of signal transduction: are they protective or pathogenic? Proteomics 15, 260–271 (2015).
Tietje, A., Maron, K. N., Wei, Y. & Feliciano, D. M. Cerebrospinal fluid extracellular vesicles undergo age dependent declines and contain known and novel non-coding RNAs. PLoS ONE 9, e113116 (2014).
Chiasserini, D. et al. Proteomic analysis of cerebrospinal fluid extracellular vesicles: a comprehensive dataset. J. Proteom. 106, 191–204 (2014).
Marzesco, A. M. et al. Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J. Cell Sci. 118, 2849–2858 (2005).
Lachenal, G. et al. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell. Neurosci. 46, 409–418 (2011).
Faure, J. et al. Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 31, 642–648 (2006). A seminal study showing that primary cultured neurons secrete EVs in response to depolarization.
Chivet, M. et al. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J. Extracell. Vesicles 3, 24722 (2014).
Goldie, B. J. et al. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res. 42, 9195–9208 (2014).
Morel, L. et al. Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J. Biol. Chem. 288, 7105–7116 (2013). A fine study documenting a potentially physiological action of an miRNA when transferred from neurons to astrocytes.
Kramer-Albers, E. M. et al. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: trophic support for axons? Proteom. Clin. Appl. 1, 1446–1461 (2007).
Fruhbeis, C. et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte–neuron communication. PLoS Biol. 11, e1001604 (2013). An elegant study documenting a potential role of EVs in glia–neuron communication in the mammalian brain.
Frohlich, D. et al. Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130510 (2014).
Hsu, C. et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J. Cell Biol. 189, 223–232 (2010).
Bakhti, M., Winter, C. & Simons, M. Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. J. Biol. Chem. 286, 787–796 (2011).
Fitzner, D. et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 124, 447–458 (2011).
Lopez-Verrilli, M. A., Picou, F. & Court, F. A. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia 61, 1795–1806 (2013). An important study documenting the role of EVs liberated from Schwann cells during axonal regeneration.
Escudero, C. A. et al. The p75 neurotrophin receptor evades the endolysosomal route in neuronal cells, favouring multivesicular bodies specialised for exosomal release. J. Cell Sci. 127, 1966–1979 (2014).
Bianco, F. et al. Astrocyte-derived ATP induces vesicle shedding and IL-1β release from microglia. J. Immunol. 174, 7268–7277 (2005).
Antonucci, F. et al. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 31, 1231–1240 (2012). An intriguing study showing that microglia- derived microvesicles might influence synaptic activity in mammals.
Turola, E., Furlan, R., Bianco, F., Matteoli, M. & Verderio, C. Microglial microvesicle secretion and intercellular signaling. Front. Physiol. 3, 149 (2012).
Darios, F. et al. Sphingosine facilitates SNARE complex assembly and activates synaptic vesicle exocytosis. Neuron 62, 683–694 (2009).
Gabrielli, M. et al. Active endocannabinoids are secreted on extracellular membrane vesicles. EMBO Rep. 16, 213–220 (2015).
Potolicchio, I. et al. Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J. Immunol. 175, 2237–2243 (2005).
Hooper, C. et al. Wnt3a induces exosome secretion from primary cultured rat microglia. BMC Neurosci. 13, 144 (2012).
Freese, J. L., Pino, D. & Pleasure, S. J. Wnt signaling in development and disease. Neurobiol. Dis. 38, 148–153 (2010).
Glebov, K. et al. Serotonin stimulates secretion of exosomes from microglia cells. Glia 63, 626–634 (2015).
Cirrito, J. R. et al. Serotonin signaling is associated with lower amyloid-β levels and plaques in transgenic mice and humans. Proc. Natl Acad. Sci. USA 108, 14968–14973 (2011).
Bianco, F. et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 28, 1043–1054 (2009).
Wang, S. et al. Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes. J. Neurosci. 31, 7275–7290 (2011).
Korkut, C. et al. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron 77, 1039–1046 (2013).
Korkut, C. et al. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell 139, 393–404 (2009).
Koles, K. et al. Mechanism of evenness interrupted (evi)-exosome release at synaptic boutons. J. Biol. Chem. 287, 16820–16834 (2012). Together with reference 47, this study provides in vivo evidence for the role of EVs in trans-synaptic communication of WNT signals.
Packard, M. et al. The Drosophila wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell 111, 319–330 (2002).
Kerr, K. S. et al. Glial wingless/Wnt regulates glutamate receptor clustering and synaptic physiology at the Drosophila neuromuscular junction. J. Neurosci. 34, 2910–2920 (2014).
Franch-Marro, X. et al. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat. Cell Biol. 10, 170–177 (2008).
Gross, J. C., Chaudhary, V., Bartscherer, K. & Boutros, M. Active Wnt proteins are secreted on exosomes. Nat. Cell Biol. 14, 1036–1045 (2012).
Savina, A., Vidal, M. & Colombo, M. I. The exosome pathway in K562 cells is regulated by Rab11. J. Cell Sci. 115, 2505–2515 (2002).
Savina, A., Fader, C. M., Damiani, M. T. & Colombo, M. I. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic 6, 131–143 (2005).
Ataman, B. et al. Rapid activity-dependent modifications in synaptic structure and function require bidirectional wnt signaling. Neuron 57, 705–718 (2008).
Yoshihara, M., Adolfsen, B., Galle, K. T. & Littleton, J. T. Retrograde signaling by Syt 4 induces presynaptic release and synapse-specific growth. Science 310, 858–863 (2005).
Budnik, V. & Salinas, P. C. Wnt signaling during synaptic development and plasticity. Curr. Opin. Neurobiol. 21, 151–159 (2011).
Zhang, L. & Wrana, J. L. The emerging role of exosomes in Wnt secretion and transport. Curr. Opin. Genet. Dev. 27, 14–19 (2014).
Wang, J. et al. C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr. Biol. 24, 519–525 (2014). A pioneering study documenting the role of EVs in inter-organismal communication.
Maguire, J. E. et al. Myristoylated CIL-7 regulates ciliary extracellular vesicle biogenesis. Mol. Biol. Cell. 26, 2823–2832 (2015).
Nakano, I., Garnier, D., Minata, M. & Rak, J. Extracellular vesicles in the biology of brain tumour stem cells — implications for inter-cellular communication, therapy and biomarker development. Semin. Cell Dev. Biol. 40, 17–26 (2015). An excellent discussion on the role of EVs in brain tumours.
Pegtel, D. M., Peferoen, L. & Amor, S. Extracellular vesicles as modulators of cell-to-cell communication in the healthy and diseased brain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130516 (2014).
Grad, L. I., Fernando, S. M. & Cashman, N. R. From molecule to molecule and cell to cell: prion-like mechanisms in amyotrophic lateral sclerosis. Neurobiol. Dis. 77, 257–265 (2015).
Aguzzi, A. & Lakkaraju, A. K. Cell biology of prions and prionoids: a status report. Trends Cell Biol. 26, 40–51 (2015).
Guest, W. C., Plotkin, S. S. & Cashman, N. R. Toward a mechanism of prion misfolding and structural models of PrP(Sc): current knowledge and future directions. J. Toxicol. Environ. Health A 74, 154–160 (2011).
Eisele, Y. S. et al. Peripherally applied Aβ-containing inoculates induce cerebral β-amyloidosis. Science 330, 980–982 (2010).
Clavaguera, F. et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 11, 909–913 (2009).
Luk, K. C. et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953 (2012).
Grad, L. I. et al. Intermolecular transmission of superoxide dismutase 1 misfolding in living cells. Proc. Natl Acad. Sci. USA 108, 16398–16403 (2011).
Coleman, B. M. & Hill, A. F. Extracellular vesicles — their role in the packaging and spread of misfolded proteins associated with neurodegenerative diseases. Semin. Cell Dev. Biol. 40, 89–96 (2015). An excellent discussion about the role of EVs in spreading misfolded proteins associated with neurodegenerative disorders.
Grad, L. I. et al. Intercellular propagated misfolding of wild-type Cu/Zn superoxide dismutase occurs via exosome-dependent and -independent mechanisms. Proc. Natl Acad. Sci. USA 111, 3620–3625 (2014).
Emmanouilidou, E. et al. Cell-produced α-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J. Neurosci. 30, 6838–6851 (2010).
Melachroinou, K. et al. Deregulation of calcium homeostasis mediates secreted α-synuclein-induced neurotoxicity. Neurobiol. Aging 34, 2853–2865 (2013).
Rajendran, L. et al. Alzheimer's disease β-amyloid peptides are released in association with exosomes. Proc. Natl Acad. Sci. USA 103, 11172–11177 (2006).
Joshi, P. et al. Microglia convert aggregated amyloid-β into neurotoxic forms through the shedding of microvesicles. Cell Death Differ. 21, 582–593 (2014). An important review highlighting the complexity of protein forms involved in neurodegeneration and the role of EVs.
Sharples, R. A. et al. Inhibition of γ-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. FASEB J. 22, 1469–1478 (2008).
Joshi, P., Benussi, L., Furlan, R., Ghidoni, R. & Verderio, C. Extracellular vesicles in Alzheimer's disease: friends or foes? Focus on aβ-vesicle interaction. Int. J. Mol. Sci. 16, 4800–4813 (2015).
Yuyama, K., Sun, H., Mitsutake, S. & Igarashi, Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia. J. Biol. Chem. 287, 10977–10989 (2012).
Asai, H. et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 18, 1584–1593 (2015).
Al-Nedawi, K. et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 10, 619–624 (2008).
Al-Nedawi, K., Meehan, B., Kerbel, R. S., Allison, A. C. & Rak, J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc. Natl Acad. Sci. USA 106, 3794–3799 (2009).
Bronisz, A. et al. Extracellular vesicles modulate the glioblastoma microenvironment via a tumor suppression signaling network directed by miR-1. Cancer Res. 74, 738–750 (2014).
Tominaga, N. et al. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood–brain barrier. Nat. Commun. 6, 6716 (2015).
Carandini, T. et al. Microvesicles: what is the role in multiple sclerosis? Front. Neurol. 6, 111 (2015).
Verderio, C. et al. Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann. Neurol. 72, 610–624 (2012).
Minagar, A. & Alexander, J. S. Blood–brain barrier disruption in multiple sclerosis. Mult. Scler. 9, 540–549 (2003).
Saenz-Cuesta, M., Osorio-Querejeta, I. & Otaegui, D. Extracellular vesicles in multiple sclerosis: what are they telling us? Front. Cell. Neurosci. 8, 100 (2014).
Prada, I., Furlan, R., Matteoli, M. & Verderio, C. Classical and unconventional pathways of vesicular release in microglia. Glia 61, 1003–1017 (2013).
Peferoen, L., Kipp, M., van der Valk, P., van Noort, J. M. & Amor, S. Oligodendrocyte–microglia cross-talk in the central nervous system. Immunology 141, 302–313 (2014).
Saenz-Cuesta, M. et al. Circulating microparticles reflect treatment effects and clinical status in multiple sclerosis. Biomark. Med. 8, 653–661 (2014).
Paschon, V. et al. Interplay between exosomes, microRNAs and Toll-like receptors in brain disorders. Mol. Neurobiol. 11, 11 (2015).
Gupta, A. & Pulliam, L. Exosomes as mediators of neuroinflammation. J. Neuroinflammation 11, 68 (2014).
Frühbeis, C., Fröhlich, D. & Krämer-Albers, E. M. Emerging roles of exosomes in neuron–glia communication. Front. Physiol. 3, 119 (2012).
An, K. et al. Exosomes neutralize synaptic-plasticity-disrupting activity of Aβ assemblies in vivo. Mol. Brain 6, 47 (2013).
Polazzi, E. & Monti, B. Microglia and neuroprotection: from in vitro studies to therapeutic applications. Prog. Neurobiol. 92, 293–315 (2010).
De Maio, A. & Vazquez, D. Extracellular heat shock proteins: a new location, a new function. Shock 40, 239–246 (2013).
Tytell, M., Greenberg, S. G. & Lasek, R. J. Heat shock-like protein is transferred from glia to axon. Brain Res. 363, 161–164 (1986).
Taylor, A. R., Robinson, M. B., Gifondorwa, D. J., Tytell, M. & Milligan, C. E. Regulation of heat shock protein 70 release in astrocytes: role of signaling kinases. Dev. Neurobiol. 67, 1815–1829 (2007).
Pusic, A. D. & Kraig, R. P. Youth and environmental enrichment generate serum exosomes containing miR-219 that promote CNS myelination. Glia 62, 284–299 (2014).
Dugas, J. C. et al. Dicer1 and miR-219 are required for normal oligodendrocyte differentiation and myelination. Neuron 65, 597–611 (2010).
Zhao, X. et al. MicroRNA-mediated control of oligodendrocyte differentiation. Neuron 65, 612–626 (2010).
Aryani, A. & Denecke, B. Exosomes as a nanodelivery system: a key to the future of neuromedicine? Mol. Neurobiol. http://dx.doi.org/10.1007/s12035-014-9054-5 (2014).
Zhuang, X. et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 19, 1769–1779 (2011).
Alvarez-Erviti, L. et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 341–345 (2011).
Yeo, R. W. et al. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev. 65, 336–341 (2013).
Park, J. S., Suryaprakash, S., Lao, Y. H. & Leong, K. W. Engineering mesenchymal stem cells for regenerative medicine and drug delivery. Methods 84, 3–16 (2015).
Xin, H. et al. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 33, 1711–1715 (2013).
Katsuda, T. et al. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci. Rep. 3, 1197 (2013).
Pusic, A. D., Pusic, K. M., Clayton, B. L. & Kraig, R. P. IFNγ-stimulated dendritic cell exosomes as a potential therapeutic for remyelination. J. Neuroimmunol. 266, 12–23 (2014).
Haney, M. J. et al. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J. Control. Release 207, 18–30 (2015).
Schwab, A. et al. Extracellular vesicles from infected cells: potential for direct pathogenesis. Front. Microbiol. 6, 1132 (2015).
Acknowledgements
V.B. is supported by a US National Institutes of Health grant (R37 MH070000).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
PowerPoint slides
Glossary
- Protein processing
-
Protein processing may involve protein folding, covalent modifications (for example, phosphorylation and acetylation) and/or cleavage of the protein product into a smaller biologically active protein.
- Reactive microglia
-
In the injured or disease brain, glia can become reactive by proliferating and upregulating a number of cytoskeletal components, leading to the formation of glial scars, which replace the damaged brain cells.
- Transforming proteins
-
Proteins that cause a cell to be transformed into a neoplastic cell, which undergoes uncontrolled cell division.
Rights and permissions
About this article
Cite this article
Budnik, V., Ruiz-Cañada, C. & Wendler, F. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci 17, 160–172 (2016). https://doi.org/10.1038/nrn.2015.29
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn.2015.29
This article is cited by
-
M2 Microglia-derived Exosomes Promote Spinal Cord Injury Recovery in Mice by Alleviating A1 Astrocyte Activation
Molecular Neurobiology (2024)
-
Extracellular vesicles derived from mesenchymal stem cells — a novel therapeutic tool in infectious diseases
Inflammation and Regeneration (2023)
-
Engineered extracellular vesicles for ischemic stroke: a systematic review and meta-analysis of preclinical studies
Journal of Nanobiotechnology (2023)
-
Focal ischemic stroke modifies microglia-derived exosomal miRNAs: potential role of mir-212-5p in neuronal protection and functional recovery
Biological Research (2023)
-
Neutral sphingomyelinase inhibition promotes local and network degeneration in vitro and in vivo
Cell Communication and Signaling (2023)