Prepulse Inhibition of the Auditory Startle Reflex Assessment as a Hallmark of Brainstem Sensorimotor Gating Mechanisms
Abstract
:1. Characteristics and Functional Implications of the Acoustic Startle Reflex
2. Acoustic Startle Modulations
2.1. Fear Potentiation of the ASR
2.2. Sensitization and Habituation of the ASR
2.3. Drugs Affecting the ASR
2.4. Prepulse Inhibition of the ASR
3. Research and Clinical Applications
4. Prepulse Inhibition as an Indicator of Neural Plasticity
4.1. Attentional Modulations of PPI
4.2. Gap-Prepulse Inhibition of the Acoustic Startle Reflex for Tinnitus Assessment
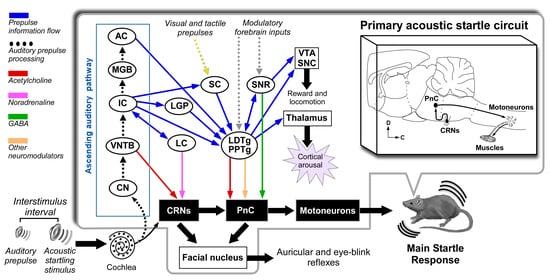
5. Neuronal Pathways of the Acoustic Startle Reflex and Its Prepulse Inhibition
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Landis, C.; Hunt, W. The Startle Pattern; Farrar & Rinehart: New York, NY, USA, 1939. [Google Scholar]
- Alvarado Ruiz, G.R.; Martínez Vázquez, R.I.; Solís Chan, M.; Plaza, M.; Gómez Ramírez, D.B. Los reflejos primitivos en el diagnóstico clínico de neonatos y lactantes. Rev. Cienc. Clín. 2009, 9, 15–26. [Google Scholar]
- Wilkins, D.E.; Hallet, M.; Wess, M.M. Audiogenic startle reflex on man and its relationship to startle syndromes. Brain 1986, 109, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Ison, J.R.; McAdam, D.W.; Hammond, G.R. Latency and amplitude changes in the acoustic startle reflex in the rat produced by variation in auditory prestimulation. Physiol. Behav. 1973, 10, 1035–1039. [Google Scholar] [CrossRef]
- Cassella, J.V.; Harty, T.; Davis, M. Fear conditioning, pre-pulse inhibition and drug modulation of a short latency startle response measured electromyographically from neck muscles in the rat. Physiol. Behav. 1986, 36, 1187–1191. [Google Scholar] [CrossRef]
- Ford, J.M.; Roth, W.T.; Isaacks, B.G.; White, P.M.; Hood, S.H.; Pfefferbaum, A. Elderly men and women are less responsive to startling noises: N1, P3 and blink evidence. Boil. Psychol. 1995, 39, 57–80. [Google Scholar] [CrossRef]
- Varty, G.B.; Hauger, R.L.; Geyer, M.A. Aging effects on the startle response and startle plasticity in Fisher F344 rats. Neurobiol. Aging 1998, 19, 243–251. [Google Scholar] [CrossRef]
- Kofler, M.; Müller, J.; Reggiani, L.; Valls-Solé, J. Influence of age on auditory startle responses in humans. Neurosci. Lett. 2001, 307, 65–68. [Google Scholar] [CrossRef]
- Lee, Y.; López, D.E.; Meloni, E.G.; Davis, M. A primary acoustic startle pathway: Obligatory role of cochlear root neurons and the nucleus reticularis pontis caudalis. J. Neurosci. 1996, 16, 3775–3789. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Nieto, R.; Horta-Júnior, J.d.A.C.; Castellano, O.; Millian-Morell, L.; Rubio, M.E.; López, D.E. Origin and function of short-latency inputs to the neural substrates underlying the acoustic startle reflex. Front. Mol. Neurosci. 2014, 8, 216. [Google Scholar] [CrossRef] [Green Version]
- Hormigo, S.; López, D.E. Adjustement of the data acquisition window for the assessment of sensorimotor mechanism in rodent. MethodsX 2019, 6, 2046–2051. [Google Scholar] [CrossRef]
- Lehmann, J.; Pryce, C.R.; Feldon, J. Sex differences in the acoustic startle response and prepulse inhibition in Wistar rats. Behav. Brain Res. 1999, 104, 113–117. [Google Scholar] [CrossRef]
- Kofler, M.; Müller, J.; Reggiani, L.; Valls-Solé, J. Influence of gender on auditory startle responses. Brain Res. 2001, 921, 206–210. [Google Scholar] [CrossRef]
- Vaillancourt, C.; Cyr, M.; Rochford, J.; Boksa, P.; Di Paolo, T. Effects of ovariectomy and estradiol on acoustic startle responses in rats. Pharmacol. Biochem. Behav. 2002, 74, 103–109. [Google Scholar] [CrossRef]
- Brown, J.S.; Kalish, H.I.; Farber, I.E. Conditioned fear as revealed by magnitude of startle response to an auditory stimulus. J. Exp. Psychol. 1951, 41, 317–328. [Google Scholar] [CrossRef]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B. Emotion, attention, and the startle reflex. Psychol. Rev. 1990, 97, 377–395. [Google Scholar] [CrossRef]
- Schmid, A.; Koch, M.; Schnitzler, H. Conditioned Pleasure Attenuates the Startle Response in Rats. Neurobiol. Learn. Mem. 1995, 64, 1–3. [Google Scholar] [CrossRef]
- Salloum, R.H.; Yurosko, C.; Santiago, L.; Sandridge, S.A.; Kaltenbach, J.A. Induction of Enhanced Acoustic Startle Response by Noise Exposure: Dependence on Exposure Conditions and Testing Parameters and Possible Relevance to Hyperacusis. PLoS ONE 2014, 9, e111747. [Google Scholar] [CrossRef]
- Pilz, P.K.; Schnitzler, H.-U. Habituation and Sensitization of the Acoustic Startle Response in Rats: Amplitude, Threshold, and Latency Measures. Neurobiol. Learn. Mem. 1996, 66, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Rinaldi, P.C.; Thompson, R.R. Age, sex and strain comparison of habituation of the startle response in the rat. Physiol. Behav. 1985, 35, 9–13. [Google Scholar] [CrossRef]
- Blanch, A.; Balada, F.; Aluja, A. Habituation in acoustic startle reflex: Individual differences in personality. Int. J. Psychophysiol. 2014, 91, 232–239. [Google Scholar] [CrossRef]
- Swerdlow, N.R.; Eastvold, A.; Karban, B.; Ploum, Y.; Stephany, N.; Geyer, M.A.; Cadenhead, K.; Auerbach, P.P. Dopamine agonist effects on startle and sensorimotor gating in normal male subjects: Time course studies. Psychopharmacology 2002, 161, 189–201. [Google Scholar] [CrossRef]
- Harris, A.C.; Gewirtz, J.C. Elevated startle during withdrawal from acute morphine: A model of opiate withdrawal and anxiety. Psychopharmacology 2004, 171, 140–147. [Google Scholar] [CrossRef]
- Bell, R.L.; Rodd, Z.A.; Hsu, C.C.; Lumeng, L.; Murphy, J.M.; McBride, W.J. Amphetamine-modified acoustic startle responding and prepulse inhibition in adult and adolescent alcohol-preferring and -nonpreferring rats. Pharmacol. Biochem. Behav. 2003, 75, 163–171. [Google Scholar] [CrossRef]
- Davis, M. Cocaine: Excitatory effects on sensorimotor reactivity measured with acoustic startle. Psychopharmacology 1985, 86, 31–36. [Google Scholar] [CrossRef]
- Farid, M.; Martinez, Z.A.; Geyer, M.A.; Swerdlow, N.R. Regulation of Sensorimotor Gating of the Startle Reflex by Serotonin 2A Receptors Ontogeny and Strain Differences. Neuropsychopharmacology 2000, 23, 623–632. [Google Scholar] [CrossRef]
- Abduljawad, K.A.J.; Langley, R.W.; Bradshaw, C.M.; Szabadi, E. Effects of clonidine and diazepam on prepulse inhibition of the acoustic startle response and the N1/P2 auditory evoked potential in man. J. Psychopharmacol. 2001, 15, 237–242. [Google Scholar] [CrossRef]
- Hutchison, K.E.; Niaura, R.; Swift, R. The effects of smoking high nicotine cigarettes on prepulse inhibition, startle latency, and subjective responses. Psychopharmacology (Berl.) 2000, 150, 244–252. [Google Scholar] [CrossRef]
- Rasmussen, D.D.; Crites, N.J.; Burke, B.L. Acoustic startle amplitude predicts vulnerability to develop post-traumatic stress hyper-responsivity and associated plasma corticosterone changes in rats. Psychoneuroendocrinology 2008, 33, 282–291. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Figueiredo, I.; Sancho, C.; Carro, J.; Castellano, O.; López, D.E. The Effects of Sertraline administration from adolescence to adulthood on physiological and emotional development in prenatally stressed rats of both sexes. Front. Behav. Neurosci. 2014, 8, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swerdlow, N.R.; Braff, D.L.; Geyer, M.A. Animal models of deficient sensorimotor gating: What we know, what we think we know, and what we hope to know soon. Behav. Pharmacol. 2000, 11, 185–204. [Google Scholar] [CrossRef]
- Braff, D.L.; Geyer, M.A.; Swerdlow, N.R. Human studies of prepulse inhibition of startle: Normal subjects, patient groups, and pharmacological studies. Psychopharmacology 2001, 156, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.S.; Wible, B.L. Role of Weak Signals in Acoustic Startle. J. Acoust. Soc. Am. 1970, 47, 489–497. [Google Scholar] [CrossRef]
- Basavaraj, S.; Yan, J. Prepulse Inhibition of Acoustic Startle Reflex as a Function of the Frequency Difference between Prepulse and Background Sounds in Mice. PLoS ONE 2012, 7, e45123. [Google Scholar] [CrossRef] [Green Version]
- Graham, F.K.; Murray, G.M. Discordant effects of weak prestimulation on magnitude and latency of the reflex blink. Physiol. Psychol. 1977, 5, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Swerdlow, N.R.; Auerbach, P.; Monroe, S.M.; Hartston, H.; Geyer, M.A.; Braff, D.L. Men are more inhibited than women by weak prepulses. Boil. Psychiatry 1993, 34, 253–260. [Google Scholar] [CrossRef]
- Swerdlow, N.R.; Hartman, P.L.; Auerbach, P.P. Changes in sensorimotor inhibition across the menstrual cycle: Implications for neuropsychiatric disorders. Boil. Psychiatry 1997, 41, 452–460. [Google Scholar] [CrossRef]
- Ison, J.R.; Bowen, G.P.; Pak, J.; Gutierrez, E. Changes in the strength of prepulse inhibition with variation in the startle baseline associated with individual differences and with old age in rats and mice. Psychobiology 1997, 25, 266–274. [Google Scholar] [CrossRef]
- Ludewig, K.; Ludewig, S.; Seitz, A.; Obrist, M.; Geyer, M.A.; Vollenweider, F.X. The acoustic startle reflex and its modulation: Effects of age and gender in humans. Boil. Psychol. 2003, 63, 311–323. [Google Scholar] [CrossRef]
- Ellwanger, J.; Geyer, M.A.; Braff, D.L. The relationship of age to prepulse inhibition and habituation of the acoustic startle response. Boil. Psychol. 2003, 62, 175–195. [Google Scholar] [CrossRef]
- Ornitz, E.M.; Guthrie, D.; Sadeghpour, M.; Sugiyama, T. Maturation of prestimulation-induced startle modulation in girls. Psychophysiology 1991, 28, 11–20. [Google Scholar] [CrossRef]
- Geyer, M.A.; Krebs-Thomson, K.; Braff, D.L.; Swerdlow, N.R. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: A decade in review. Psychopharmacology (Berl.) 2001, 156, 117–154. [Google Scholar] [CrossRef]
- Kumari, V.; Gray, J.A. Smoking withdrawal, nicotine dependence and prepulse inhibition of the acoustic startle reflex. Psychopharmacology (Berl.) 1999, 141, 11–15. [Google Scholar] [CrossRef]
- Li, L.; Du, Y.; Li, N.; Wu, X.; Wu, Y. Top–down modulation of prepulse inhibition of the startle reflex in humans and rats. Neurosci. Biobehav. Rev. 2009, 33, 1157–1167. [Google Scholar] [CrossRef]
- Cadenhead, K.S.; Carasso, B.S.; Swerdlow, N.R.; Geyer, M.A.; Braff, D.L. Prepulse inhibition and habituation of the startle response are stable neurobiological measures in a normal male population. Boil. Psychiatry 1999, 45, 360–364. [Google Scholar] [CrossRef]
- Davis, M. The Role of the Amygdala in Fear and Anxiety. Annu. Rev. Neurosci. 1992, 15, 353–375. [Google Scholar] [CrossRef]
- Halty, L.; Martínez, A.; Requena, C.; Santos, J.M.; Ortiz, T. Psicopatía en niños y adolescentes: Modelos, teorías y últimas investigaciones. Rev. Neurol. 2011, 52 (Suppl. 1), S19–S27. [Google Scholar] [CrossRef]
- Howard, R.; Ford, R. From the jumping Frenchmen of Maine to posttraumatic stress disorder: The startle response in neuropsychiatry. Psychol. Med. 1992, 22, 695–707. [Google Scholar] [CrossRef]
- Andermann, F.; Andermann, E. Startle disorders of man: Hyperekplexia, jumping and startle epilepsy. Brain Dev. 1988, 10, 213–222. [Google Scholar] [CrossRef]
- Dreissen, Y.E.M.; Tijssen, M.A.J. The startle syndromes: Physiology and treatment. Epilepsia 2012, 53 (Suppl. 7), 3–11. [Google Scholar] [CrossRef]
- Kofler, M.; Müller, J.; Wenning, G.K.; Reggiani, L.; Hollosi, P.; Bösch, S.; Ransmayr, G.; Valls-Solé, J.; Poewe, W. The auditory startle reflex in parkinsonian disorders. Mov. Disord. 2001, 16, 62–71. [Google Scholar] [CrossRef]
- Frauscher, B.; Löscher, W.N.; Högl, B.; Poewe, W.; Kofler, M. Auditory startle reaction is disinhibited in idiopathic restless legs syndrome. Sleep 2007, 30, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Capute, A.J. Early Neuromotor Reflexes in Infancy. Pediatr. Ann. 1986, 15, 217–226. [Google Scholar] [CrossRef]
- Young, J.S.; Fechter, L.D. Reflex inhibition procedures for animal audiometry: A technique for assessing ototoxicity. J. Acoust. Soc. Am. 1983, 73, 1686–1693. [Google Scholar] [CrossRef]
- Swerdlow, N.R.; Braff, D.L.; Geyer, M.A. Sensorimotor gating of the startle reflex: What we said 25 years ago, what has happened since then, and what comes next. J. Psychopharmacol. 2016, 30, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Kumari, V.; Soni, W.; Sharma, T. Normalization of information processing deficits in schizophrenia. Am. J. Psychiatry 1999, 156, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, N.R.; Keith, V.A.; Braff, D.L.; Geyer, M.A. Effects of spiperone, raclopride, SCH 23390 and clozapine on apomorphine inhibition of sensorimotor gating of the startle response in the rat. J. Pharmacol. Exp. Ther. 1991, 256, 530–536. [Google Scholar]
- Davis, M.; Gendelman, P.M. Plasticity of the acoustic startle response in the acutely decerebrate rat. J. Comp. Physiol. Psychol. 1977, 91, 549–563. [Google Scholar] [CrossRef]
- Millian-Morell, L.; López-Alburquerque, T.; Rodriguez-Rodríguez, A.; Gómez-Nieto, R.; Carro, J.; Meilán, J.J.G.; Martínez-Sánchez, F.; Sancho, C.; López, D.E. Relations between sensorimotor integration and speech disorders in Parkinson’s disease. Curr. Alzheimer Res. 2018, 15, 149–156. [Google Scholar] [CrossRef]
- Ludewig, S.; Ludewig, K.; Geyer, M.A.; Hell, D.; Vollenweider, F.X. Prepulse inhibition deficits in patients with panic disorder. Depress. Anxiety 2002, 15, 55–60. [Google Scholar] [CrossRef]
- Comasco, E.; Gulinello, M.; Hellgren, C.; Skalkidou, A.; Sylvén, S.; Sundström-Poromaa, I. Sleep duration, depression, and oxytocinergic genotype influence prepulse inhibition of the startle reflex in postpartum women. Eur. Neuropsychopharmacol. 2016, 26, 767–776. [Google Scholar] [CrossRef]
- Pereira-Figueiredo, I.; Castellano, O.; Riolobos, A.S.; Ferreira-Dias, G.; López, D.E.; Sancho, C. Long-term sertraline intake reverses the behavioral changes induced by prenatal stress in rats in a sex-dependent way. Front. Behav. Neurosci. 2017, 11, 99. [Google Scholar] [CrossRef]
- Schmajuk, N.A.; Larrauri, J.A.; De La Casa, L.G.; Levin, E.E. Attenuation of auditory startle and prepulse inhibition by unexpected changes in ambient illumination through dopaminergic mechanisms. Behav. Brain Res. 2009, 197, 251–261. [Google Scholar] [CrossRef]
- Breedh, J.; Comasco, E.; Hellgren, C.; Papadopoulos, F.C.; Skalkidou, A.; Poromaa, I.S. Hypothalamic-pituitary-adrenal axis responsiveness, startle response, and sensorimotor gating in late pregnancy. Psychoneuroendocrinology 2019, 106, 1–8. [Google Scholar] [CrossRef]
- Bannbers, E.; Kask, K.; Wikstrom, J.; Sundström-Poromaa, I. Lower levels of prepulse inhibition in luteal phase cycling women in comparison with postmenopausal women. Psychoneuroendocrinology 2010, 35, 422–429. [Google Scholar] [CrossRef]
- Molina, V.; Cortés, B.; Pérez, J.; Martín, C.; Villa, R.; López, D.E.; Sancho, C. No association between prepulse inhibition of the startle reflex and neuropsychological deficit in chronic schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2010, 260, 609–615. [Google Scholar] [CrossRef]
- Hormigo, S.; Cardoso, A.; Sancho, C.; López, D.E.; Moreno, C. Associations between neural sensorimotor gating mechanisms and athletic performance in a variety of physical conditioning tests. Eur. J. Appl. Physiol. 2019, 119, 921–932. [Google Scholar] [CrossRef]
- Graham, F.K. The More or Less Startling Effects of Weak Prestimulation. Psychophysiology 1975, 12, 238–248. [Google Scholar] [CrossRef]
- Blumenthal, T.D.; Reynolds, J.Z.; Spence, T.E. Support for the interruption and protection hypotheses of prepulse inhibition of startle: Evidence from a modified Attention Network Test. Psychophysiology 2014, 52, 397–406. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, N.; Gao, Y.; Wu, Z.; Li, L. The role of the deeper layers of the superior colliculus in attentional modulations of prepulse inhibition. Behav. Brain Res. 2019, 364, 106–113. [Google Scholar] [CrossRef]
- Brandão, M.L.; Troncoso, A.C.; de Souza Silva, M.A.; Huston, J.P. The relevance of neuronal substrates of defense in the midbrain tectum to anxiety and stress: Empirical and conceptual considerations. Eur. J. Pharmacol. 2003, 463, 225–233. [Google Scholar] [CrossRef]
- Hazlett, E.A.; Buchsbaum, M.S.; Tang, C.Y.; Fleischman, M.B.; Wei, T.-C.; Byne, W.; Haznedar, M.M. Thalamic activation during an attention-to-prepulse startle modification paradigm: A functional MRI study. Boil. Psychiatry 2001, 50, 281–291. [Google Scholar] [CrossRef]
- Anthony, B.J.; Graham, F.K. Blink reflex modification by selective attention: Evidence for the modulation of ‘automatic’ processing. Boil. Psychol. 1985, 21, 43–59. [Google Scholar] [CrossRef]
- Kumari, V.; Aasen, I.; Sharma, T. Sex differences in prepulse inhibition deficits in chronic schizophrenia. Schizophr. Res. 2004, 69, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Schell, A.M.; Wynn, J.K.; Dawson, M.E.; Sinaii, N.; Niebala, C.B. Automatic and controlled attentional processes in startle eyeblink modification: Effects of habituation of the prepulse. Psychophysiology 2000, 37, 409–417. [Google Scholar] [CrossRef]
- Aasen, I.; Kolli, L.; Kumari, V. Sex effects in prepulse inhibition and facilitation of the acoustic startle response: Implications for pharmacological and treatment studies. J. Psychopharmacol. 2005, 19, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Plappert, C.F.; Pilz, P.K.D.; Schnitzler, H.U. Factors governing prepulse inhibition and prepulse facilitation of the acoustic startle response in mice. Behav. Brain Res. 2004, 152, 403–412. [Google Scholar] [CrossRef]
- Vollenweider, F.X.; Csomor, P.A.; Knappe, B.; Geyer, M.A.; Quednow, B.B. The effects of the preferential 5-HT2A agonist psilocybin on prepulse inhibition of startle in healthy human volunteers depend on interstimulus interval. Neuropsychopharmacology 2007, 32, 1876–1887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansbach, R.S.; Geyer, M.A. Parametric determinants in pre-stimulus modification of acoustic startle: Interaction with ketamine. Psychopharmacology (Berl.) 1991, 105, 162–168. [Google Scholar] [CrossRef]
- Zenner, H.-P.; Pfister, M.; Birbaumer, N. Tinnitus sensitization: Sensory and psychophysiological aspects of a new pathway of acquired centralization of chronic tinnitus. Otol. Neurotol. 2006, 27, 1054–1063. [Google Scholar] [CrossRef]
- Dehmel, S.; Eisinger, D.; Shore, S.E. Gap prepulse inhibition and auditory brainstem-evoked potentials as objective measures for tinnitus in guinea pigs. Front. Syst. Neurosci. 2012, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Hayes, S.H.; Radziwon, K.E.; Stolzberg, D.J.; Salvi, R.J. Behavioral Models of Tinnitus and Hyperacusis in Animals. Front. Neurol. 2014, 5, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, J.G.; Bauer, C.A.; Parrish, J.L.; Myers, K.; Hughes, L.F.; Caspary, D.M. Gap Detection Deficits in Rats With Tinnitus: A Potential Novel Screening Tool. Behav. Neurosci. 2006, 120, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Hickox, A.E.; Liberman, M.C. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J. Neurophysiol. 2014, 111, 552–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, C.A.; Berger, J.I.; de Boer, J.; Sereda, M.; Palmer, A.R.; Hall, D.A.; Wallace, M.N. Gap-induced inhibition of the post-auricular muscle response in humans and guinea pigs. Hear. Res. 2019, 374, 13–23. [Google Scholar] [CrossRef]
- Galazyuk, A.; Hébert, S. Gap-prepulse inhibition of the acoustic startle reflex (GPIAS) for tinnitus assessment: Current status and future directions. Front. Neurol. 2015, 6, 88. [Google Scholar] [CrossRef] [Green Version]
- Nodal, F.R.; López, D.E. Direct input from cochlear root neurons to pontine reticulospinal neurons in albino rat. J. Comp. Neurol. 2003, 460, 80–93. [Google Scholar] [CrossRef]
- Osen, K.K.; López, D.E.; Slyngstad, T.A.; Ottersen, O.P.; Storm-Mathisen, J. GABA-like and glycine-like immunoreactivities of the cochlear root nucleus in rat. J. Neurocytol. 1991, 20, 17–25. [Google Scholar] [CrossRef]
- López, D.E.; Saldaña, E.; Nodal, F.R.; Merchán, M.A.; Warr, W.B. Projections of cochlear root neurons, sentinels of the rat auditory pathway. J. Comp. Neurol. 1999, 415, 160–174. [Google Scholar] [CrossRef]
- Gokin, A.P. Relay levels of acoustic and tactile startle s reflexes in the reticular formation of the cat. Neirofiziologiia 1985, 17, 703–707. [Google Scholar]
- Valls-Solé, J. Assessment of excitability in brainstem circuits mediating the blink reflex and the startle reaction. Clin. Neurophysiol. 2012, 123, 13–20. [Google Scholar] [CrossRef]
- Edinger, L.; Fisher, B. Ein Mensch ohne Grohirn. Pfluegers Ges Physiol. 1913, 152, 535–562. [Google Scholar] [CrossRef]
- Fendt, M.; Li, L.; Yeomans, J.S. Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology (Berl.) 2001, 56, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Azzopardi, E.; Louttit, A.G.; Deoliveira, C.; Laviolette, S.R.; Schmid, S. The Role of Cholinergic Midbrain Neurons in Startle and Prepulse Inhibition. J. Neurosci. 2018, 38, 8798–8808. [Google Scholar] [CrossRef] [PubMed]
- MacLaren, D.A.A.; Markovic, T.; Clark, S.D. Assessment of sensorimotor gating following selective lesions of cholinergic pedunculopontine neurons. Eur. J. Neurosci. 2014, 40, 3526–3537. [Google Scholar] [CrossRef]
- Koch, M.; Fendt, M.; Kretschmer, B.D. Role of the substantia nigra pars reticulata in sensorimotor gating, measured by prepulse inhibition of startle in rats. Behav. Brain Res. 2000, 117, 153–162. [Google Scholar] [CrossRef]
- Rohleder, C.; Jung, F.; Mertgens, H.; Wiedermann, D.; Sue, M.; Neumaier, B.; Graf, R.; Leweke, F.M.; Endepols, H. Neural correlates of sensorimotor gating: A metabolic positron emission tomography study in awake rats. Front. Behav. Neurosci. 2014, 8, 178. [Google Scholar] [CrossRef]
- Rohleder, C.; Wiedermann, D.; Neumaier, B.; Drzezga, A.; Timmermann, L.; Graf, R.; Leweke, F.M.; Endepols, H. The Functional Networks of Prepulse Inhibition: Neuronal Connectivity Analysis Based on FDG-PET in Awake and Unrestrained Rats. Front. Behav. Neurosci. 2016, 10, 148. [Google Scholar] [CrossRef] [Green Version]
- Korte, S.M.; Jaarsma, D.; Luiten, P.G.M.; Bohus, B. Mesencephalic cuneiform nucleus and its ascending and descending projections serve stress-related cardiovascular responses in the rat. J. Auton. Nerv. Syst. 1992, 41, 157–176. [Google Scholar] [CrossRef]
- Saitoh, K.; Tilson, H.A.; Shaw, S.; Dyer, R.S. Possible role of the brainstem in the mediation of prepulse inhibition in the rat. Neurosci. Lett. 1987, 75, 216–222. [Google Scholar] [CrossRef]
- Hoffman, H.S.; Ison, J.R. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol. Rev. 1980, 87, 175–189. [Google Scholar] [CrossRef]
- Gómez-Nieto, R.; Rubio, M.E.; López, D.E. Cholinergic input from the ventral nucleus of the trapezoid body to cochlear root neurons in rats. J. Comp. Neurol. 2008, 506, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Nieto, R.; Horta-Júnior, J.A.; Castellano, O.; Sinex, D.G.; López, D.E. Auditory prepulse inhibition of neuronal activity in the rat cochlear root nucleus. In The Neurophysiological Bases of Auditory Perception; Palmer, A.R., Meddis, R., López Poveda, E.A., Eds.; Springer: New York, NY, USA, 2010; pp. 79–90. [Google Scholar] [CrossRef]
- Gómez-Nieto, R.; Sinex, D.G.; Horta-Júnior, J.d.A.C.; Castellano, O.; Herrero-Turrión, J.M.; López, D.E. A fast cholinergic modulation of the primary acoustic startle circuit in rats. Brain Struct. Funct. 2014, 219, 1555–15573. [Google Scholar] [CrossRef]
- Hormigo, S.; Gómez-Nieto, R.; Castellano, O.; Herrero-Turrión, J.M.; López, D.E.; Horta-Júnior, J.A.C. The noradrenergic projection from the locus coeruleus to the cochlear root neurons in rats. Brain Struct. Funct. 2014, 220, 1477–1496. [Google Scholar] [CrossRef]
- Yeomans, J.S.; Lee, J.; Yeomans, M.H.; Steidl, S.; Li, L. Midbrain pathways for prepulse inhibition and startle activation in rat. Neuroscience 2006, 142, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Fendt, M.; Koch, M. Cholinergic modulation of the acoustic startle response in the caudal pontine reticular nucleus of the rat. Eur. J. Pharmacol. 1999, 370, 101–107. [Google Scholar] [CrossRef]
- Weible, A.P.; Moore, A.K.; Liu, C.; DeBlander, L.; Wu, H.; Kentros, C.G.; Wehr, M. Perceptual gap detection is mediated by gap termination responses in auditory cortex. Curr. Biol. 2014, 24, 1447–1455. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Patil, K.V.; Han, C.; Fabella, B.; Canlon, B.; Someya, S.; Cederroth, C.R. GLAST deficiency in mice exacerbates gap detection deficits in a model of salicylate-induced tinnitus. Front. Behav. Neurosci. 2016, 10, 158. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Paublete, R.; Canlon, B.; Cederroth, C.R. Differential Neural Responses Underlying the Inhibition of the Startle Response by Pre-Pulses or Gaps in Mice. Front. Cell. Neurosci. 2017, 11, 19. [Google Scholar] [CrossRef]
- Swerdlow, N.R.; Geyer, M.A.; Braff, D.L. Neural circuit regulation of prepulse inhibition of startle in the rat: Current knowledge and future challenges. Psychopharmacology 2001, 156, 194–215. [Google Scholar] [CrossRef]
- Swerdlow, N.R.; Caine, S.B.; Braff, D.L.; Geyer, M.A. The neural substrates of sensorimotor gating of the startle reflex: A review of recent findings and their implications. J. Psychopharmacol. 1992, 6, 176–190. [Google Scholar] [CrossRef]
- Kumari, V.; Antonova, E.; Geyer, M.A.; Ffytche, D.; Williams, S.C.; Sharma, T. A fMRI investigation of startle gating deficits in schizophrenia patients treated with typical or atypical antipsychotics. Int. J. Neuropsychopharmacol. 2006, 10, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Kumari, V.; Gray, J.A.; Geyer, M.A.; Ffytche, D.; Soni, W.; Mitterschiffthaler, M.T.; Vythelingum, G.N.; Simmons, A.; Williams, S.C.; Sharma, T. Neural correlates of tactile prepulse inhibition: A functional MRI study in normal and schizophrenic subjects. Psychiatry Res. Neuroimaging 2003, 122, 99–113. [Google Scholar] [CrossRef]
- Campbell, L.E.; Hughes, M.; Budd, T.W.; Cooper, G.; Fulham, W.R.; Karayanidis, F.; Hanlon, M.C.; Stojanov, W.; Johnston, P.; Case, V.; et al. Primary and secondary neural networks of auditory prepulse inhibition: A functional magnetic resonance imaging study of sensorimotor gating of the human acoustic startle response. Eur. J. Neurosci. 2007, 26, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Postma, P.; Gray, J.A.; Sharma, T.; Geyer, M.; Mehrotra, R.; Das, M.; Zachariah, E.; Hines, M.; Williams, S.C.; Kumari, V. A behavioural and functional neuroimaging investigation into the effects of nicotine on sensorimotor gating in healthy subjects and persons with schizophrenia. Psychopharmacology (Berl). 2006, 184, 589–599. [Google Scholar] [CrossRef]
- Burgess, H.A.; Granato, M. Sensorimotor Gating in Larval Zebrafish. J. Neurosci. 2007, 27, 4984–4994. [Google Scholar] [CrossRef] [Green Version]
- Sipes, T.E.; Geyer, M.A. Functional behavioral homology between rat 5-HT1B and guinea pig 5-HT1D receptors in the modulation of prepulse inhibition of startle. Psychopharmacology (Berl.) 1996, 125, 231–237. [Google Scholar] [CrossRef]
- Vaillancourt, C.; Boksa, P. Birth insult alters dopamine-mediated behavior in a precocial species, the guinea pig. Implications for schizophrenia. Neuropsychopharmacology 2000, 23, 654–666. [Google Scholar] [CrossRef]
- Lind, N.M.; Arnfred, S.M.; Hemmingsen, R.P.; Hansenet, K.A. Prepulse inhibition of the acoustic startle reflex in pigs and its disruption by d-amphetamine. Behav. Brain Res. 2004, 155, 217–222. [Google Scholar] [CrossRef]
- Linn, G.S.; Negi, S.S.; Gerum, S.V.; Javitt, D.C. Reversal of phencyclidine induced prepulse inhibition deficits by clozapine in monkeys. Psychopharmacology 2003, 169, 234–239. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Nieto, R.; Hormigo, S.; López, D.E. Prepulse Inhibition of the Auditory Startle Reflex Assessment as a Hallmark of Brainstem Sensorimotor Gating Mechanisms. Brain Sci. 2020, 10, 639. https://doi.org/10.3390/brainsci10090639
Gómez-Nieto R, Hormigo S, López DE. Prepulse Inhibition of the Auditory Startle Reflex Assessment as a Hallmark of Brainstem Sensorimotor Gating Mechanisms. Brain Sciences. 2020; 10(9):639. https://doi.org/10.3390/brainsci10090639
Chicago/Turabian StyleGómez-Nieto, Ricardo, Sebastián Hormigo, and Dolores E. López. 2020. "Prepulse Inhibition of the Auditory Startle Reflex Assessment as a Hallmark of Brainstem Sensorimotor Gating Mechanisms" Brain Sciences 10, no. 9: 639. https://doi.org/10.3390/brainsci10090639