Laboratory of Behavioral Neuroscience, Intramural Research Program, National Institute of Mental Health,
Bethesda, MD, USA
The rich repertoire of mouse social behaviors makes it possible to use mouse models to study neurodevelopmental disorders characterized by social deficits. The fact that mice are naturally nocturnal animals raises a critical question of whether behavioral experiments should be strictly conducted in the dark phase and whether light phase testing is a major methodologically mistake. Although mouse social tasks have been performed in both phases in different laboratories, there seems to be no general consensus on whether testing phase is a critical factor or not. A recent study from our group showed remarkably similar social scores obtained from inbred mice tested in the light and the dark phase, providing evidence that light phase testing could yield reliable results as robust as dark phase testing for the sociability test. Here we offer a comprehensive review on mouse social behaviors measured in light and dark phases and explain why it is reasonable to test laboratory mice in experimental social tasks in the light phase.
Mice are naturally nocturnal animals (McLennan and Taylor-Jeffs, 2004
; Refinetti, 2004
) which tend to be active after dark and rest during the day (Arakawa et al., 2007
; Laviola et al., 1994
; Panksepp and Lahvis, 2007
; Terranova et al., 1998
). The dark and light phases are sometimes referred to as “active phase” and “inactive phase”, respectively, reflecting the nocturnal nature of the mouse (Arakawa et al., 2007
). Numerous studies have described circadian variations in physiological processes (Arraj and Lemmer, 2006
; Castillo et al., 2005
; Kohsaka et al., 2007
; Li et al., 2006
; Refinetti, 2007
), circulating hormones (Li et al., 2006
; Malisch et al., 2008
), levels of gene expression (Kalamatianos et al., 2004
; Nakamura et al., 2008
; Sheward et al., 2007
; Yambe et al., 2002
), wheel running activities (Kopp, 2001
; Kriegsfeld et al., 2008
; Meng et al., 2008
; Valentinuzzi et al., 2000
), cognitive performances (Chaudhury and Colwell, 2002
; Eckel-Mahan et al., 2008
; Roedel et al., 2006
; Valentinuzzi et al., 2004
) and social behaviors (Arakawa et al., 2007
; Van Loo et al., 2004
) in laboratory rats and mice.
In mice, active social behaviors mostly occur after dark. A recent study by Arakawa et al. (2007)
provides a useful description of spontaneous social behaviors of inbred mice in a laboratory
environment across the circadian cycle. Groups of 3-4 C57BL/6J (B6) mice were placed in a semi-natural living environment – a large apparatus built to resemble the burrow systems in which small rodent species dwell in the wild. Spontaneous social behaviors were recorded in both light and dark phases for 2 weeks and scored from the videotapes afterwards. Time sampling results showed that mice exhibit more social approach towards other animals in the dark phase and more huddling during the light hours. This study confirmed that standard laboratory inbred mice, like wild-dwelling mice, prefer to engage in social activities in the dark phase. Another important implication from this study is that sociability might be an enduring trait that manifests differently in opposite phases. Active approach may be the major social behavior in the dark phase, while inactive huddling predominates as a reflection of sociability in the light phase.
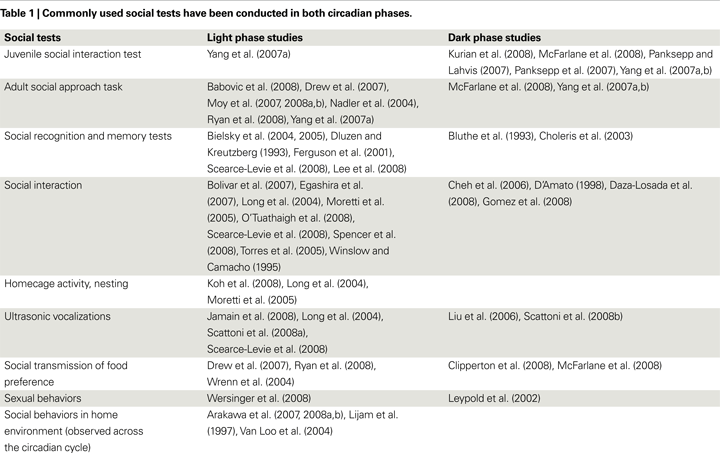
Although most people are aware of the fact that mice are nocturnal, many researchers conduct behavioral tests in the light phase because of practical difficulties. As such, the current literature on mouse social behaviors
consists of studies done in both phases. As shown in Table 1
, while dark phase experiments continue to contribute to our current knowledge of mouse social behaviors (Arakawa et al., 2008a
, b
; Bluthe et al., 1993
; Cheh et al., 2006
; Clipperton et al., 2008
; D’Amato, 1998
; Daza-Losada et al., 2008
; Gomez et al., 2008
; Kurian et al., 2008
; Leypold et al., 2002
; Liu et al., 2006
; McFarlane et al., 2008
; McNaughton et al., 2008
; Panksepp and Lahvis, 2007
; Panksepp et al., 2007
; Scattoni et al., 2008b
; Torres et al., 2005
; Yang et al., 2007b
), light phase experiments have also been producing remarkable findings on genetic, neuroanatomical, and environmental factors that are important for mouse social behaviors (Babovic et al., 2008
; Bielsky et al., 2004
, 2005
; Dluzen and Kreutzberg, 1993
; Egashira et al., 2007
; Ehninger et al., 2008
; Fairless et al., 2008
; Ferguson et al., 2001
; Jamain et al., 2008
; Koh et al., 2008
; Lee et al., 2008
; Long et al., 2004
; Moretti et al., 2005
; Moy et al., 2007
, 2008
; O’Tuathaigh et al., 2008
; Refinetti, 2004
; Ryan et al., 2008
; Scattoni et al., 2008a
; Scearce-Levie et al., 2008
; Spencer et al., 2008
; Stack et al., 2008
; Wersinger et al., 2008
; Winslow and Camacho, 1995
; Wrenn et al., 2004
).
A prevailing concern is that social scores obtained from experiments done in opposite phases may not be comparable. To address this concern, we compared social scores reported in light and dark phase studies. In the social recognition test, the subject is presented with a novel mouse for multiple short trials separated by 20–30 min intervals. In a dark phase studies, the baseline sniff time was approximately 120 s in DBA/2 mice tested in a 4-min trial (Bluthe et al., 1993
) and 150 s in wild type mice tested in a 5-min trial (Choleris et al., 2003
). In light phase experiments, the baseline sniff time was approximately 90 s in CD-1 mice tested in a 2-min trial (Dluzen and Kreutzberg, 1993
) and 60 s in 129X1/SvJ mice tested in a 90-s trial (Scearce-Levie et al., 2008
). Significant reductions in sniff time towards the re-introduced stimulus mouse was found in mice tested in both phase (Bluthe et al., 1993
; Choleris et al., 2003
; Dluzen and Kreutzberg, 1993
; Scearce-Levie et al., 2008
). Moreover, in a study that directly compared the social recognition test done in opposite phases, phase effect was not significantly different between light and dark phases for the level of social sniffing, and similar strain differences were found in both phases (Hossain et al., 2004
). Taken together, these findings supports the interpretation that mice tested in both circadian phases exhibit comparable levels of active social investigation (i.e. social sniff) towards novel social stimuli, and that light phase testing
can be used to evaluate social recognition/memory.
In our three-chambered social approach test (Chadman et al., 2008
; Crawley et al., 2007
; McFarlane et al., 2008
; Moy et al., 2004
, 2007
, 2008
; Nadler et al., 2004
; Ryan et al., 2008
; Stack et al., 2008
; Yang et al., 2007a
,b
), more time spent in the chamber containing a novel mouse than in the chamber containing a novel object indicates the presence of sociability (i.e. greater interest in interacting with a novel conspecific than with a novel inanimate object). Studies published since the invention of this task have consistently showed high sociability in B6 mice. Interestingly, both light phase studies (Moy et al., 2007
, 2008
; Nadler et al., 2004
; Ryan et al., 2008
) and dark phase experiments (McFarlane et al., 2008
; Yang et al., 2007b
) showed that B6 mice spend around 300 s in the chamber containing the novel mouse and about 200 s in the chamber containing the novel objects in a 600-s test session, indicating that social approach scores are similar in animals tested in opposite phases, as described in more detail below.
Taken together, evidence summarized above indicates that the levels of social behaviors are actually quite similar between light and dark phase studies.
The standard daytime working hours of humans overlap with the “inactive” phase of mice. The large body of literature documenting murine nocturnality
has led many investigators to believe that dark phase mouse behaviors are more analogous to daytime human behaviors and that dark phase testing promises superior outcomes as compared to light phase testing (Hossain et al., 2004
). While there is no doubt that dark phase testing is ethologically correct and theoretically ideal, this approach generates a number of practical difficulties. First, dark phase testing, which commonly requires reversing or shifting the light/dark cycles (Blanchard et al., 2005
; Yang et al., 2007a
), can not be easily accommodated in many research facilities. Many researchers have to share animal housing rooms with several other investigators who may not want to reverse the LD cycle. Second, dark phase testing takes more space. For light phase experiments, subjects waiting to be tested can be held in communal acclimating room or even in the hallway outside the experimental room. Dark phase testing, which requires dedicated dark rooms for acclimating subjects and/or mice used as novel social stimuli, is simply not practical for researchers with tight experimental space (not an uncommon situation). Third, dark phase testing increases difficulties in conducting behavioral tests. Cages need to be covered with light-proof materials when transferring animals between rooms and whenever the door is opened. The red light generated from commonly used incandescent red light bulbs is less than ideal for human visual perception (McLennan and Taylor-Jeffs, 2004
), making it difficult to perform tasks that require visual acuity, e.g. identifying animals (by tattoos or ear tags), live scoring behaviors, and taking notes. Also, catching escaped animals in a dark room can be quite difficult. In experiments in which the some subjects are hyperactive or irritable, this problem could cause frequent interruptions to the experiment and unnecessary disruptions to the subjects.
High sociability in B6 mice and low sociability in BTBR mice have been consistently reported in a number of recent studies (McFarlane et al., 2008
; Moy et al., 2007
, 2008
; Yang et al., 2007a
, b
), some of which were done in the light phase (Moy et al., 2007
, 2008
) and others in the dark phase (McFarlane et al., 2008
; Yang et al., 2007b
). Two questions arise: (1) In the three-chambered social approach apparatus, would mice be more interested in interacting with the novel mouse in dark phase than in the light phase? (2) Would testing social approach behaviors in opposite circadian phases yield quantitatively different outcomes? We addressed these questions in the primary publication (Yang et al., 2007a
). Social approach scores were compared between two cohorts of B6 and BTBR males, one cohort raised in a conventional 12:12 light/dark cycle (lights on at 6:00 a.m.) and tested in the light phase, under fluorescent light, and the other cohort raised in the reversed 12:12 light/dark cycles (lights on at 9:00 p.m.) and tested in the dark phase, under incandescent red light illumination. Data obtained from the two cohorts were strikingly similar. B6 tested in the light phase spent as much time in the chamber with the novel mouse as B6 tested in the dark phase, and displayed similar time spent sniffing the novel mouse. Moreover, qualitatively and quantitatively similar strain differences in chamber time and sniff time were found in both phases, indicating that testing sociability in the light phase produced results highly comparable to dark phase experiments.
The second part of our study (Yang et al., 2007a
) described a series of five experiments to test whether circadian phase affect the expression of social behaviors in mice bearing mutations of the vasopressin receptor subtype 1b gene (Avpr1b). In addition, we assessed the extent to which results of the social approach task are replicable in our laboratory. Two cohorts of Avpr1b mice were tested in the light phase and three cohorts in the dark phase. One light phase experiment reported genotype differences in chamber time but not in sniff time. The second light phase experiment and all three dark phase experiments found no genotype differences in either chamber time or sniff time. Thus, as in the B6 vs. BTBR experiments, similar levels of chamber time and sniff time were found in Avpr1b mice tested in the two circadian phases, as well as across cohorts. These highly consistent results from the Avpr1b experiments indicate that circadian phase is not likely to affect the outcome of a genetic study.
The last part of our study (Yang et al., 2007a
) compared juvenile play behaviors of Apvr1b mice tested in the two circadian phases. To increase the motivation of social interaction, pups were taken from the home cage and isolated in clean cages for 1 h before the play test. Two non-sibling pups were then placed in a novel area (Noldus PhenoTyper Observer 3000 chamber, Noldus, Leesburg, Virginia) and allowed to interact freely for 30 min. Social behaviors were recorded and scored afterwards. Juvenile Avpr1b of all genotypes tested in the two circadian phases exhibited similar levels of active social investigation and play soliciting behaviors, indicating that light phase testing is acceptable for studying juvenile social behaviors as well (Yang et al., 2007a
).
Taken together, our findings demonstrate that mice can actively perform the social approach task in the light phase, and that results from light and dark phases are comparable.
With a substantial body of literature clearly showing circadian variations in social behaviors in rodents, how does one explain the similar levels of sociability in mice tested in opposite phases, and the fact that many light phase studies have yielded meaningful results? Field studies have shown that many species of nocturnal rodents are able to adjust their activities according to non-photic factors
including predation and conspecific competition (Daily and Ehrlich, 1996
; Mistlberger and Skene, 2004
; Mrosovsky, 2003
; Shkolnik, 1971
). For laboratory mice, human activities in the vivarium and test facility may act analogously as circadian entrainers. In standard commercial and research facilities, routine cleaning, feeding, cage changing, and inspection of mice are conducted during the light phase. These animal husbandry requirements represent unavoidable disruptions to mice during their natural resting phase. It is probable that animals that are better at adapting to such daily disruption have enjoyed reproductive success superior to those individuals who were unable to adapt well. Consequently, modern laboratory mouse colonies might largely consist of animals that have evolved to be able to adjust physiologically and behaviorally to the demands of the vivarium environment. Further, the ethological importance of investigating a novel conspecific may override the tendency of mice to sleep during the light phase. Thus, social interaction assays may be among the least sensitive to circadian phase, at least for laboratory mice.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supported by the National Institute of Mental Health Intramural Research Program.
Babovic, D., O’Tuathaigh, C. M., O’Connor, A. M., O’Sullivan, G. J., Tighe, O., Croke, D. T., Karayiorgou, M., Gogos, J. A., Cotter, D., and Waddington, J. L. (2008). Phenotypic characterization of cognition and social behavior in mice with heterozygous versus homozygous deletion of catechol-O-methyltransferase. Neuroscience 155, 1021–1029.
Crawley, J. N., Chen, T., Puri, A., Washburn, R., Sullivan, T. L., Hill, J. M., Young, N. B., Nadler, J. J., Moy, S. S., Young, L. J., Caldwell, H. K., and Young, W. S. (2007). Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides 41, 145–163.
Jamain, S., Radyushkin, K., Hammerschmidt, K., Granon, S., Boretius, S., Varoqueaux, F., Ramanantsoa, N., Gallego, J., Ronnenberg, A., Winter, D., Frahm, J., Fischer, J., Bourgeron, T., Ehrenreich, H., and Brose, N. (2008). Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc. Natl. Acad. Sci. U.S.A. 105, 1710–1715.
Malisch, J. L., Breuner, C. W., Gomes, F. R., Chappell, M. A., and Garland, T., Jr. (2008). Circadian pattern of total and free corticosterone concentrations, corticosteroid-binding globulin, and physical activity in mice selectively bred for high voluntary wheel-running behavior. Gen. Comp. Endocrinol. 156, 210–217.
Meng, Q. J., Logunova, L., Maywood, E. S., Gallego, M., Lebiecki, J., Brown, T. M., Sladek, M., Semikhodskii, A. S., Glossop, N. R., Piggins, H. D., Chesham, J. E., Bechtold, D. A., Yoo, S. H., Takahashi, J. S., Virshup, D. M., Boot-Handford, R. P., Hastings, M. H., and Loudon, A. S. (2008). Setting clock speed in mammals: the CK1 epsilon tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron 58, 78–88.
O’Tuathaigh, C. M., O’Connor, A. M., O’Sullivan, G. J., Lai, D., Harvey, R., Croke, D. T., and Waddington, J. L. (2008). Disruption to social dyadic interactions but not emotional/anxiety-related behaviour in mice with heterozygous ‚knockout’ of the schizophrenia risk gene neuregulin 1. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 462–466.
Wrenn, C. C., Kinney, J. W., Marriott, L. K., Holmes, A., Harris, A. P., Saavedra, M. C., Starosta, G., Innerfield, C. E,, Jacoby, A. S., Shine, J., Iismaa, T. P., Wenk, G. L., and Crawley, J. N. (2004). Learning and memory performance in mice lacking the GAL-R1 subtype of galanin receptor. Eur. J. Neurosci. 19, 1384–1396.
Yang, M., Scattoni, M. L., Zhodzishsky, V., Chen, T., Caldwell, H. K,, Young, W. S., McFarlane, H. G., and Crawley, J. N. (2007a). Similar social approach behaviors in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B knockout mice tested on conventional versus reverse light cycles, and in replications across cohorts. Front. Behav. Neurosci. 1, 9.