- 1Biological Psychiatry Laboratory, Department of Psychiatry, Jiangxi Mental Hospital/Affiliated Mental Hospital of Nanchang University, Nanchang, China
- 2Department of Pharmacy, Jiangxi Maternal and Child Health Hospital, Nanchang, China
- 3Department of Neurology, Second Affiliated Hospital of Nanchang University, Nanchang, China
- 4Department of Pharmacology, College of Medical Science, China Three Gorges University, Yichang, China
- 5Laboratory of Fear and Anxiety Disorders, Institute of Life Science, Nanchang University, Nanchang, China
As an endogenous neuromodulator, hydrogen sulfide (H2S) exerts multiple biological effects in the brain. Previous studies have shown that H2S is involved in the regulation of neural synaptic plasticity and cognition in healthy rodents. It is well known that there is a progressive decline of cognitive function that occurs with increased age. The purpose of this study was to investigate the role of H2S in aging-associated amygdalar synaptic plasticity and cued fear memory deficits as well as to explore the underlying mechanisms. We found that H2S levels in the amygdala were significantly lower in aged rats when compared with healthy adult rates, which displayed significant deficits in long-term potentiation (LTP) in the thalamo-lateral amygdala (LA) pathway and amygdala-dependent cued fear memory. Bath application of an H2S donor, sodium hydrogen sulfide (NaHS), significantly reversed the impaired LTP in brain slices from aged rats, and intra-LA infusion of NaHS restored the cued fear memory in aged rats. Mechanismly, we found that H2S treatment significantly enhanced NMDAR-mediated synaptic responses in the thalamo-LA pathway of aged rats. Notably, GluN2B-containing NMDARs, but not GluN2A-containing NMDARs, contributed to the effects of H2S on aging-associated impairments of amygdalar LTP and fear memory, because applying GluN2B antagonist could abolish the beneficial effects of NaHS treatment on amygdalar LTP and cognitive performance in aged rats. Collectively, these results show that H2S can reverse aging-associated amygdalar synaptic plasticity and fear memory deficits by restoring the function of GluN2B-containing NMDARs, suggesting that supplement of H2S might be a therapeutic approach for aging-related cognitive disorders.
Introduction
Cognitive decline is a natural part of aging (Corey-Bloom et al., 1996; Bishop et al., 2010) and memory is normally the first cognitive domains to decline as individuals age (Singh-Manoux et al., 2012). As a particular kind of memory, fear memory has been shown to be severely affected during the aging process (Deptula et al., 1993; Gould and Feiro, 2005; Fukushima et al., 2008; Kaczorowski et al., 2012; Zeng et al., 2012). Aging-related processes, including inflammatory, oxidative stress, endocrine and immune changes cause functional and anatomical alterations of the amygdala, a key brain area for fear memories, in turn contributing to aging-associated fear memory impairments (McGaugh, 2000; von Bohlen Und Halbach and Unsicker, 2002; Roozendaal et al., 2008; Kumar, 2015).
Synaptic plasticity, which includes long-term potentiation (LTP) and depression (LTD), is a broadly utilized cellular model of memory and learning (Lynch, 2004). Numerous studies have demonstrated a decline in LTP with increased age, and this defect in LTP is believed to underlie age-associated memory impairment (Mothet et al., 2006; Yang et al., 2010; Haxaire et al., 2012). The N-methyl-d-aspartate receptor (NMDAR) is one of the excitatory glutamate receptors known to play a significant role in both memory and learning (Collingridge, 1987; Kumar, 2015). Mounting evidence has indicated that aging is associated with hypofunction of NMDARs in regions of the brain associated with synaptic plasticity, memory, and learning (Kumar, 2015). For instance, NMDAR-mediated excitatory postsynaptic potentials (EPSPs) in the hippocampus are decreased in aged rodents (Yang et al., 2010; Haxaire et al., 2012) and reduced protein expression of NMDARs is observed in the hippocampus of aged animals (Liu et al., 2008; Zhao et al., 2009; Marquez Loza et al., 2017). Nowadays, it is generally accepted that hypofunction of NMDARs contributes to impediments in memory and learning that occur with increased age (Das and Magnusson, 2011; Foster, 2012; Kumar and Foster, 2013; Li et al., 2017) and augmenting the expression and functional activity of the NMDAR subunit could overcome the cognitive impairments in aged animals (Slutsky et al., 2010; Robillard et al., 2011; Brim et al., 2013; Wang et al., 2014).
Hydrogen sulfide (H2S) is a highly toxic and flammable gas that is colorless in appearance. Currently, there is mounting evidence to suggest that H2S may function as an endogenous gasotransmitter because it regulates several physiological and pathophysiological activities in different biochemical processes (Paul and Snyder, 2015, 2017). There are two mechanisms by which H2S produced at high levels (50–160 μmol/L) in the brain, either by the union of cysteine aminotransferase with 3-mercaptopyruvate sulfurtransferase (3-MST) or by the enzyme cystathionine-β-synthase (CBS) (Hu et al., 2011). Abe and Kimura (1996) were the first researchers to investigate the influences of H2S on synaptic plasticity and the function of NMDAR function. They showed that physiological concentrations of H2S could specifically enhance NMDAR activity and facilitate LTP induction in the hippocampus (Abe and Kimura, 1996). Dysfunction of H2S signaling contributes to cognitive impairments in degenerative disorders, such as ischemic cerebral stroke and Alzheimer’s disease (AD) (Li et al., 2011; He et al., 2014; Yang et al., 2016). Aged rats showed decreased hippocampal levels of H2S and exogenous H2S could alleviate the impaired hippocampal NMDAR-dependent LTP (Li et al., 2017). However, the role that H2S may play in aging-associated amygdalar synaptic plasticity and deficits in fear memory remains unknown.
Previously, we have shown that H2S can exert a regulatory role in amygdalar LTP and cued fear memory in rats (Wang et al., 2015; Chen et al., 2017). Specifically, treatment with H2S promoted cued fear memory and amygdalar LTP by improving NMDAR function in normal rats (Wang et al., 2015); inhibition of endogenous H2S generation reduced the synaptic responses of amygdalar NMDARs and impaired cued fear memory in rats (Chen et al., 2017). The aim of this current study was to investigate if H2S could reverse aging-associated amygdalar synaptic plasticity and cued fear memory impairments in aged rats. H2S levels in the amygdala of aged rats were first examined. Then we investigated the influence of H2S donor on aging-associated amygdalar LTP and cued fear memory impairments. Next, NMDAR functions were determined to investigate the mechanisms by which H2S exerts beneficial effects on aging.
Materials and Methods
Animals
Thirty-two adult (3–4 months) and 60 aged (22–24 months) male Sprague-Dawley rats were obtained from the Hunan SJA Laboratory Animal Company (Changsha, Hunan, China). Rats were fed in a room with controlled light-dark cycle (12:12) and steady temperature (22 ± 2°C). Water and food were supplied ad libitum. This research was carried out in accordance with the EU Directive 2010/63/EU and was approved by the Review Committee for the Use of Human or Animal Subjects of Jiangxi Mental Hospital.
Measurement of H2S
The content of H2S in amygdala tissue was examined according to a method described in previous studies (Yang et al., 2016; Chen et al., 2017). In brief, the tissue of amygdala was isolated and homogenized in ice-cold KHPO4 buffer (pH 7.4, 10 μL buffer per mg tissue). The homogenate was centrifuged, and then 200 μL of supernatant was added to sealed Eppendorf tubes containing 200 μL zinc acetate (1% w/v). Then, 150 μL N,N-dimethyl-p-phenylenediamine sulfate (20 mM) in 7.2 M HCl and 100 μL FeCl3 (30 mM) in 1.2 M HCl were added. Reactions were terminated by TCA (10% w/v, 250 μL) after 15 min color development. The resulting solutions were transferred to 96-well plates, and the absorbance of the mixture was measured at 670 nm.
Electrophysiological Recording
Field potentials recording was used to record LTP and NMDAR-mediated synaptic responses in the thalamo-LA pathway of rats. These procedures were conducted as previously described (Chen et al., 2017). In brief, the brain of the rat was quickly removed, and coronal slices (350 μm) containing amygdala were cut using a vibratome in ice-cold artificial cerebrospinal fluid (ACSF). Slices were recovered for at least 1.5 h by putting them in a holding chamber filled with oxygenated ACSF at 28°C. Then, a single slice was transferred to the perfusion-type recording chamber which was continuously superfused with ACSF pre-gassed with 95% O2/5% CO2. A bipolar electrode was placed in the internal capsule and a 3.0 M NaCl-filled glass electrode was placed in the LA region to record EPSPs. The stimulation frequency was 0.033 Hz and the stimulating intensity was set to produce an EPSP with 1/3 of the maximal response. High-frequency stimulation (HFS) was used to induce LTP. It consists of five trains at 100 Hz for 1 s, and the interval between trains is 90 s. APV (50 μM), a selective NMDAR antagonist, was used to test whether this LTP was NMDAR-dependent. To isolate NMDAR-mediated synaptic responses, ACSF was changed to magnesium-free ACSF containing glycine (10 μM) and CNQX (10 μM), an AMPA/kainate receptor antagonist (Yang et al., 2016; Chen et al., 2017). 30 μM bicuculline was added into the ACSF to block the activity of GABAA receptors when slices were recorded.
Fear Conditioning Task
Fear conditioning task was performed according to our previously described method (Chen et al., 2017). One day before fear training, animals were taken into the experimental room and handled for 5-min to make them familiarize the stimuli in the room. On the training day, rats were put into the training chamber for a 3-min acclimatizing period, and then received two pairings of a conditioned stimulus (CS: tone, 80 dB, 30 s) and unconditioned stimulus (US: electric foot shock, 0.75 mA, 1 s). CS and US were co-terminated, and the intertrial interval (ITI) between two trials was 90 s. Rats were stayed in the chamber for 30 s after termination of the procedure and then returned to their cage. 24 h later, cued fear memory was tested. During the test, the rat was placed in a chamber which differentiated from the training chamber for a 3-min acclimatizing period, and then received eight tones (30 s each) with an ITI of 10 s. Freezing was measured as the complete absence of activity except for respiratory movement. Fear memory was assessed by calculating the time spent freezing during the test periods. The freezing behavior was measured by a trained researcher who was blinded from the treatment.
Measurement of Pain Threshold
The pain threshold was measured according to a method described in our previous study (Chen et al., 2017). After the fear conditioning experiment, the rat was placed into another conditioning chamber for a 3-min acclimatizing period. The electric foot-shocks (1 s) were applied, starting at an intensity of 0.1 mA. The current intensity was increased stepwise by 0.05 mA. The respective current intensity of shock at which the rat began to jump was taken as the pain threshold.
Open Field Test
The open field test was conducted according to our previously described protocol (Wang et al., 2015). Briefly, an individual rat was allowed to freely behave in an open field arena (40 cm × 40 cm × 40 cm) monitored by a video tracking system. The locomotor activity and the time rats spent in the center region (20 cm × 20 cm) during the 3-min test period were monitored and assessed. The time taken in the central square is used for measuring anxiety-like behaviors.
Surgery and Injection
The procedures of surgery and injection were performed according to our previous study (Chen et al., 2017). A rat was placed in a stereotaxic apparatus after being anesthetized. Two 22-gauge cannulas were bilaterally implanted in the LA region (+2.8 AP, ±5.0 ML, -8.0 DV from bregma) and secured to the skull with dental acrylic. Rats were recovered for at least 7 days before the behavioral experiments started. When injection was performed, the inner sealing wire was replaced by a 33-gauge injector and drugs were infused into the LA in freely moving rats at a rate of 0.5 μL per min with total volume of 0.5 μL per side. The injector was left for 1.5-min after injection to minimize dragging of injected liquid along the injection track.
Statistical Analysis
All data are presented as the mean ± SEM and were analyzed using SPSS 18.0 software. The results were statistically analyzed using Student’s t-tests or one-way analysis of variance (ANOVA). For ANOVA, post hoc comparisons were performed using Bonferroni or Dunnett’s T3 post hoc tests, depending on the presence of equal or unequal variance in the groups, respectively. Statistical significance was set at p < 0.05.
Results
Aged Rats Display Deficits in Amygdalar Synaptic Plasticity and Cued Fear Memory
The content of H2S in amygdala tissue of aged rats was first measured. We found that amygdalar H2S levels in aged rats were lower than those detected in adult rats (p < 0.05; Figure 1A). H2S is a modulator for NMDAR function. We then adopted field potentials recording to examine NMDAR-dependent LTP in the thalamo-LA pathway of aged rats. HFS evoked a stable LTP in the thalamo-LA pathway from the adult rat slices (137.3 ± 7.1% of baseline), while pre-incubation with D-APV (50 μM), a NMDAR antagonist, for 10 min obviously blocked the LTP induction (101.4 ± 7.0% of baseline; p < 0.01 vs. control; n = 6–7 slices per group), suggesting that the LTP evoked by HFS was NMDAR-dependent (Figures 1B,C). In agreement with a previous study (Zeng et al., 2012), a significant suppression of NMDAR-dependent LTP was observed in the thalamo-LA pathway of aged rats in this study (adult rat 139.2 ± 7.9%, aged rat 105.4 ± 7.3%; p < 0.01; n = 8 slices per group; Figures 1D,E). Paired-pulse facilitation (PPF) is a common indicator used for evaluating presynaptic function. There was no significant difference in PPF between the two groups (p > 0.05; Figure 1F), suggesting that impairment of amygdalar LTP in aged rats should attribute to a modification in postsynaptic responsiveness, but not be from the altered release of the presynaptic neurotransmitter.
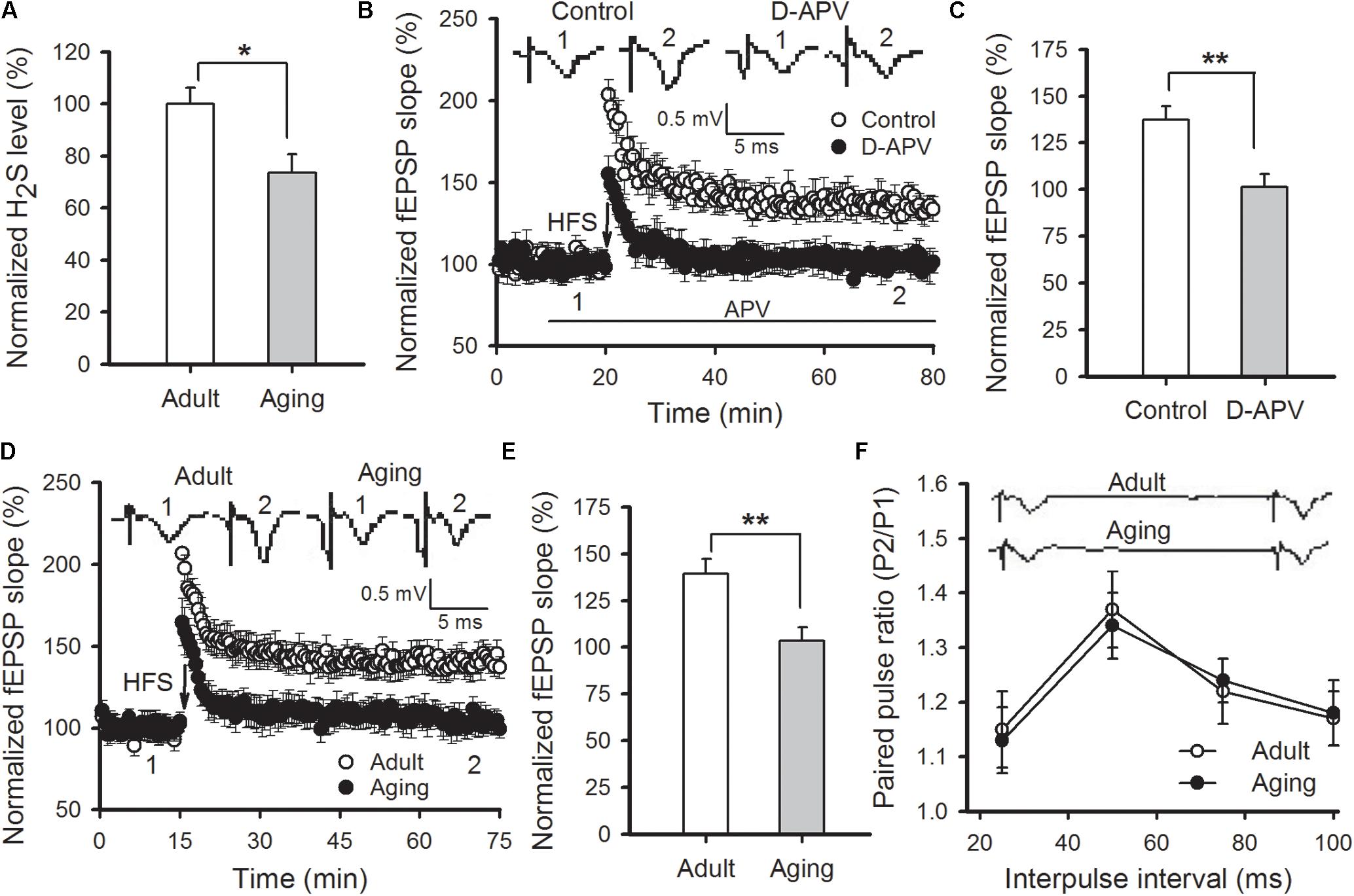
FIGURE 1. Aged rats display reduced hippocampal H2S level and impaired NMDAR-dependent LTP. (A) Amygdalar H2S levels were lower in aged rats in comparison to healthy adult rats (∗p < 0.05; n = 4 rats per group). (B) D-APV (50 μM) effectively blocked HFS-induced LTP in the thalamo-LA pathway of adult rats (n = 6–7 slices per group). (C) The mean EPSP slope, as depicted through histograms, from 50 to 60 min post-HFS in control and APV-treated slices (∗∗p < 0.01). (D) HFS failed to induce LTP in the thalamo-LA pathway of aged rats (n = 8 slices per group). (E) The mean EPSP slope, as depicted by histograms, from 50 to 60 min post-HFS in slices obtained from adult and aged rats (∗∗p < 0.01). (F) There was no significant difference in PPF between the healthy adult and aged rats (n = 6 slices per group). In (B,D,F), the insets are sample traces from the indicated times on the graph. All data are expressed as the mean ± SEM.
Then classical fear conditioning paradigm was conducted to evaluate changes in amygdala-dependent memory in aged rats. On the day of conditioning, rats were given two-tone (CS) – footshock (US). The aged rats displayed normal acquisition during the training phase, and a similar number of freezing responses was observed in aged rats compared to the adults (p > 0.05). At 24 h post-conditioning, the rats were put into a new chamber and the auditory conditioned stimulus tone was delivered 3 min later. Significantly increased levels of freezing elicited by the tone were observed in the adult rats, whereas the aged rats displayed a significant reduction in the number of freezing responses when compared with the adult rats (p < 0.01) (Figure 2A). Notably, the reduction in freezing behavior in aged rats was not due to adjustments in their pain threshold, state of anxiety, or locomotion of animals because no difference in these indexes was found between the two groups (Figures 2B,C). Altogether, our results confirm that aged rats exhibited deficits in cued fear memory and amygdalar NMDAR-dependent LTP.
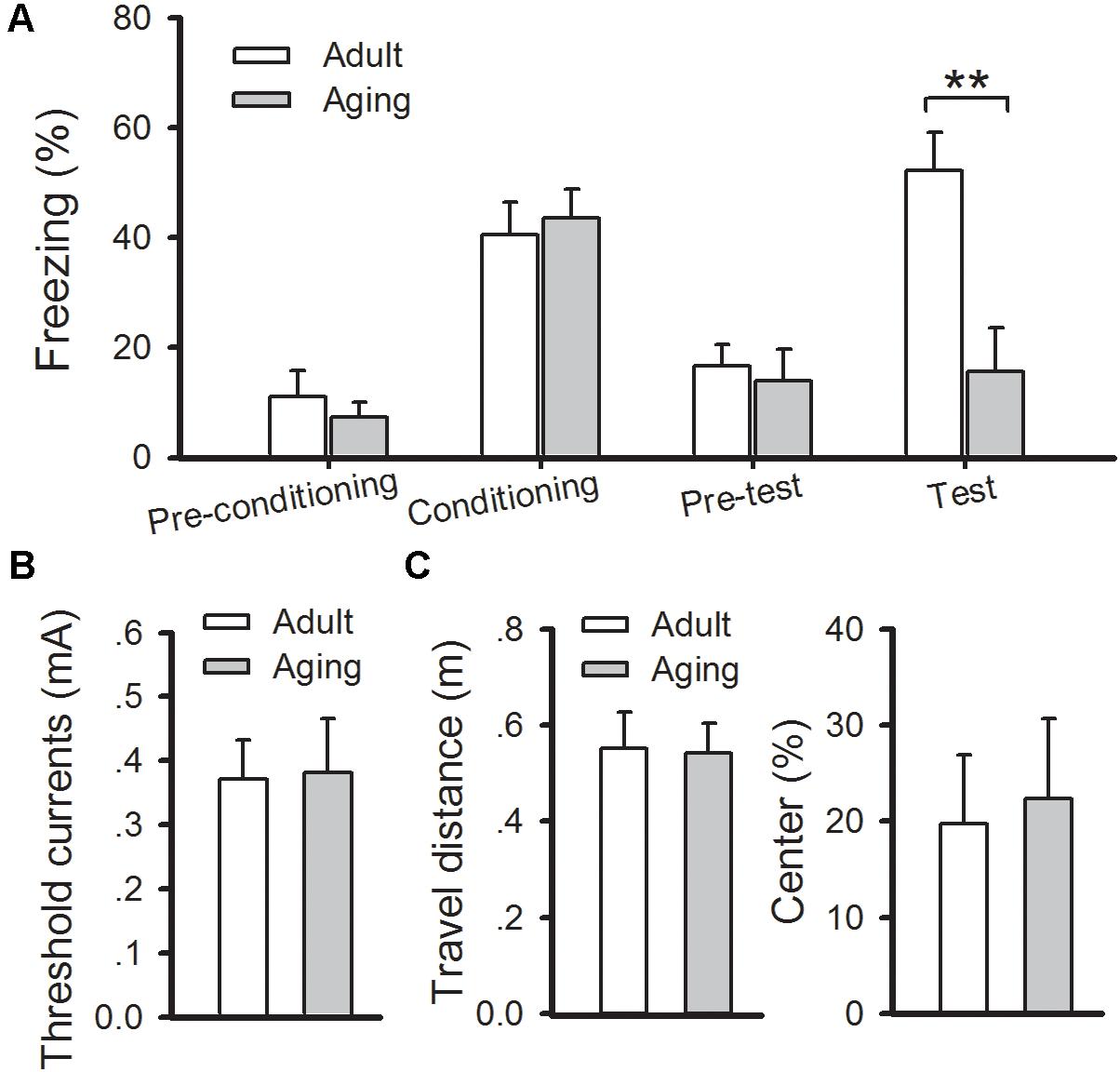
FIGURE 2. Aged rats exhibit a deficit in cued fear memory. (A) During fear training, there was no observable difference in freezing rates between healthy adult and aged rats, while freezing responses were significantly decreased in aged rats during cued memory test (∗∗p < 0.01). (B) There was no significant difference in the pain threshold between the two groups. (C) There was no significant difference in either locomotion (left) or anxiety (right) between the two groups. N = 7 rats per group. All data are expressed as the mean ± SEM.
H2S Donor Reverses the Impaired Amygdalar LTP and Fear Memory in Aged Rats
Next, we investigated whether application of an H2S donor could undo the amygdalar LTP and cued fear memory impairments in aged rats. NaHS is a commonly utilized exogenous donor for H2S. Exposure to 75 μM NaHS via bath application in slices of aged rats did not affect the basal neurotransmission (Figure 3A), but significantly enhanced the amygdalar NMDAR-dependent LTP to a level commonly found in adult rats (p < 0.05 vs. aging control; n = 6–9 slices per group; Figures 3B,C). In accordance with the electrophysiological findings, intra-LA infusion of 0.5 μL of 75 μM NaHS (per side) in aged rats 30 min prior to fear conditioning improved the freezing rate to that found in healthy adult rats [ANOVA, F(2,18) = 5.130, p = 0.017; n = 6–9 rats per group; Figures 3D,E]. As previously reported (Wang et al., 2015), treatment with NaHS did not significantly impact anxiety levels, sensitivity to pain, or locomotion in aged rats, ruling out the possibility that the effect of NaHS in aged rats was a gross change in pain sensitivity or anxiety (data not shown). Statistical analyses revealed a significant difference in mean freezing rates between ACSF-treated aged rats and NaHS-treated aged rats (p = 0.048), and the freezing rate of the NaHS-treated aged rats was indistinguishable from the level of the adult group (p > 0.05). These data suggest that the application of H2S may undo impaired amygdalar LTP and cued fear memory in aged rats.
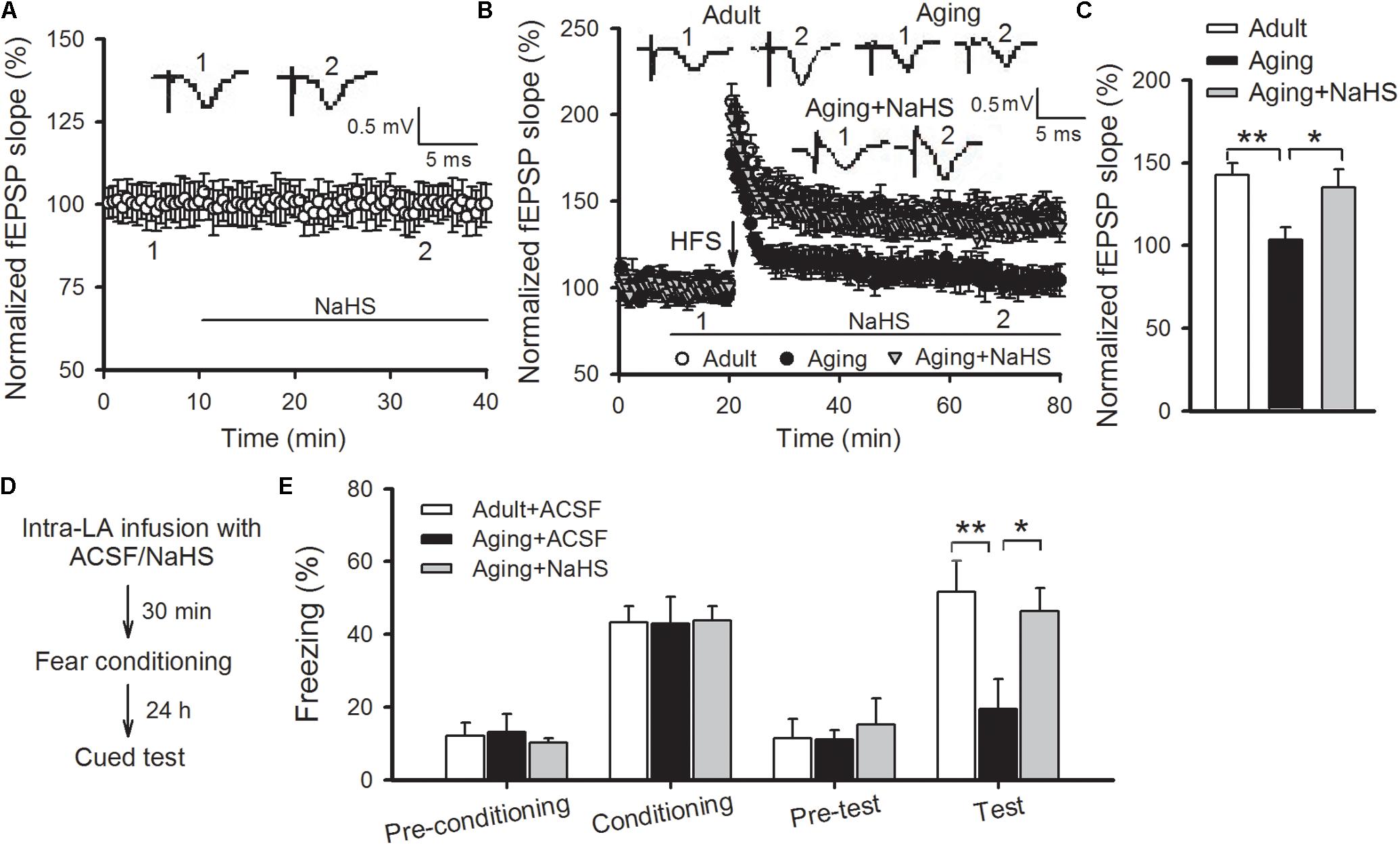
FIGURE 3. NaHS reverses the impaired amygdalar LTP and cued fear memory in aged rats. (A) Bath-applied NaHS (75 μM) had no effects on the basal EPSPs recorded in the thalamo-LA pathway of aged rats (n = 8 slices). (B) NaHS restored LTP in the thalamo-LA pathway in aged rats (n = 6–9 slices per group). (C) The mean EPSP slope, as illustrated by histograms, from 50 to 60 min post-HFS (∗p < 0.05, ∗∗p < 0.01). (D) Schematic describing the behavioral experiment setup. (E) Freezing responses were unaffected by intra-LA infusion with NaHS (75 μM, 0.5 μL per side) in aged rats during fear conditioning; however, the freezing rate was increased to levels consistently found in ACSF-treated adult rats during cued memory testing (∗p < 0.05, ∗∗p < 0.01; n = 5–8 rats per group). All data are expressed by mean ± SEM.
H2S Donor Improves Amygdalar GluN2B-Containing NMDAR Function in Aged Rats
Previous studies have revealed that H2S can regulate NMDAR function in LA neurons as well as amygdalar synaptic plasticity and cued fear memory (Wang et al., 2015; Chen et al., 2017). Hence, we tested whether the benefits of H2S in LTP and fear memory in aged rats arose from its regulatory role in NMDAR function. The NMDAR-mediated synaptic potentials were isolated by exchanging the normal ACSF for Mg2+-free ACSF, which contained 10 μM glycine and 10 μM of an AMPA receptor antagonist known as CNQX (Figure 4A). We found that NaHS treatment (75 μM) increased the amplitude of NMDAR-mediated EPSPs in the thalamo-LA synapses of aged rats [ANOVA, main effect of NaHS F(1,6) = 333.416, p < 0.001; Figure 4B]. Then input-output curves for NMDAR-EPSPs were conducted to further evaluate the effect of H2S on NMDAR function in aged rats. The amplitude of NMDAR-mediated EPSPs increased with incremental stimulus intensity (Figure 4C); however, the mean amplitude of NMDAR-mediated EPSP recorded in the slices obtained from aged rats was significantly lower than that of the healthy adult controls when the same stimulation was delivered. Also, bath application of NaHS in slices from aged rats increased the NMDAR-mediated EPSP to a level that was comparable to that of adult rats (n = 5 slices per group). The ANOVA (mixed model) for input-output curves showed a significant aging main effect [F(1,9) = 18.219, p < 0.001] and a significant NaHS treatment effect [F(1,9) = 9.720, p = 0.003], suggesting that administration of H2S could rescue amygdalar NMDAR function in aged rats.
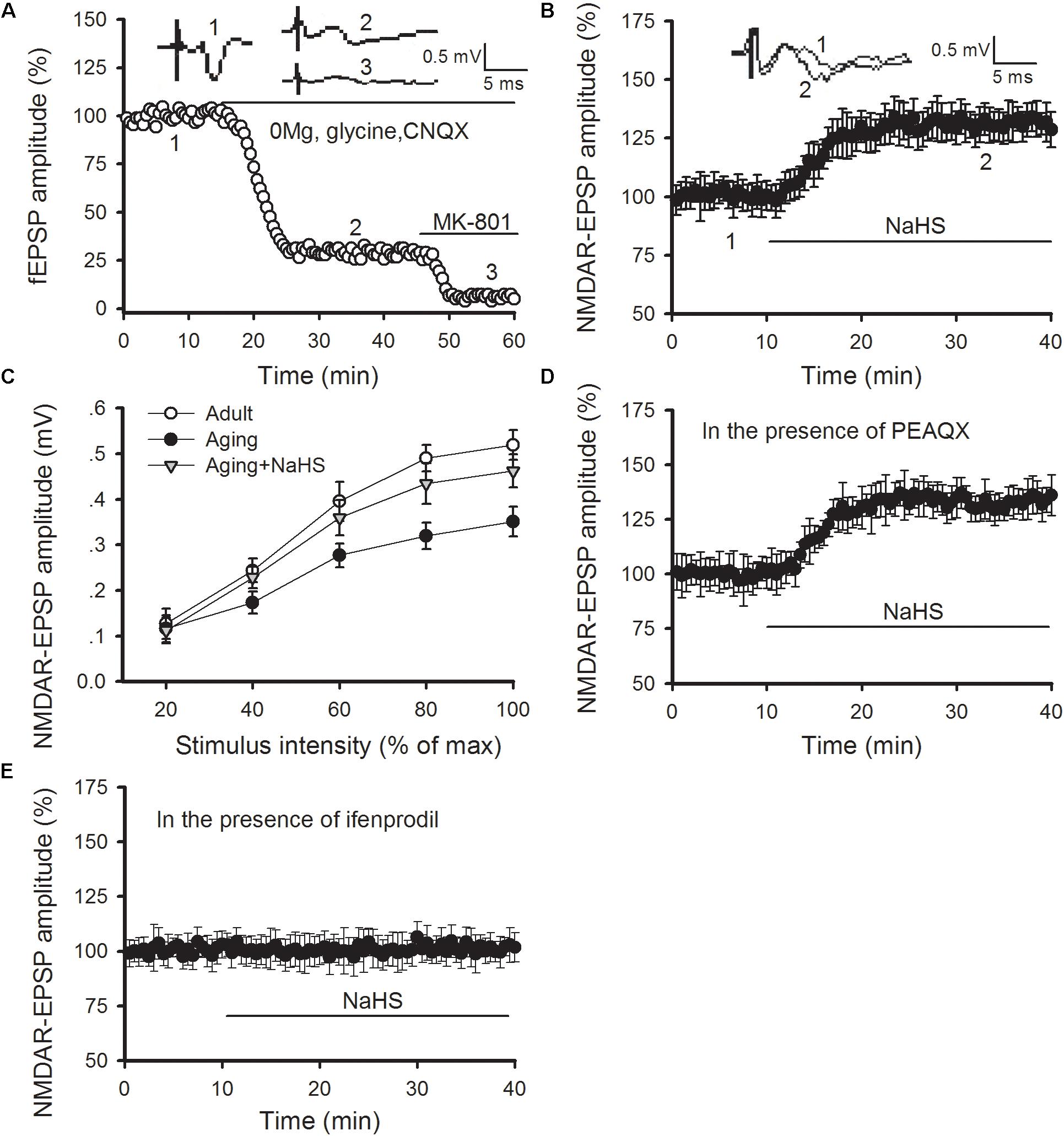
FIGURE 4. NaHS restores amygdalar NMDAR-mediated synaptic responses by regulating GluN2B-containing NMDAR function in aged rats. (A) NMDAR-mediated EPSPs in the thalamo-LA pathway were isolated by pharmacological intervention. Treatment with MK-801, an NMDAR non-competitive inhibitor, showed complete inhibition of EPSPs. (B) NaHS (75 μM) in slices obtained from aged rats increased the amplitude of NMDAR-EPSPs in the thalamo-LA pathway (n = 7 slices). (C) As shown by input–output curves, NaHS increased the amplitude of NMDAR-EPSPs to that of healthy adult controls in the slices from aged rats (p < 0.05 vs. aging alone) (n = 5 slices per group). (D) PEAQX (0.4 μM) pre-treatment did not impact the effect of NaHS on NMDAR-mediated EPSPs in aged rats (n = 6 slices). (E) Ifenprodil (3 μM) pre-treatment inhibited the action of NaHS on NMDAR-mediated EPSPs in aged rats (n = 6 slices). All data are expressed by mean ± SEM.
We further explored the influence of H2S on GluN2A- and GluN2B-containing NMDAR function by pharmacologic manipulation. PEAQX is a specific antagonist of GluN2A-containing NMDARs. In the presence of PEAQX (0.4 μM), NaHS treatment could still increase the NMDAR-EPSPs recorded in the thalamo-LA synapses of aged rats [ANOVA, main effect of NaHS F(1,5) = 374.676, p < 0.001; Figure 4D]. However, NaHS treatment failed to cause an increase in NMDAR-EPSPs in the thalamo-LA pathway in the presence of ifenprodil (3 μM), which is a specific antagonist of GluN2B subunit (Figure 4E). Together, these findings indicate that the GluN2B subunit is likely involved in the mechanism by which H2S effects amygdalar NMDAR function in aged rats.
Blockade of GluN2B Abolishes the Beneficial Effects of H2S on Amygdalar LTP and Fear Memory in Aged Rats
We then investigated whether blocking GluN2B-containing NMDARs modulated the beneficial effects of H2S in aged rats. As shown in Figures 5A,B, pretreating slices of aged rats with ifenprodil (3 μM) observably abolished the rescued effect of NaHS on the induction of amygdalar LTP (p < 0.05 vs. NaHS treatment alone; n = 6–8 slices per group). In a separate set of experiments, aged rats were bilateral intra-LA infused with ifenprodil or ACSF (0.2 μg per side) at 15 prior to NaHS infusion, and 30 min following NaHS treatment, were fear conditioned (Figure 5C). We found that treatment with ifenprodil in aged rats did not affect the behavioral performance during the training session, yet it observably blocked the enhancement effect of NaHS on cued fear memory [ANOVA, F(2,16) = 7.848, p = 0.005; Figure 5D]. Post hoc comparisons using Bonferroni’s test revealed that the freezing level in the NaHS-treated aged rats that were pre-infused with ifenprodil was not different from the freezing rate in ACSF-treated aged rats during fear memory test (p > 0.05), indicating that the GluN2B subunit may mediate the beneficial effects of H2S in aged rats.
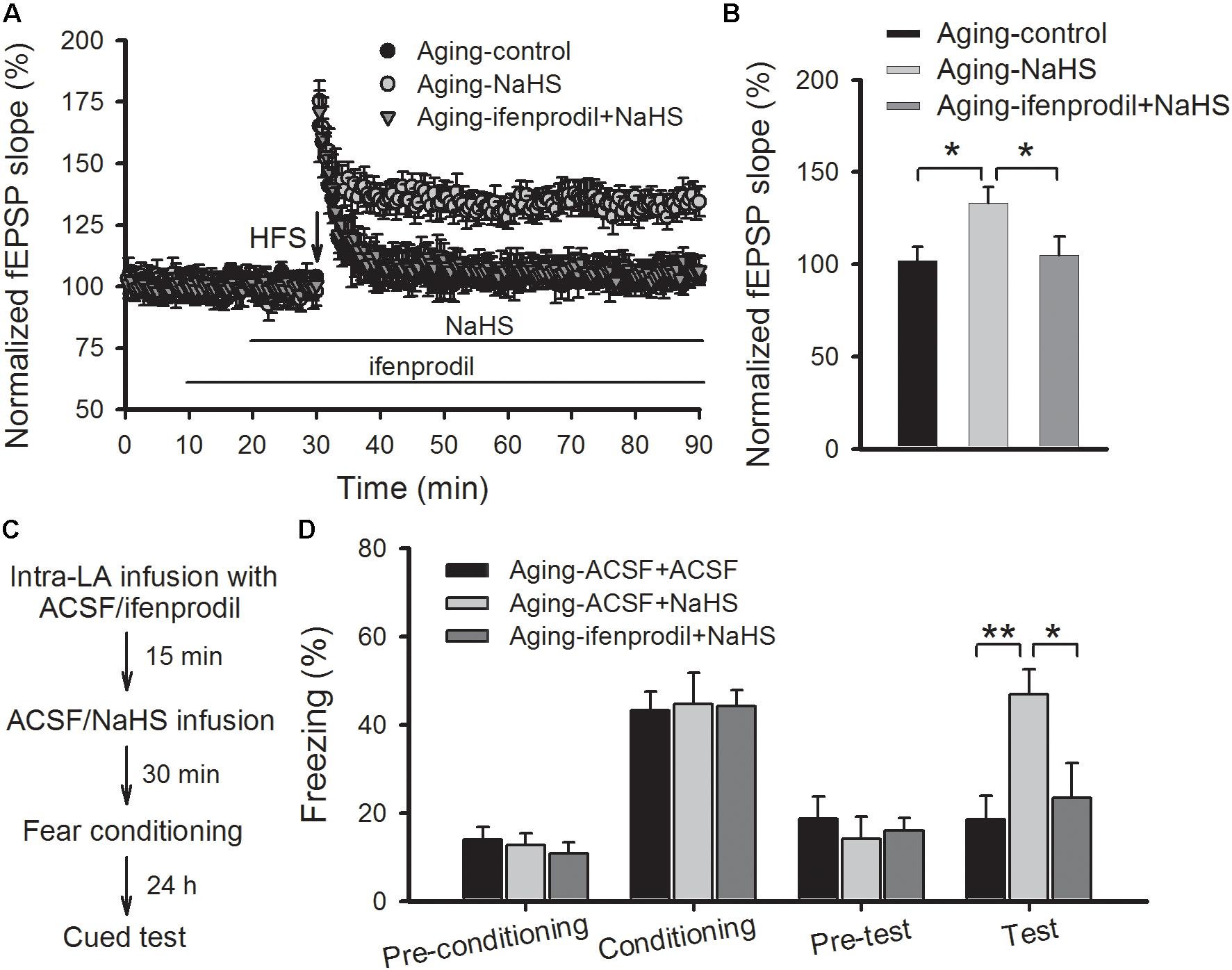
FIGURE 5. Treatment with a GluN2B antagonist abolishes the benefits of NaHS treatment on amygdalar LTP and fear memory in aged rats. (A) Pre-treatment with ifenprodil (3 μM) eliminated the benefits of NaHS on NMDAR-dependent LTP in the thalamo-LA pathway of aged rats (n = 6–8 slices per group). (B) The mean EPSP, as illustrated by histograms, from 50 to 60 min post-HFS (∗p < 0.05). (C) Schematic describing the behavioral experiment setup. (D) The mean freezing rate of rats during fear training and memory test sessions. Treatment with ifenprodil (0.2 μg, 0.5 μL per side) has no effect on behavioral performance in the training session but abolished the improvements, that resulted from NaHS treatment, on cued fear memory in aged rats (∗p < 0.05, ∗∗p < 0.01; n = 5–7 rats per group). All data are expressed by mean ± SEM.
Discussion
In this study, our primary finding was that supplementing aged rats with H2S can reverse deficits in cued fear memory and amygdalar NMDAR-dependent LTP, which is a validated model of memory and learning. The H2S effects may be associated with the upregulation of GluN2B-containing NMDAR because treatment with NaHS enhanced the synaptic responsiveness of GluN2B-containing NMDARs in the thalamo-LA pathway of aged rats and a GluN2B-specific antagonist could abolish the benefits of H2S in amygdalar LTP and cognitive performance. These findings suggest that H2S supplementation may be a promising strategy for the treatment of age-related fear memory deficits.
H2S acts as an endogenous neuromodulator in the brain (Hu et al., 2011). H2S at physiological concentration can promote NMDAR-dependent LTP, which is correlated with behaviorally relevant memory functions, in both amygdala and hippocampus (Abe and Kimura, 1996; Wang et al., 2015). An increase of H2S signal in the limbic system in vivo can improve fear memory in aged rats (Wang et al., 2015; Li et al., 2017). Accumulating studies have demonstrated a link between dysfunction of H2S signaling and the pathogenesis of age-related neurodegenerative disorders, including Parkinson’s disease (PD), AD and cerebral ischemia (Hu et al., 2011; Li et al., 2011; Yang et al., 2016). For instance, decreased H2S levels have been noted in the brains of AD animal models (He et al., 2014; Yang et al., 2016) and administration of H2S can alleviate the neuropathophysiological changes and undo the deficits in cognitive and synaptic plasticity in AD animal models (Giuliani et al., 2013; He et al., 2014; Yang et al., 2016). In this study, we demonstrate that amygdalar H2S levels were significantly decreased in aged rats and NaHS treatment could reverse the impairments in amygdalar NMDAR-dependent LTP and cued fear memory, suggesting that endogenous H2S has an important influence in aging-associated fear memory deficits.
What are the molecular mechanisms underlying the beneficial role that H2S plays in the synaptic and cognitive impairments of aged rats? NMDAR is a known action target of H2S in neurons. H2S could improve NMDAR-mediated synaptic responses in both amygdala and hippocampus (Abe and Kimura, 1996; Wang et al., 2015). Previously, NMDAR function was found to be impaired when endogenous H2S levels were suppressed (Chen et al., 2017). Hypofunction of NMDARs was shown to contribute to the cognitive impairments resulting from aging and many aging-related neurodegenerative diseases (Barnes et al., 1997; Kumar and Foster, 2013; Yang et al., 2016). Therefore, we surmise that the benefits of H2S in aged rats arise from its regulatory role in amygdalar NMDARs. This assumption was tested by recording NMDAR-mediated synaptic responses (Chen et al., 2017). The input-output responses of NMDAR-mediated potentials in the thalamo-LA pathway of aged rats were much lower than those of adult rats. In slices obtained from aged rats, application with H2S donor could observably increase NMDAR-mediated EPSP amplitude and return the NMDAR-EPSPs to levels found in healthy adult rats. The expression of GluN2A and GluN2B, two essential modulatory subunits of NMDAR, was decreased in multiple brain regions of aged rodents (Magnusson et al., 2007; Liu et al., 2008; Zhao et al., 2009). In this study, we showed that H2S donor could increase NMDAR-mediated synaptic potentials in the thalamo-LA synapses of aged rats with the presence of GluN2A specific antagonist, whereas no increase was observed in NMDAR-mediated EPSPs with the presence of GluN2B antagonist, suggesting that H2S specifically regulated the activity of GluN2B-containing NMDARs in the amygdala of aged rats. Furthermore, antagonizing GluN2B could abolish the benefits of H2S in the impairments of amygdalar LTP and cued fear memory in aged rats, suggesting that GluN2B-containing NMDARs may be a critical molecular target of H2S action.
GluN2B-containing NMDARs are proposed to mainly localize on the extrasynaptic site and considered as a crucial factor involved in apoptosis (Hardingham and Bading, 2010; Zhou et al., 2015). However, there is growing evidence suggesting that GluN2B-containing NMDARs may be functional at the synapse of the amygdala (Lopez de Armentia and Sah, 2003; Miwa et al., 2008; Duan et al., 2015). For instance, blockade of the receptors leads to deficits in amygdala-dependent LTP and memory (Rodrigues et al., 2001; Muller et al., 2009) and enhancement of the receptors promotes amygdalar synaptic plasticity and fear memory in rats (Abumaria et al., 2011; Wang et al., 2015). The GluN2B subunit is severely affected by the aging process (Magnusson et al., 2007; Zhao et al., 2009). Our results show that H2S treatment selectively enhanced GluN2B-mediated synaptic responses in the thalamo-LA pathway of aged rats and specific GluN2B antagonist abolished the benefits of H2S donor in amygdalar NMDAR-dependent cued fear memory and LTP, not only indicate that upregulation of GluN2B function is responsible for the effects of H2S in aged rats but also suggest that hypofunction of GluN2B-containing NMDARs contribute to aging-associated amygdalar synaptic plasticity and cognitive defects. However, the mechanisms by which GluN2B subunits are regulated by the H2S in the amygdala requires further investigations.
Activation of GluN2B and GluN2A is thought to be needed for hippocampal LTD and LTP, respectively (Liu et al., 2004). However, mounting evidence has indicated that GluN2B subunit is required for the induction of amygdalar LTP (Miwa et al., 2008; Muller et al., 2009; Wang et al., 2015). Perhaps the differences in biophysical properties and protein expression of the two subunits between amygdala and hippocampus could be responsible for this discrepancy. Specifically, more GluN2B subunits were found in the synapses of LA neurons compare to the CA1 region (Lopez de Armentia and Sah, 2003). The ratio of NMDA-EPSCs to AMPA-EPSCs in the LA neurons is significantly larger than that in the CA1 region and the effect of Mg2+ on the NMDARs is much lower in the LA neurons than in the CA1 neurons (Miwa et al., 2008). Our previous study showed that improvements in amygdalar LTP and emotional memory by H2S treatment were dependent on the activation of GluN2B-containing NMDARs (Wang et al., 2015). In the study, the restoration of GluN2B-containing NMDARs is shown to contribute to the beneficial properties of H2S on amygdalar LTP and fear memory in aged rats, also providing evidence for the importance of GluN2B subunit in amygdalar synaptic plasticity and memory.
There are some limitations in this study. First, due to the technological difficulties of performing whole-cell patch clamp recording in brain neurons of aged animals, we adopted field potential recording to detect a NMDAR-mediated synaptic response in the thalamo-LA synapses by pharmacologic manipulation. In the future, whole-cell patch clamp recording may allow for the direct measurement of NMDAR-mediated excitatory postsynaptic current in LA neurons to confirm the influence of H2S on NMDAR function in aged rats. Second, a single concentration and dosage of NaHS, selected from our previous studies (Wang et al., 2015; Chen et al., 2017), was used to investigate the influence of H2S on the impaired fear memory and synaptic plasticity in the amygdala. In the future, other concentration and dosage should be investigated to explore the effective range of H2S in the treatment of aging-associated amygdalar LTP and fear memory deficits. Moreover, whether the beneficial effects of H2S found in aged rats could be generalized to other animal species, especially for human being remains unknown. In addition, considering the lethality of H2S at high concentrations, determining the lowest effective dose will help with treatment planning. H2S could enhance the excitotoxicity of glutamate after a stroke and aggravate seizure-like events (Qu et al., 2006; Luo et al., 2014). Thus, the potential risks of brain damage and epileptic seizures should also be assessed during treatment planning.
Conclusion
We show that H2S can reverse amygdalar NMDAR-dependent LTP and fear memory deficits in aged rats, and the mechanism might be correlated to upregulation of GluN2B-containing NMDAR function. H2S is a gaseous molecule that can rapidly cross the blood-brain barrier. Inhaled H2S has been shown to produce a similar cognitive regulatory effect to that of the amygdala-specific delivery of the H2S donor (Wang et al., 2015). Thus, H2S inhalation may be an excellent therapy for synaptic plasticity and cognitive impairments in the elderly population.
Author Contributions
Y-JY and BW wrote the paper and designed the research. J-QZ, L-LZ, H-BC, WW, BR, J-RC, and X-FL performed the research. BY, TW, B-XP, and Y-JY analyzed the data.
Funding
This study was supported by grants from the National Natural Science Foundation of China to Y-JY (81600939 and 81560232), BW (81760254), and WW (81760256). It was also supported by the Natural Science Foundation of Jiangxi Province of China (20171BAB205020) and the Natural Science Foundation of Hubei Province of China (2014CFB186).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abe, K., and Kimura, H. (1996). The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 16, 1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996
Abumaria, N., Yin, B., Zhang, L., Li, X. Y., Chen, T., Descalzi, G., et al. (2011). Effects of elevation of brain magnesium on fear conditioning, fear extinction, and synaptic plasticity in the infralimbic prefrontal cortex and lateral amygdala. J. Neurosci. 31, 14871–14881. doi: 10.1523/JNEUROSCI.3782-11.2011
Barnes, C. A., Rao, G., and Shen, J. (1997). Age-related decrease in the N-methyl-D-aspartateR-mediated excitatory postsynaptic potential in hippocampal region CA1. Neurobiol. Aging 18, 445–452. doi: 10.1016/S0197-4580(97)00044-4
Bishop, N. A., Lu, T., and Yankner, B. A. (2010). Neural mechanisms of ageing and cognitive decline. Nature 464, 529–535. doi: 10.1038/nature08983
Brim, B. L., Haskell, R., Awedikian, R., Ellinwood, N. M., Jin, L., Kumar, A., et al. (2013). Memory in aged mice is rescued by enhanced expression of the GluN2B subunit of the NMDA receptor. Behav. Brain Res. 238, 211–226. doi: 10.1016/j.bbr.2012.10.026
Chen, H. B., Wu, W. N., Wang, W., Gu, X. H., Yu, B., Wei, B., et al. (2017). Cystathionine-beta-synthase-derived hydrogen sulfide is required for amygdalar long-term potentiation and cued fear memory in rats. Pharmacol. Biochem. Behav. 155, 16–23. doi: 10.1016/j.pbb.2017.03.002
Collingridge, G. (1987). Synaptic plasticity. The role of NMDA receptors in learning and memory. Nature 330, 604–605. doi: 10.1038/330604a0
Corey-Bloom, J., Wiederholt, W. C., Edelstein, S., Salmon, D. P., Cahn, D., and Barrett-Connor, E. (1996). Cognitive and functional status of the oldest old. J. Am. Geriatr. Soc. 44, 671–674. doi: 10.1111/j.1532-5415.1996.tb01830.x
Das, S. R., and Magnusson, K. R. (2011). Changes in expression of splice cassettes of NMDA receptor GluN1 subunits within the frontal lobe and memory in mice during aging. Behav. Brain Res. 222, 122–133. doi: 10.1016/j.bbr.2011.03.045
Deptula, D., Singh, R., and Pomara, N. (1993). Aging, emotional states, and memory. Am. J. Psychiatry 150, 429–434. doi: 10.1176/ajp.150.3.429
Duan, Y., Zhou, S., Ma, J., Yin, P., and Cao, X. (2015). Forebrain NR2B overexpression enhancing fear acquisition and long-term potentiation in the lateral amygdala. Eur. J. Neurosci. 42, 2214–2223. doi: 10.1111/ejn.13008
Foster, T. C. (2012). Dissecting the age-related decline on spatial learning and memory tasks in rodent models: N-methyl-D-aspartate receptors and voltage-dependent Ca2+ channels in senescent synaptic plasticity. Prog. Neurobiol. 96, 283–303. doi: 10.1016/j.pneurobio.2012.01.007
Fukushima, H., Maeda, R., Suzuki, R., Suzuki, A., Nomoto, M., Toyoda, H., et al. (2008). Upregulation of calcium/calmodulin-dependent protein kinase IV improves memory formation and rescues memory loss with aging. J. Neurosci. 28, 9910–9919. doi: 10.1523/JNEUROSCI.2625-08.2008
Giuliani, D., Ottani, A., Zaffe, D., Galantucci, M., Strinati, F., Lodi, R., et al. (2013). Hydrogen sulfide slows down progression of experimental Alzheimer’s disease by targeting multiple pathophysiological mechanisms. Neurobiol. Learn. Mem. 104, 82–91. doi: 10.1016/j.nlm.2013.05.006
Gould, T. J., and Feiro, O. R. (2005). Age-related deficits in the retention of memories for cued fear conditioning are reversed by galantamine treatment. Behav. Brain Res. 165, 160–171. doi: 10.1016/j.bbr.2005.06.040
Hardingham, G. E., and Bading, H. (2010). Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat. Rev. Neurosci. 11, 682–696. doi: 10.1038/nrn2911
Haxaire, C., Turpin, F. R., Potier, B., Kervern, M., Sinet, P. M., Barbanel, G., et al. (2012). Reversal of age-related oxidative stress prevents hippocampal synaptic plasticity deficits by protecting D-serine-dependent NMDA receptor activation. Aging Cell 11, 336–344. doi: 10.1111/j.1474-9726.2012.00792.x
He, X. L., Yan, N., Zhang, H., Qi, Y. W., Zhu, L. J., Liu, M. J., et al. (2014). Hydrogen sulfide improves spatial memory impairment and decreases production of Abeta in APP/PS1 transgenic mice. Neurochem. Int. 67, 1–8. doi: 10.1016/j.neuint.2014.01.004
Hu, L. F., Lu, M., Hon Wong, P. T., and Bian, J. S. (2011). Hydrogen sulfide: neurophysiology and neuropathology. Antioxid. Redox Signal. 15, 405–419. doi: 10.1089/ars.2010.3517
Kaczorowski, C. C., Davis, S. J., and Moyer, J. R. Jr. (2012). Aging redistributes medial prefrontal neuronal excitability and impedes extinction of trace fear conditioning. Neurobiol. Aging 33, 1744–1757. doi: 10.1016/j.neurobiolaging.2011.03.020
Kumar, A. (2015). NMDA receptor function during senescence: implication on cognitive performance. Front. Neurosci. 9:473. doi: 10.3389/fnins.2015.00473
Kumar, A., and Foster, T. C. (2013). Linking redox regulation of NMDAR synaptic function to cognitive decline during aging. J. Neurosci. 33, 15710–15715. doi: 10.1523/JNEUROSCI.2176-13.2013
Li, Y. L., Wu, P. F., Chen, J. G., Wang, S., Han, Q. Q., Li, D., et al. (2017). Activity-dependent sulfhydration signal controls N-Methyl-D-aspartate subtype glutamate receptor-dependent synaptic plasticity via increasing d-serine availability. Antioxid. Redox Signal. 27, 398–414. doi: 10.1089/ars.2016.6936
Li, Z., Wang, Y., Xie, Y., Yang, Z., and Zhang, T. (2011). Protective effects of exogenous hydrogen sulfide on neurons of hippocampus in a rat model of brain ischemia. Neurochem. Res. 36, 1840–1849. doi: 10.1007/s11064-011-0502-6
Liu, L., Wong, T. P., Pozza, M. F., Lingenhoehl, K., Wang, Y., Sheng, M., et al. (2004). Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 304, 1021–1024. doi: 10.1126/science.1096615
Liu, P., Smith, P. F., and Darlington, C. L. (2008). Glutamate receptor subunits expression in memory-associated brain structures: regional variations and effects of aging. Synapse 62, 834–841. doi: 10.1002/syn.20563
Lopez de Armentia, M., and Sah, P. (2003). Development and subunit composition of synaptic NMDA receptors in the amygdala: NR2B synapses in the adult central amygdala. J. Neurosci. 23, 6876–6883. doi: 10.1523/JNEUROSCI.23-17-06876.2003
Luo, Y., Wu, P. F., Zhou, J., Xiao, W., He, J. G., Guan, X. L., et al. (2014). Aggravation of seizure-like events by hydrogen sulfide: involvement of multiple targets that control neuronal excitability. CNS Neurosci. Ther. 20, 411–419. doi: 10.1111/cns.12228
Lynch, M. A. (2004). Long-term potentiation and memory. Physiol. Rev. 84, 87–136. doi: 10.1152/physrev.00014.2003
Magnusson, K. R., Scruggs, B., Zhao, X., and Hammersmark, R. (2007). Age-related declines in a two-day reference memory task are associated with changes in NMDA receptor subunits in mice. BMC Neurosci. 8:43. doi: 10.1186/1471-2202-8-43
Marquez Loza, A., Elias, V., Wong, C. P., Ho, E., Bermudez, M., and Magnusson, K. R. (2017). Effects of ibuprofen on cognition and NMDA receptor subunit expression across aging. Neuroscience 344, 276–292. doi: 10.1016/j.neuroscience.2016.12.041
McGaugh, J. L. (2000). Memory–a century of consolidation. Science 287, 248–251. doi: 10.1126/science.287.5451.248
Miwa, H., Fukaya, M., Watabe, A. M., Watanabe, M., and Manabe, T. (2008). Functional contributions of synaptically localized NR2B subunits of the NMDA receptor to synaptic transmission and long-term potentiation in the adult mouse CNS. J. Physiol. 586, 2539–2550. doi: 10.1113/jphysiol.2007.147652
Mothet, J. P., Rouaud, E., Sinet, P. M., Potier, B., Jouvenceau, A., Dutar, P., et al. (2006). A critical role for the glial-derived neuromodulator D-serine in the age-related deficits of cellular mechanisms of learning and memory. Aging Cell 5, 267–274. doi: 10.1111/j.1474-9726.2006.00216.x
Muller, T., Albrecht, D., and Gebhardt, C. (2009). Both NR2A and NR2B subunits of the NMDA receptor are critical for long-term potentiation and long-term depression in the lateral amygdala of horizontal slices of adult mice. Learn. Mem. 16, 395–405. doi: 10.1101/lm.1398709
Paul, B. D., and Snyder, S. H. (2015). Modes of physiologic H2S signaling in the brain and peripheral tissues. Antioxid. Redox Signal. 22, 411–423. doi: 10.1089/ars.2014.5917
Paul, B. D., and Snyder, S. H. (2017). Gasotransmitter hydrogen sulfide signaling in neuronal health and disease. Biochem. Pharmacol. 149, 101–109. doi: 10.1016/j.bcp.2017.11.019
Qu, K., Chen, C. P., Halliwell, B., Moore, P. K., and Wong, P. T. (2006). Hydrogen sulfide is a mediator of cerebral ischemic damage. Stroke 37, 889–893. doi: 10.1161/01.STR.0000204184.34946.41
Robillard, J. M., Gordon, G. R., Choi, H. B., Christie, B. R., and Macvicar, B. A. (2011). Glutathione restores the mechanism of synaptic plasticity in aged mice to that of the adult. PLoS One 6:e20676. doi: 10.1371/journal.pone.0020676
Rodrigues, S. M., Schafe, G. E., and Ledoux, J. E. (2001). Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning. J. Neurosci. 21, 6889–6896. doi: 10.1523/JNEUROSCI.21-17-06889.2001
Roozendaal, B., Barsegyan, A., and Lee, S. (2008). Adrenal stress hormones, amygdala activation, and memory for emotionally arousing experiences. Prog. Brain Res. 167, 79–97. doi: 10.1016/S0079-6123(07)67006-X
Singh-Manoux, A., Kivimaki, M., Glymour, M. M., Elbaz, A., Berr, C., Ebmeier, K. P., et al. (2012). Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ 344:d7622. doi: 10.1136/bmj.d7622
Slutsky, I., Abumaria, N., Wu, L. J., Huang, C., Zhang, L., Li, B., et al. (2010). Enhancement of learning and memory by elevating brain magnesium. Neuron 65, 165–177. doi: 10.1016/j.neuron.2009.12.026
von Bohlen Und Halbach, O., and Unsicker, K. (2002). Morphological alterations in the amygdala and hippocampus of mice during ageing. Eur. J. Neurosci. 16, 2434–2440. doi: 10.1046/j.1460-9568.2002.02405.x
Wang, C. M., Yang, Y. J., Zhang, J. T., Liu, J., Guan, X. L., Li, M. X., et al. (2015). Regulation of emotional memory by hydrogen sulfide: role of GluN2B-containing NMDA receptor in the amygdala. J. Neurochem. 132, 124–134. doi: 10.1111/jnc.12961
Wang, D., Jacobs, S. A., and Tsien, J. Z. (2014). Targeting the NMDA receptor subunit NR2B for treating or preventing age-related memory decline. Expert Opin. Ther. Targets 18, 1121–1130. doi: 10.1517/14728222.2014.941286
Yang, Y. J., Wu, P. F., Long, L. H., Yu, D. F., Wu, W. N., Hu, Z. L., et al. (2010). Reversal of aging-associated hippocampal synaptic plasticity deficits by reductants via regulation of thiol redox and NMDA receptor function. Aging Cell 9, 709–721. doi: 10.1111/j.1474-9726.2010.00595.x
Yang, Y. J., Zhao, Y., Yu, B., Xu, G. G., Wang, W., Zhan, J. Q., et al. (2016). GluN2B-containing NMDA receptors contribute to the beneficial effects of hydrogen sulfide on cognitive and synaptic plasticity deficits in APP/PS1 transgenic mice. Neuroscience 335, 170–183. doi: 10.1016/j.neuroscience.2016.08.033
Zeng, Y., Liu, Y., Wu, M., Liu, J., and Hu, Q. (2012). Activation of TrkB by 7,8-dihydroxyflavone prevents fear memory defects and facilitates amygdalar synaptic plasticity in aging. J. Alzheimers Dis. 31, 765–778. doi: 10.3233/JAD-2012-120886
Zhao, X., Rosenke, R., Kronemann, D., Brim, B., Das, S. R., Dunah, A. W., et al. (2009). The effects of aging on N-methyl-D-aspartate receptor subunits in the synaptic membrane and relationships to long-term spatial memory. Neuroscience 162, 933–945. doi: 10.1016/j.neuroscience.2009.05.018
Keywords: aging, hydrogen sulfide, amygdala, synaptic plasticity, fear memory, NMDA receptor
Citation: Zhan J-Q, Zheng L-L, Chen H-B, Yu B, Wang W, Wang T, Ruan B, Pan B-X, Chen J-R, Li X-F, Wei B and Yang Y-J (2018) Hydrogen Sulfide Reverses Aging-Associated Amygdalar Synaptic Plasticity and Fear Memory Deficits in Rats. Front. Neurosci. 12:390. doi: 10.3389/fnins.2018.00390
Received: 30 January 2018; Accepted: 22 May 2018;
Published: 07 June 2018.
Edited by:
Lee E. Eiden, National Institutes of Health (NIH), United StatesReviewed by:
Tamas Kozicz, Radboud University Nijmegen, NetherlandsBen Nephew, Tufts University, United States
Copyright © 2018 Zhan, Zheng, Chen, Yu, Wang, Wang, Ruan, Pan, Chen, Li, Wei and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Wei, jxmh_wb@163.com Yuan-Jian Yang, yuanjimyang@yeah.net
† These authors have contributed equally to this work.
 Jin-Qiong Zhan1†
Jin-Qiong Zhan1† Bin-Xing Pan
Bin-Xing Pan Yuan-Jian Yang
Yuan-Jian Yang