Basal ganglia—thalamus and the “crowning enigma”
- Okinawa Institute of Science and Technology Graduate University, Okinawa, Japan
When Hubel (1982) referred to layer 1 of primary visual cortex as “… a ‘crowning mystery’ to keep area-17 physiologists busy for years to come …” he could have been talking about any cortical area. In the 80’s and 90’s there were no methods to examine this neuropile on the surface of the cortex: a tangled web of axons and dendrites from a variety of different places with unknown specificities and doubtful connections to the cortical output neurons some hundreds of microns below. Recently, three changes have made the crowning enigma less of an impossible mission: the clear presence of neurons in layer 1 (L1), the active conduction of voltage along apical dendrites and optogenetic methods that might allow us to look at one source of input at a time. For all of those reasons alone, it seems it is time to take seriously the function of L1. The functional properties of this layer will need to wait for more experiments but already L1 cells are GAD67 positive, i.e., inhibitory! They could reverse the sign of the thalamic glutamate (GLU) input for the entire cortex. It is at least possible that in the near future normal activity of individual sources of L1 could be detected using genetic tools. We are at the outset of important times in the exploration of thalamic functions and perhaps the solution to the crowning enigma is within sight. Our review looks forward to that solution from the solid basis of the anatomy of the basal ganglia output to motor thalamus. We will focus on L1, its afferents, intrinsic neurons and its influence on responses of pyramidal neurons in layers 2/3 and 5. Since L1 is present in the whole cortex we will provide a general overview considering evidence mainly from the somatosensory (S1) cortex before focusing on motor cortex.
Layer 1: The Crowning Enigma
Our interest in layer 1 (L1) of cortex sprang from the knowledge that it was the final destination of basal ganglia output. In spite of the fact that the whole cortex is represented in striatum and movement is certainly not its only function, here we review thalamic output to motor cortex because of our long-standing interest in movement and the basal ganglia. Similarly L1 is not only present in motor cortex but invests the complete cortex. In theorizing about basal ganglia and their influence on the “crowning enigma” it is as important to remember that movement is only the most obvious output and the easiest outcome to measure from both the dark basements of the brain and its crowing glory. With its plastic spines and input from so many parts of the environment L1 might be the best place to provide the necessary context for movements, or for perceptions, both of which, like all decisions of the cortex, need information from many sources including historical experience that can be coded in the pattern of spines.
Neurons Intrinsic to Layer 1
L1 is recognized throughout the entire cerebral cortex. Cajal (1890) described for the first time horizontal cells in L1 later called Cajal-Retzius and Lorente De Nó (1922) using Golgi staining in mouse auditory cortex provided the first evidence of “non-specific” thalamic afferents to L1. For a review see Marin-Padilla and Marin-Padilla (1982).
From work performed in mice and rat neocortex (rostral, central and caudal areas) three types of neurons in L1 are described as non-pyramidal GABAergic: Cajal-Retzius, elongated neurogliaform and single bouquet. Cajal-Retzius are the earliest born (embryonic 10–11 days; Soda et al., 2003; Anstotz et al., 2014) and they have an oval shape with a prominent long spiny dendrite (two occasionally) that runs horizontally along L1 (Imamoto et al., 1994). Their horizontal axon extends about 1.7 mm and serves as anchor of dendritic tufts of pyramidal neurons of layers 2/3 and 5 (Anstotz et al., 2014). These neurons participate in layering and connectivity during development (Zecevic and Rakic, 2001; Soda et al., 2003). During the second postnatal week most Cajal-Retzius cells suffer apoptotic death (del Río et al., 1995; Chowdhury et al., 2010) and have nearly disappeared at P14 (Anstotz et al., 2014).
The elongated neurogliaform type comprises 30–40% of neurons in L1, they have a characteristic dense axonal arbor confined to L1 and are coupled electrically. These cells express GABAAδ receptors and mostly display non-adapting late spiking action potentials. In the monkey sensory-motor cortex intense GABAA immunostaining outlines somas of pyramidal and non-pyramidal cells in layers 1–3 (Huntley et al., 1990). Single bouquet cells express vasoactive intestinal peptide (VIP) and mostly display adapting early spiking to depolarizing current injections (Jiang et al., 2013; Ma et al., 2014).
Following the Petilla terminology for interneuron firing patterns (Ascoli et al., 2008) there are four different types of interneurons in L1: neurogliaform, classical-accomodating, fast-spiking and burst-spiking (Wozny and Williams, 2011). The most common type is the fast spiking, with no frequency adaptation and pronounced fast afterhyperpolarizations (Zhou and Hablitz, 1996; Wozny and Williams, 2011; Li et al., 2012; Muralidhar et al., 2013). Correlation of function and morphology with colocalization of neuronal markers and specific neuronal proteins has produced four different subtypes of agranular neocortical GABAergic neurons. Two are found in L1: the calretinin/alpha-actin-2 and somatostatin subtypes (Kubota et al., 2011).
Afferents to Layer 1
It is estimated that 4000–5000 glutamate (GLU) containing axons reach any given square millimeter of rat L1 (Rubio-Garrido et al., 2009) to selectively target apical dendritic tufts (Herkenham, 1986; Arbuthnott et al., 1990; Lu and Lin, 1993), Figure 1 illustrates the extension of neocortical L1 projections from ventromedial (VM) that include forelimb and hindlimb areas. Dendritic tufts of layer 5 corticospinal, corticostriatal and corticothalamic neurons are all subject to modulation from L1 (Gao and Zheng, 2004). Moreover, important modulation of dendritic tufts of layer 2/3 pyramidal neurons takes place in L1 (see “Responses Mediated by Activation of Layer 1” Section).
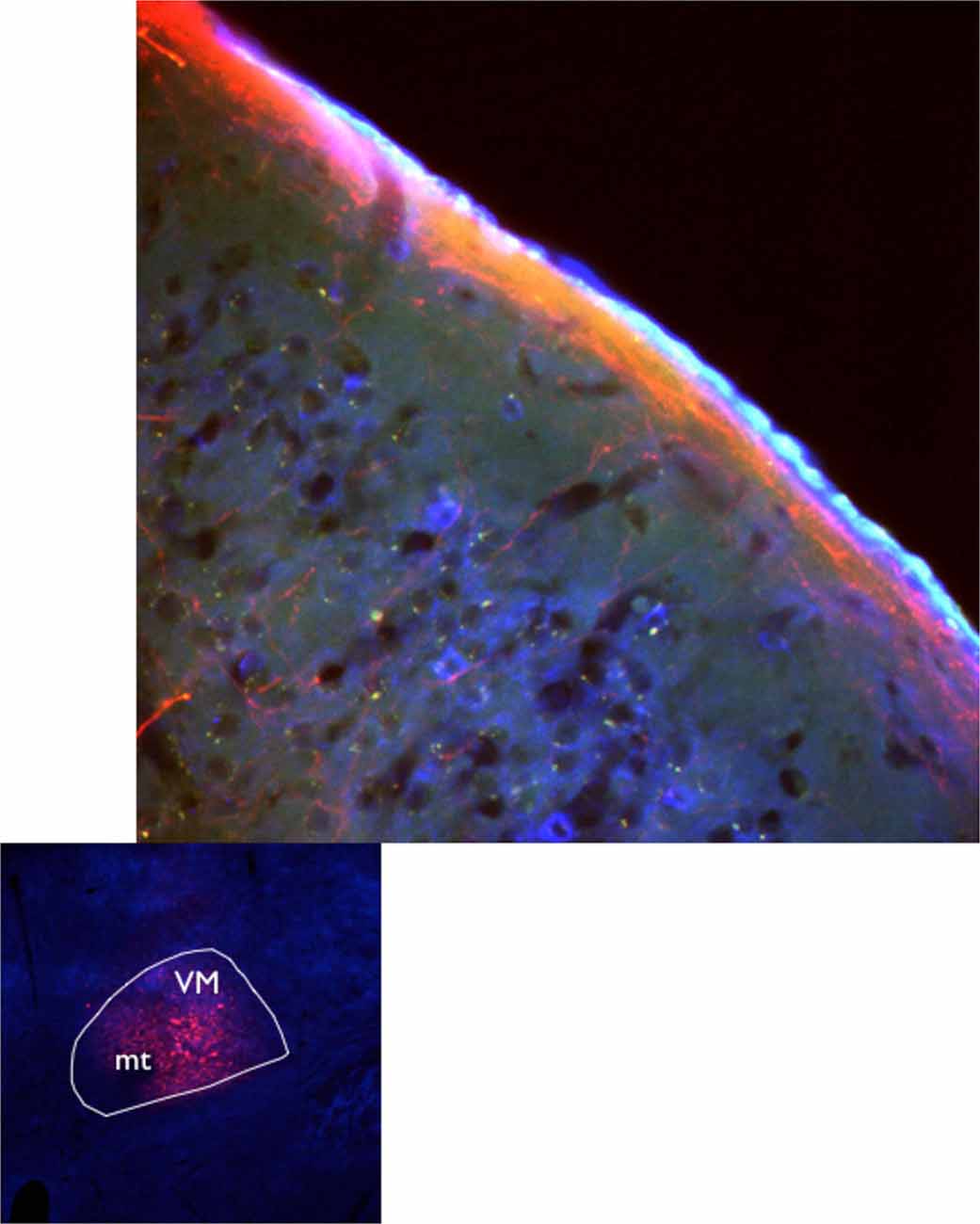
Figure 1. Axons from the ventromedial nucleus of the motor thalamus delineate layer 1 in motor cortex. Axon terminal fields in cortical L1 seen after VM injection of AAV10 containing turbo red and GCamp6 in C57Bl6J mice (Jáidar Benavides and Arbuthnott, 2013; Jáidar et al., 2015).
In motor cortex afferents reaching L1 are exclusively from motor thalamus (MT) and midline rhomboid nucleus (Ohtake and Yamada, 1989; Van Der Werf et al., 2002; Vertes et al., 2015). Other midline/intralaminar nuclei (i.e., centrolateral, centromedian, paracentral, posterior and parafascicular) terminate although not exclusively, in L1 of motor related areas (Royce and Mourey, 1985; Royce et al., 1989; Jones, 2007; Mohammed and Jain, 2014).
Neuronal Processes that Mingle in Layer 1
Axons that run along L1 originate in higher cortical areas, thalamic specific and non-specific nuclei (Mitchell and Cauller, 2001) and brainstem specific neurotransmitter producing nuclei. Norepinephrine (NE) originates in the pontine locus coeruleus, serotonin (5HT) in the midbrain raphe nuclei, dopamine (DA) in the ventral mesencephalon and acetylcholine (ACh) in the basal forebrain. In general these neurotransmitter-containing fibers enter below layer 6 and ascend sending collateral branches at all levels. L1 is particularly filled with dense axonal terminals and long branching collaterals (Levitt and Moore, 1978, 1979). Cortical NE innervates the marginal zone at embryonic 18–21 days and its participation in pyramidal cell development and layering was highlighted following locus coeruleus lesions in newborn rats (Felten et al., 1982). Similarly, 5HT is related to neuronal development, differentiation and migration (Rubenstein, 1998). The participation of DA and ACh will be indicated below (see “Modulatory Role of Neurotrasmitters Released in L1” Section) associated to responses mediated by activation of L1.
Other neuronal processes in L1 come from cortical interneurons, mainly from axons of somatostatin-positive Martinotti cells contained in layers 2–6 (Thomson and Lamy, 2007; Muralidhar et al., 2013), vertical dendrites from bipolar interneurons (layers 1–3) that run horizontally once in L1, and apical dendrites of layer 5 pyramidal cells (Larsen and Callaway, 2006) and layer 2/3 (Walcott and Langdon, 2001). Intrinsic axonal arborizations from L1 run either in the horizontal plane along the layer or descend in the vertical plane to frequently synapse with interneurons of deeper layers (Zhu and Zhu, 2004; Jiang et al., 2013).
Motor Thalamus
Motor thalamus (MT) is considered the area where afferents from globus pallidus (GPi or entopeduncular nucleus, EP), substantia nigra reticulata (SNR) and deep cerebellar nuclei form terminal fields in separate nuclei of the ventral thalamus: ventrolateral (VL), ventral anterior (VA) and ventromedial (VM). According to Scheibel and Scheibel (1967) the best way to conceptualize MT is to look at a horizontal section of the brain through the rostral half of thalamus.
MT Inputs
Terminal sites of afferent axons to MT are conserved across species (Antal et al., 2014) and establish multiple synapses with neurons in VM (Kultas-Ilinsky and Ilinsky, 1990; Kuroda and Price, 1991; Sakai et al., 1998; Tsumori et al., 2002; Bodor et al., 2008).
The use of a new anatomical technique with a resolution like “the old Golgi staining” (Furuta et al., 2001) has refined previous findings of inputs to MT (Deniau et al., 1978; Uno et al., 1978; Bava et al., 1979; MacLeod et al., 1980; Chevalier and Deniau, 1982; Matsuda and Nakamura, 1982; Ueki, 1983). As a result the VA/VL complex is divided in two sections: the rostromedial area immunoreactive to calbindin and GAD67 and the caudolateral area immunoreactive to VGluT2. These results sparked the idea of associating the neurotransmitter markers with the sites of origin calling the GABAergic GAD67-immunoreactive neurons the “inhibitory zone” and the glutamatergic VGluT2-immunoreactive neurons the “excitatory zone”. It is important to note that although immunureactivities to GAD67 and VGluT2 vary in intensity, they can be found at variable levels throughout MT. VM and VA/VL contain axon terminals of both GABA and GLU in different proportions. GABAergic terminals from SNR and GPi(EP) terminate in VM and the rostroventral VA/VL and cerebellar GLU terminals in the caudodorsal portion of VA/VL (Kuramoto et al., 2011).
In rats and monkeys, the GAD67-immunoreactive axon terminals are large (Bodor et al., 2008; Kuramoto et al., 2011) with a synaptic arrangement of the typical thalamic “detonator or driver”-type input that favors neurotransmitter spillover and volume transmission (Destexhe and Sejnowski, 1995; Agnati and Fuxe, 2000; Diamond, 2002; Agnati et al., 2008) and provides an ideal form of communication between neighboring neurons as has been observed in other thalamic areas (Bright and Brickley, 2008; Errington et al., 2011; Bright and Smart, 2013; Herd et al., 2013; Ye et al., 2013).
MT Outputs
MT projects to prefrontal cortex (Middleton and Strick, 1994), motor cortex (Hoover and Strick, 1999), supplementary motor area (SMA) and pre-SMA (Akkal et al., 2007). Axonal processes from ventromedial (VM) and ventral anterior (VA) thalamic nuclei terminate in L1 (Donoghue and Ebner, 1981; Arbuthnott et al., 1990; Desbois and Villanueva, 2001; Mitchell and Cauller, 2001; Kuramoto et al., 2009, 2015; Rubio-Garrido et al., 2009).
VM neurons project to extensive motor associated cortical areas including the forelimb and hindlimb regions (Tennant et al., 2011; Deffeyes et al., 2015). These results are consistent with the findings of Arbuthnott et al. (1990) following antidromically driven VM neurons over a similarly extensive cortical area. The other areas that receive fibers from VM according to Kuramoto et al. (2009) are primary somatosensory (S1) and associated sensory orbital and cingulate areas. Figure 2 presents a sketch of afferents to L1, its neuronal types some of neuronal processes found at this level.
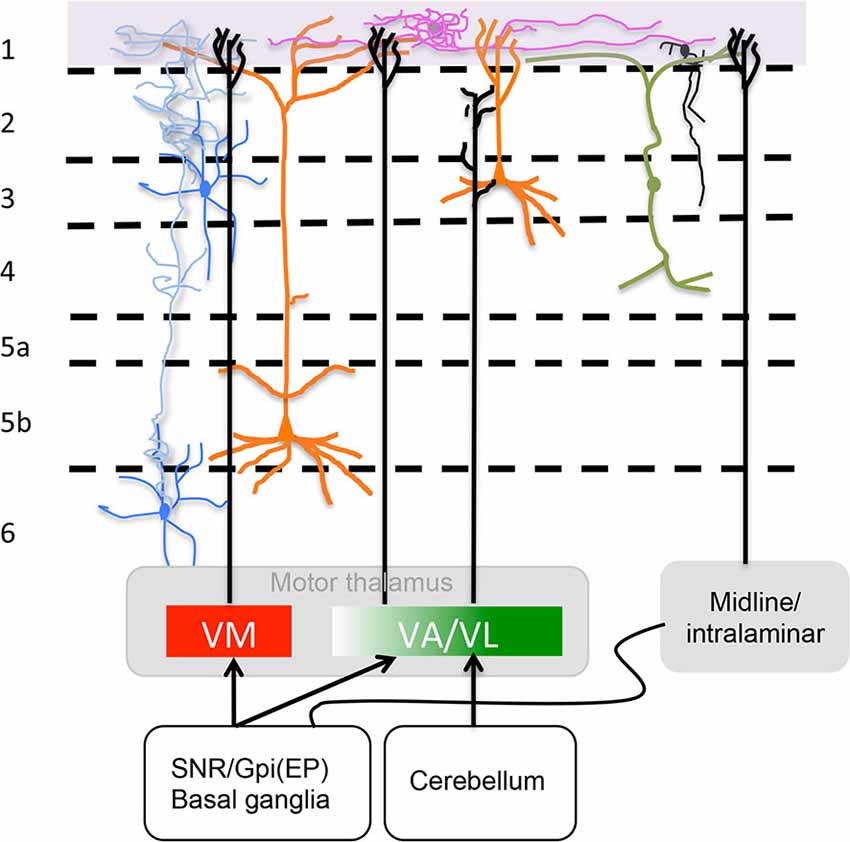
Figure 2. Motor thalamus (MT) and related midline/intralaminar thalamic connections to layer 1. Depicted in the rectangles at the bottom are projections from MT divided according to a direct VM and rostromedial VA projection to L1 and a caudolateral VA/VL projection that does not reach L1 as described by Kuramoto et al. (2015) (see “MT Inputs” Section ) illustrated also is the minor input from midline/intralaminar nuclei to L1 via the basal ganglia (see “Midline/Intralaminar Nuclei” Section). Specific L1 neurons are depicted neurogliaform (purple) and single bouquet (black) (see “Layer 1: The Crowning Enigma” Section). The rectangle at the top marks where axons from specific neurotransmitter producing nuclei (i.e., ACh, NE, 5HT and DA) run along L1. Sketched are also important neuronal processes mingled in L1 from axons of Martinotti cells (blue), vertical dendrites from bipolar interneurons that run horizontally once in layer 1 (green) and apical dendritic tufts of layers 2/3 and 5 pyramidal cells (orange) (see “Neuronal Processes that Mingle in Layer 1” Section). For accurate afferent arborizations consult Arbuthnott et al. (1990), Kuramoto et al. (2009) and Cruikshank et al. (2012).
MT Functional Output
The motor function of MT reflects the function of its afferent nuclei: optimization of motor sequences, sensory motor control, switching of attention and decision-making of cerebellum e.g., D’Angelo et al. (2011) and attention, implicit learning, habit formation and selection of appropriate motor activity of basal ganglia e.g., Lanciego and Vázquez (2012). How this information is consolidated and expressed depends not only on integrative processes in MT but also on the reentrant cortical input from thalamus (Magill et al., 2004; Bosch-Bouju et al., 2013; Nakamura et al., 2014) and the anatomical reality of a reentrant thalamic pathway to L1.
Initial functional evidence of MT (VA, VM) in relation to basal ganglia output indicated that increases in GPi(EP) and SNR activity resulted in decreased VM activity (Deniau et al., 1978; Patino and Garcia-Munoz, 1985) and increased cortical activity (Tanibuchi et al., 2009). Monosynaptic inhibitory postsynaptic potentials were recorded in VM to stimulation of SNR or GPi(EP) (MacLeod et al., 1980; Ueki, 1983). Recently it has been reported that the similar electrophysiological characteristics of VA and VM suggest they could form a single nucleus recipient of inputs from basal ganglia (Nakamura et al., 2014). Coherence between cortical oscillatory activity (electrocorticograms, ECoG) and action potentials has been reported for cortex-basal ganglia (Magill et al., 2004) or cortex-thalamus (Nakamura et al., 2014). Nakamura et al. (2014) observed that MT neuronal spike discharges are phase-locked to ongoing cortical slow oscillations, and that the two neuronal populations of MT (defined by their GLU or GABA inputs) preferentially discharge at the ascending phase of the cortical oscillation.
VM and VA are assumed to carry information about movement from basal ganglia, though it will be strongly modified information. Deep brain stimulation in the subthalamic nucleus changes dramatically MT responses, decreases beta oscillations and improves Parkinson’s disease symptoms (Anderson et al., 2015) possibly via cortex. Moreover, thalamic deep brain stimulation is not only an effective treatment for movement disorders but also for pain and epilepsy. Nociceptive neurons are located in the lateral parts of VM (lateral to MT) they respond to painful stimulation of the whole body in rats and project to the entire layer 1 of the dorsolateral neocortex (Monconduit and Villanueva, 2005) and cortical epileptic activity during absence seizures is accompanied by rhythmic burst activity in VM (Paz et al., 2007), although the terminals seem widely spread in cortex L1 inhibitory neurons could target specific pyramidal cells.
Midline/Intralaminar Nuclei
The lateral, ventral and posterior groups of midline and intralaminar nuclei interact with MT and its afferent sites according to Van Der Werf et al. (2002). The ascribed functions of these three groups are cognitive for the lateral (centrolateral, CL; centromedian anterior, CM; and paracentral, PC nuclei), sensory for the ventral (rhomboid, Rh; reuniens, Re; centromedian, CM and posterior, Po) and multisensory for the posterior group (parafascicular, Pf). Among the midline/intralaminar thalamic nuclei Rh, Re and Pf are consistently reported to project to striatum and also to cortical L1 (Berendse and Groenewegen, 1990; Mohammed and Jain, 2014). Optical stimulation of midline thalamic neurons (e.g., Re, PC, CM and Rh) preferentially drives L1 interneurons that often trigger feedforward inhibition of other L1 interneurons and L2/3 pyramidal neurons in medial prefrontal and secondary motor cortex (Cruikshank et al., 2012).
Projections from midline/intralaminar thalamus on their way to cortex on occasions bifurcate to reenter basal ganglia (Killackey and Ebner, 1973; Cesaro et al., 1979; Jinnai and Matsuda, 1981; Royce, 1983; Macchi et al., 1984) and in other cases separate groups of neurons send axons to striatal and cortical targets (Sadikot et al., 1992). Figure 2 illustrates ascending inputs to L1 (motor areas) from MT and midline/ intralaminar nuclei. The topic of dual projections is discussed by Jones and Leavitt (1974). Afferents from these nuclei to striatum have been reported in monkeys (Macchi et al., 1984; Nakano et al., 1990; Fenelon et al., 1991; Sadikot et al., 1992; Gimenéz-Amaya et al., 1995) cats (Sato et al., 1979; Beckstead, 1984; Takada et al., 1986) and rodents (Dube et al., 1988).
Gimenéz-Amaya et al. (1995) raised important questions regarding the double thalamic projection to striatum and cortex: Is their function modulatory at both ends? Are the contacted neurons a subset of neurons with common outputs or functions? Axonal branches from thalamic afferents that synapse in striatum on their way to cortex may contribute to striatal multisensory responses (Reig and Silberberg, 2014). Since the early work it was suggested that some afferents on L1 originated from the midline/intralaminar nuclei (Jones and Powell, 1969, 1970a,b; Strick, 1973; Strick and Sterling, 1974). Medium to large caliber axons from VA afferents converged with fine caliber afferents from intralaminar nuclei in L1 (Killackey and Ebner, 1973). Concurrent afferent information from MT and intralaminar nuclei to L1 and striatum may contribute to the animal’s awareness of position in space necessary for posture and head orientation (Barter et al., 2015) and also contribute to improvement of symptoms of Parkinson’s disease induced by deep brain stimulation (Jouve et al., 2010).
Responses Mediated by Activation of Layer 1
The evidence presented in this section is a summary of the neuronal circuitry activated by L1 or compilation of experimental observations from different cortical areas i.e., S1, medial prefrontal, neocortical and of course specific motor cortex responses relevant to this review. Although the summary underlies general features, important anatomical distinctions between motor/frontal cortex and other cortical areas must be considered (Weiler et al., 2008).
Activation of the Distal Tuft of Pyramidal Cells in L1
Electrophysiological responses of L1 interneurons are mediated by excitatory GLU (i.e., AMPA, kainite and NMDA) and inhibitory GABAA receptors (Li et al., 2012). Thalamic afferents are in intimate contact with the distal part of the apical dendrite or dendritic tuft that reach L1 and provide a substantial AMPA and NMDA-mediated excitatory synaptic drive that generates subthreshold and suprathreshold voltage responses through the tuft. AMPA receptors mediate unitary depolarizing potentials and NMDA receptors mediate an extensive depolarizing input that leads to the generation in the dendritic tuft of calcium-dependent regenerative action potentials. In summary, inputs to L1 produce regenerative calcium spikes that can induce sodium axosomatic action potentials (Larkum and Zhu, 2002; Larkum et al., 2009). A general organization of cortical synaptic interactions emphasizes a descending influence on cortical output from layer 2/3 to layer 5 for rat S1 (Jiang et al., 2013) as well as motor cortex (Weiler et al., 2008) previously believed to have a predominantly horizontal synaptic interaction.
Influence of the Distal Dendritic Tuft in L1 on Cortical Output
The presence of a cooperative integration between the distal dendritic tuft and the axosomatic compartment of pyramidal cells has been studied following co-activation of both compartments. It has been observed for example, that axosomatic action potentials can be generated by the coincidence of back propagation of action potentials and the effect of distal dendritic excitatory potentials (Larkum et al., 2004) and that volleys of excitatory postsynaptic potentials generated from distal apical dendritic sites facilitate action potential discharges (Williams, 2005).
Expression of specific channels in dendritic tufts that mediate different conductances such as the voltage-activated potassium outward conductance (Kv channels) or the hyperpolarization-activated current (Ih) plus the effects of specific activation of neurotransmitter receptors are important mechanisms for microcircuit control. Kv channels in the distal apical dendrite of layer 5 pyramidal neurons control the interaction between subthreshold tuft excitatory input and trunk spike generation (Harnett et al., 2013). These channels are co-localized with hyperpolarization activated cyclic nucleotide-gated nonselective cation (HCN) channels that are expressed at a high density in the tuft (≈85 channels/μm2) and at low density in the somatic region with two different effects: an inhibitory action in the tuft that controls initiation and propagation of dendritic spikes, and an excitatory action in the soma that decreases the threshold for action potentials (Harnett et al., 2015). Also Ih-mediated currents make corticospinal neurons susceptible to attenuation of GLU responses but not corticostriatal neurons. Similarly, the blockade of Ih results in increased layer 2/3-driven spiking in corticospinal, but not in corticostriatal neurons (Sheets et al., 2011). This emphasizes differential influences of L1 on cortical output.
Modulatory Role of Neurotrasmitters Released in L1
The neuromodulatory role of axons from several neurotransmitter systems (e.g., DA, NE, 5HT, ACh) on L1 is also important in the control of tuft currents and local interneurons. For example, α-adrenergic agents decrease Ih in corticospinal neurons thereby amplifying the impact of excitatory postsynaptic potentials with an increase in action potentials (Sheets et al., 2011). Likewise, the synergistic action of DA D1 and D2 receptors induces a depolarizing shift in the Ih activation curve that results in depolarization of L1 interneurons that can alter tonic cortical inhibition (Wu and Hablitz, 2005; Zhou et al., 2013).
Other neurotransmitters acting on L1 are also relevant for cortical function. For example, ascending ACh axons extend preferential terminations in L1 (Kristt et al., 1985) where neurons express high concentrations of nicotinic receptors (Komal et al., 2015), and an inhibitory interaction of DA D1/5 receptor on α7 nicotinic receptors has been observed in prefrontal L1 (Komal et al., 2015). This DA-Ach interaction can have important consequences in learning and memory. Optical stimulation of cholinergic axons induces excitatory potentials in L1 neurons and generates di-synaptic nicotine receptor-induced prolonged postsynaptic inhibition in layer 2/3 that could be associated with the effects of nicotine on arousal (Arroyo et al., 2012).
Activation of Layer 1 in Motor Cortex
L1 at the level of motor cortex contains afferents from MT specifically VM and VA (Herkenham, 1979; Arbuthnott et al., 1990; Kuramoto et al., 2009, 2015; Rubio-Garrido et al., 2009) and from midline/intralaminar nuclei (e.g., Re, PC, CM, Pf and Rh; Berendse and Groenewegen, 1990; Cruikshank et al., 2012). It seems that stimulation of these afferents in L1 can produce changes in synaptic efficacy in motor cortex, for a review see Yu and Zuo (2011). Reorganization of motor representations is a common phenomenon seen after peripheral transection of nerves, repetitive limb movement or motor learning results (Elbert et al., 1995; Sanes and Donoghue, 2000; Harms et al., 2008; Plowman and Kleim, 2010).
Long-term Changes in Dendritic Spines of Pyramidal Neurons are Facilitated by L1 Stimulation
Direct and indirect activation of tufts of apical dendrites by brief stimulation of L1 in vitro induces long-term depression of layer 2/3 neurons (Walcott and Langdon, 2001). These changes in postsynaptic response are associated to structural changes likely related to activation of immediate early genes for instance, the activity-regulated cytoskeleton-associated proteins (Arc) and cFos proteins are increased in layer 2/3 and 180 μm from pia in motor cortex as a consequence of learning complex motor tasks (Kleim et al., 1996; Cao et al., 2015). Structural changes include increases in dendritic spine density and cortical map expansion of limb or paw representation. Increases in synapse/neuron ratio are reported for motor cortex layers 5 and 2/3 contralateral to the trained forelimb (Kleim et al., 1996) likely related to consolidation of learning (Kleim et al., 2004). Enlargement of dendritic spines in L1 (Harms et al., 2008) are also a good indicator of modifications of synaptic connectivity and the influence of this layer on the apical tuft of pyramidal neurons.
Recent in vivo visualization of dendritic spines has revealed specific neuronal changes related to motor activity and learned tasks in layer 2/3 of motor cortex in mice under head fixation. Ensembles of neurons display calcium transients in layer 2/3 during performance of a learned task (Komiyama et al., 2010; Huber et al., 2012; Hira et al., 2013; Masamizu et al., 2014) and a parallel pruning and growth of dendritic spines likely important for task performance occurs in the apical tuft of neurons active during acquisition of a task in layer 5 (Xu et al., 2009; Yang et al., 2009) and layer 2/3 (Peters et al., 2014; Ma et al., 2015). The presence of DA is crucial for dendritic spine turnover of layer 5 pyramidal tuft (Guo et al., 2015).
Neurotransmitters Released in L1 Modulate Plasticity in Motor Cortex
Learning changes information processing in cortical microcircuits; interest on how neuronal interactions between cortical layers ultimately result in behavior is of outmost relevance. Letzkus et al. (2011) have studied how Ach released in L1 following aversive foot shocks modifies cortical microcircuits involved in learning. Ach produces a nicotinic-dependent excitation of L1 neurons that results in inhibition of layer 2/3 palvalbumin interneurons. This inhibition in turn disinhibits pyramidal neurons. In order for mice to associate a sound with foot shock and express fear, the disinhibition of pyramidal neurons associated with foot shock must take place; pharmacological or optical blockade of pyramidal neuron disinhibition prevents learning. Most likely, similar effects induced by other neurotransmitters released in L1 (e.g., DA and 5HT) are involved in microcircuits involved in learning.
Horizontal L1 Connections between Motor and Sensorimotor Cortex
L1 forms cortico-cortical connections that provide feedback during behavioral tasks. Whisker touch activates L1 of vibrissal motor cortex that in turn sends excitatory input to L1 of S1 barrel cortex to excite dendritic tufts of layer 5 pyramidal neurons (Xu et al., 2012). The large calcium signals recorded in L1 in the S1 cortex during active facial whisker-object contact recorded by these authors in another set of experiments reinforce the concept of a top-down processing of behavioral relevant outputs as well as the horizontal cortico-cortical influence (Harnett et al., 2013).
Summary and Conclusions
Highlights on Advances in the Field
• Motor-related information from basal ganglia and cerebellum enters motor cortex via MT. This thalamic region has recently received a different demarcation thanks to new anatomical methods with very good resolution. As a result VA/VL has been divided in rostromedial and caudolateral areas immunoreactive to GAD67 and VGluT2, respectively. VM and rostromedial VA/VL receive GABAergic input from basal ganglia (SNR and GPi(EP)) and caudolateral VA/VL receives GLU terminals from cerebellum. VM and rostromedial VA/VL project to L1 and caudolateral VA/VL to layer 2/3.
• Apart from cerebellar and basal ganglia influences relayed to motor cortex via MT, indirect cerebellar inputs reach basal ganglia through midline/intralaminar outputs.
• Plastic related changes in spines of pyramidal dendritic tufts make learning-related structural changes a likely substrate for learning in L1.
Voids in the Field that will be Interesting to Fill
• What is the role of the large synaptic terminals from SNR to VM described as “detonator drives” that typically favor neurotransmitter spillover and volume transmission?
• What is the function of the synaptic contacts made in basal ganglia from MT and midline/intralaminar axons as they travel to motor cortex? Which neurons are being contacted?
• What is the function of the electrically coupled elongated neurogliaform interneurons?
• Is 5HT released in L1 involved in learning-induced dendritic changes as are other neurotransmitters released in the area?
Speculations on the Function of Layer 1
The excitatory input of L1 as studied in many cortical areas regulates the active regenerative electrical activity of dendrites of pyramidal cells of layer 2/3 and 5. This excitatory top down control on the dendritic tuft is crucial for integration and further generation of axosomatic discharge.
Recent combined electrophysiological and behavioral observations indicate that L1 can be considered as a driver of pyramidal neurons with important behavioral consequences such as attention, expectation, anticipation, and action command.
We would like to consider projections from MT and midline/intralaminar nuclei to L1 as a regulatory entity of pyramidal cell excitability in motor cortex. These projections can provide the necessary binding input to trigger or suppress final patterns of activity that would ultimately generate appropriate behavioral responses. Motor thalamus has already been labeled a “super integrator” (Bosch-Bouju et al., 2013) and the MT and midline/intralaminar nuclei projections to L1 provide a step further in the integration of motor, cognitive and motivational aspects to produce, in collaboration with thalamic inputs that ultimately reach layer 5, an appropriate response according to the surrounding environment. Hence, projections from MT and midline/intalaminar nuclei could resolve conflicting alternative response patterns and give continuity to cortical function as proposed by Edelman and Gally (2013) and Damasio (1989).
A source of concern when dealing with MT and midline/intralaminar afferents to L1 is the extent of their projection as observed in other neocortical areas. It is hard to conceive the point-to-point modulation suggested by some electrophysiological results e.g., Jiang et al. (2013). Perhaps there are two kinds of inputs, one that involves a restricted command and another that provides a wide-range informative action as suggested by anatomical studies e.g., Arbuthnott et al. (1990). A mundane example could be a “specific” reverse 911-emergency phone call ordering individual immediate evacuation vs. a “general” regional sound alarm of an approaching hurricane. Does L1 command both types of system? There is still plenty of mystery in the superficial cortical layer but at least forefront tools are shedding new light and making the future an exciting time to be studying this mysterious crown of the cortex.
Changes in dendritic spines induced by motor activity and learning observed in dendritic tufts in L1 underline the functional rewiring observed as changes in spine morphology and neuronal dynamics (Yuste and Bonhoeffer, 2001; Holtmaat and Svoboda, 2009).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Funding for this work was provided by the Okinawa Institute of Science and Technology Graduate University, Japan.
References
Agnati, L. F., and Fuxe, K. (2000). Volume transmission as a key feature of information handling in the central nervous system possible new interpretative value of the Turing’s B-type machine. Prog. Brain Res. 125, 3–19. doi: 10.1016/s0079-6123(00)25003-6
Agnati, L. F., Guidolin, D., Carone, C., Dam, M., Genedani, S., and Fuxe, K. (2008). Understanding neuronal molecular networks builds on neuronal cellular network architecture. Brain Res. Rev. 58, 379–399. doi: 10.1016/j.brainresrev.2007.11.002
Akkal, D., Dum, R. P., and Strick, P. L. (2007). Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar output. J. Neurosci. 27, 10659–10673. doi: 10.1523/jneurosci.3134-07.2007
Anderson, C. J., Sheppard, D. T., Huynh, R., Anderson, D. N., Polar, C. A., and Dorval, A. D. (2015). Subthalamic deep brain stimulation reduces pathological information transmission to the thalamus in a rat model of parkinsonism. Front. Neural Circuits 9:31. doi: 10.3389/fncir.2015.00031
Anstotz, M., Cosgrove, K. E., Hack, I., Mugnaini, E., Maccaferri, G., and Lübke, J. H. (2014). Morphology, input-output relations and synaptic connectivity of Cajal-Retzius cells in layer 1 of the developing neocortex of CXCR4-EGFP mice. Brain Struct. Funct. 219, 2119–2139. doi: 10.1007/s00429-013-0627-2
Antal, M., Beneduce, B. M., and Regehr, W. G. (2014). The substantia nigra conveys target-dependent excitatory and inhibitory outputs from the basal ganglia to the thalamus. J. Neurosci. 34, 8032–8042. doi: 10.1523/JNEUROSCI.0236-14.2014
Arbuthnott, G. W., MacLeod, N. K., Maxwell, D. J., and Wright, A. K. (1990). Distribution and synaptic contacts of the cortical terminals arising from neurons in the rat ventromedial thalamic nucleus. Neuroscience 38, 47–60. doi: 10.1016/0306-4522(90)90373-c
Arroyo, S., Bennett, C., Aziz, D., Brown, S. P., and Hestrin, S. (2012). Prolonged disynaptic inhibition in the cortex mediated by slow, non-alpha7 nicotinic excitation of a specific subset of cortical interneurons. J. Neurosci. 32, 3859–3864. doi: 10.1523/JNEUROSCI.0115-12.2012
Ascoli, G. A., Alonso-Nanclares, L., Anderson, S. A., Barrionuevo, G., Benavides-Piccione, R., Burkhalter, A., et al. (2008). Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 9, 557–568. doi: 10.1038/nrn2402
Barter, J. W., Li, S., Sukharnikova, T., Rossi, M. A., Bartholomew, R. A., and Yin, H. H. (2015). Basal ganglia outputs map instantaneous position coordinates during behavior. J. Neurosci. 35, 2703–2716. doi: 10.1523/JNEUROSCI.3245-14.2015
Bava, A., Cicirata, F., Licciardello, S., Li Volsi, G., and Panto, M. R. (1979). Fastigial nuclei projections on the ventralis lateralis (VL) thalamic nucleus neurons. Brain Res. 168, 169–175. doi: 10.1016/0006-8993(79)90134-3
Beckstead, R. M. (1984). The thalamostriatal projection in the cat. J. Comp. Neurol. 223, 313–346. doi: 10.1002/cne.902230302
Berendse, H. W., and Groenewegen, H. J. (1990). Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J. Comp. Neurol. 299, 187–228. doi: 10.1002/cne.902990206
Bodor, A. L., Giber, K., Rovó, Z., Ulbert, I., and Acsady, L. (2008). Structural correlates of efficient GABAergic transmission in the basal ganglia-thalamus pathway. J. Neurosci. 28, 3090–3102. doi: 10.1523/JNEUROSCI.5266-07.2008
Bosch-Bouju, C., Hyland, B. I., and Parr-Brownlie, L. C. (2013). Motor thalamus integration of cortical, cerebellar and basal ganglia information: implications for normal and parkinsonian conditions. Front. Comput. Neurosci. 7:163. doi: 10.3389/fncom.2013.00163
Bright, D. P., and Brickley, S. G. (2008). Acting locally but sensing globally: impact of GABAergic synaptic plasticity on phasic and tonic inhibition in the thalamus. J. Physiol. 586, 5091–5099. doi: 10.1113/jphysiol.2008.158576
Bright, D. P., and Smart, T. G. (2013). Protein kinase C regulates tonic GABA(A) receptor-mediated inhibition in the hippocampus and thalamus. Eur. J. Neurosci. 38, 3408–3423. doi: 10.1111/ejn.12352
Cajal, S. R. (1890). Sobre la existencia de células merviosas especiales de la primera capa de las circonvoluciones cerebrales. Gac. Med. Catalana 15, 225–228.
Cao, V. Y., Ye, Y., Mastwal, S., Ren, M., Coon, M., Liu, Q., et al. (2015). Motor learning consolidates arc-expressing neuronal ensembles in secondary motor cortex. Neuron 86, 1385–1392. doi: 10.1016/j.neuron.2015.05.022
Cesaro, P., Nguyen-Legros, J., Berger, B., Alvarez, C., and Albe-Fessard, D. (1979). Double labelling of blanched neurons in the central nervous system of the rat by retrograde axonal transport of horseradish peroxidase and iron dextran complex. Neurosci. Lett. 15, 1–7. doi: 10.1016/0304-3940(79)91519-2
Chevalier, G., and Deniau, J. M. (1982). Inhibitory nigral influence on cerebellar evoked responses in the rat ventromedial thalamic nucleus. Exp. Brain Res. 48, 369–376. doi: 10.1007/bf00238613
Chowdhury, T. G., Jimenez, J. C., Bomar, J. M., Cruz-Martin, A., Cantle, J. P., and Portera-Cailliau, C. (2010). Fate of cajal-retzius neurons in the postnatal mouse neocortex. Front. Neuroanat. 4:10. doi: 10.3389/neuro.05.010.2010
Cruikshank, S. J., Ahmed, O. J., Stevens, T. R., Patrick, S. L., Gonzalez, A. N., Elmaleh, M., et al. (2012). Thalamic control of layer 1 circuits in prefrontal cortex. J. Neurosci. 32, 17813–17823. doi: 10.1523/JNEUROSCI.3231-12.2012
Damasio, A. R. (1989). The brain binds entities and events by multiregional activation from convergence zones. Neural Comput. 1, 123–132. doi: 10.1162/neco.1989.1.1.123
D’Angelo, E., Mazzarello, P., Prestori, F., Mapelli, J., Solinas, S., Lombardo, P., et al. (2011). The cerebellar network: from structure to function and dynamics. Brain Res. Rev. 66, 5–15. doi: 10.1016/j.brainresrev.2010.10.002
Deffeyes, J. E., Touvykine, B., Quessy, S., and Dancause, N. (2015). Interactions between rostral and caudal cortical motor areas in the rat. J. Neurophysiol. 113, 3893–3904. doi: 10.1152/jn.00760.2014
del Río, J. A., Martínez, A., Fonseca, M., Auladell, C., and Soriano, E. (1995). Glutamate-like immunoreactivity and fate of Cajal-Retzius cells in the murine cortex as identified with calretinin antibody. Cereb. Cortex 5, 13–21. doi: 10.1093/cercor/5.1.13
Deniau, J. M., Lackner, D., and Feger, J. (1978). Effect of substantia nigra stimulation on identified neurons in the VL-VA thalamic complex: comparison between intact and chronically decorticated cats. Brain Res. 145, 27–35. doi: 10.1016/0006-8993(78)90793-x
Desbois, C., and Villanueva, L. (2001). The organization of lateral ventromedial thalamic connections in the rat: a link for the distribution of nociceptive signals to widespread cortical regions. Neuroscience 102, 885–898. doi: 10.1016/s0306-4522(00)00537-6
Destexhe, A., and Sejnowski, T. J. (1995). G protein activation kinetics and spillover of gamma-aminobutyric acid may account for differences between inhibitory responses in the hippocampus and thalamus. Proc. Natl. Acad. Sci. U S A 92, 9515–9519. doi: 10.1073/pnas.92.21.9515
Diamond, J. S. (2002). A broad view of glutamate spillover. Nat. Neurosci. 5, 291–292. doi: 10.1038/nn0402-291
Donoghue, J. P., and Ebner, F. F. (1981). The laminar distribution and ultrastructure of fibers projecting from three thalamic nuclei to the somatic sensory-motor cortex of the opossum. J. Comp. Neurol. 198, 389–420. doi: 10.1002/cne.901980303
Dube, L., Smith, A. D., and Bolam, J. P. (1988). Identification of synaptic terminals of thalamic or cortical origin in contact with distinct medium-size spiny neurons in the rat neostriatum. J. Comp. Neurol. 267, 455–471. doi: 10.1002/cne.902670402
Edelman, G. M., and Gally, J. A. (2013). Reentry: a key mechanism for integration of brain function. Front. Integr. Neurosci. 7:63. doi: 10.3389/fnint.2013.00063
Elbert, T., Pantev, C., Wienbruch, C., Rockstroh, B., and Taub, E. (1995). Increased cortical representation of the fingers of the left hand in string players. Science 270, 305–307. doi: 10.1126/science.270.5234.305
Errington, A. C., Di Giovanni, G., Crunelli, V., and Cope, D. W. (2011). mGluR control of interneuron output regulates feedforward tonic GABAA inhibition in the visual thalamus. J. Neurosci. 31, 8669–8680. doi: 10.1523/JNEUROSCI.0317-11.2011
Felten, D. L., Hallman, H., and Jonsson, G. (1982). Evidence for a neurotropic role of noradrenaline neurons in the postnatal development of rat cerebral cortex. J. Neurocytol. 11, 119–135. doi: 10.1007/bf01258008
Fenelon, G., Francois, C., Percheron, G., and Yelnik, J. (1991). Topographic distribution of the neurons of the central complex (centre median-parafascicular complex) and of other thalamic neurons projecting to the striatum in macaques. Neuroscience 45, 495–510. doi: 10.1016/0306-4522(91)90244-i
Furuta, T., Tomioka, R., Taki, K., Nakamura, K., Tamamaki, N., and Kaneko, T. (2001). In vivo transduction of central neurons using recombinant sindbis virus: golgi-like labeling of dendrites and axons with membrane-targeted fluorescent proteins. J. Histochem. Cytochem. 49, 1497–1508. doi: 10.1177/002215540104901203
Gao, W. J., and Zheng, Z. H. (2004). Target-specific differences in somatodendritic morphology of layer V pyramidal neurons in rat motor cortex. J. Comp. Neurol. 476, 174–185. doi: 10.1002/cne.20224
Gimenéz-Amaya, J. M., McFarland, N. R., de las Heras, S., and Haber, S. N. (1995). Organization of thalamic projections to the ventral striatum in the primate. J. Comp. Neurol. 354, 127–149. doi: 10.1002/cne.903540109
Guo, L., Xiong, H., Kim, J. I., Wu, Y. W., Lalchandani, R. R., Cui, Y., et al. (2015). Dynamic rewiring of neural circuits in the motor cortex in mouse models of parkinson’s disease. Nat. Neurosci. 18, 1299–1309. doi: 10.1038/nn.4082
Harms, K. J., Rioult-Pedotti, M. S., Carter, D. R., and Dunaevsky, A. (2008). Transient spine expansion and learning-induced plasticity in layer 1 primary motor cortex. J. Neurosci. 28, 5686–5690. doi: 10.1523/JNEUROSCI.0584-08.2008
Harnett, M. T., Magee, J. C., and Williams, S. R. (2015). Distribution and function of HCN channels in the apical dendritic tuft of neocortical pyramidal neurons. J. Neurosci. 35, 1024–1037. doi: 10.1523/JNEUROSCI.2813-14.2015
Harnett, M. T., Xu, N. L., Magee, J. C., and Williams, S. R. (2013). Potassium channels control the interaction between active dendritic integration compartments in layer 5 cortical pyramidal neurons. Neuron 79, 516–529. doi: 10.1016/j.neuron.2013.06.005
Herd, M. B., Brown, A. R., Lambert, J. J., and Belelli, D. (2013). Extrasynaptic GABA(A) receptors couple presynaptic activity to postsynaptic inhibition in the somatosensory thalamus. J. Neurosci. 33, 14850–14868. doi: 10.1523/JNEUROSCI.1174-13.2013
Herkenham, M. (1979). The afferent and efferent connections of the ventromedial thalamic nucleus in the rat. J. Comp. Neurol. 183, 487–517. doi: 10.1002/cne.901830304
Herkenham, M. (1986). “New perspectives on the organization and evolution of nonspecific thalamocortical projections,” in Cerebral Cortex, ed. E. G. Jones (New York: Plenum), 403–445.
Hira, R., Ohkubo, F., Ozawa, K., Isomura, Y., Kitamura, K., Kano, M., et al. (2013). Spatiotemporal dynamics of functional clusters of neurons in the mouse motor cortex during a voluntary movement. J. Neurosci. 33, 1377–1390. doi: 10.1523/JNEUROSCI.2550-12.2013
Holtmaat, A., and Svoboda, K. (2009). Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 10, 647–658. doi: 10.1038/nrn2699
Hoover, J. E., and Strick, P. L. (1999). The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1. J. Neurosci. 19, 1446–1463.
Hubel, D. H. (1982). Cortical neurobiology: a slanted historical perspective. Annu. Rev. Neurosci. 5, 363–370. doi: 10.1146/annurev.ne.05.030182.002051
Huber, D., Gutnisky, D. A., Peron, S., O’Connor, D. H., Wiegert, J. S., Tian, L., et al. (2012). Multiple dynamic representations in the motor cortex during sensorimotor learning. Nature 484, 473–478. doi: 10.1038/nature11039
Huntley, G. W., de Blas, A. L., and Jones, E. G. (1990). GABAA receptor immunoreactivity in adult and developing monkey sensory-motor cortex. Exp. Brain Res. 82, 519–535. doi: 10.1007/bf00228794
Imamoto, K., Karasawa, N., Isomura, G., and Nagatsu, I. (1994). Cajal-Retzius neurons identified by GABA immunohistochemistry in layer I of the rat cerebral cortex. Neurosci. Res. 20, 101–105. doi: 10.1016/0168-0102(94)90027-2
Jáidar Benavides, O., and Arbuthnott, G. W. (2013). Section 3.2: The Thalamocortical Output from Basal Ganglia, Figure 2. Available online at: https://groups.oist.jp/bmbu
Jáidar, O., Roome, C. J., Nakano, Y., Garcia-Munoz, M., Bernd, K., and Arbuthnott, G. W. (2015). “Thalamocortical axons and deep cortical layer dendritic arbor in motor cortex layer I display a dynamic array of calcium transients during locomotion and systemic blockade of dopamine receptors,” in British Neuroscience Association Festival of Neuroscience, Vol. 23, Poster Ref. P1-C-008, Theme: C: Sensory and Motor Systems, Edinburgh.
Jiang, X., Wang, G., Lee, A. J., Stornetta, R. L., and Zhu, J. J. (2013). The organization of two new cortical interneuronal circuits. Nat. Neurosci. 16, 210–218. doi: 10.1038/nn.3305
Jinnai, K., and Matsuda, Y. (1981). Thalamocaudate projection neurons with a branching axon to the cerebral motor cortex. Neurosci. Lett. 26, 95–99. doi: 10.1016/0304-3940(81)90332-3
Jones, E. G., and Leavitt, R. Y. (1974). Retrograde axonal-transport and demonstration of nonspecific projections to cerebral-cortex and striatum from thalamic intralaminar nuclei in rat, cat and monkey. J. Comp. Neurol. 154, 349–377. doi: 10.1002/cne.901540402
Jones, E. G., and Powell, T. P. (1969). The cortical projection of the ventroposterior nucleus of the thalamus in the cat. Brain Res. 13, 298–318. doi: 10.1016/0006-8993(69)90289-3
Jones, E. G., and Powell, T. P. (1970a). Connexions of the somatic sensory cortex of the rhesus monkey. 3. Thalamic connexions. Brain 93, 37–56. doi: 10.1093/brain/93.1.37
Jones, E. G., and Powell, T. P. (1970b). An electron microscopic study of the laminar pattern and mode of termination of afferent fibre pathways in the somatic sensory cortex of the cat. Philos. Trans. R. Soc. Lond. B Biol. Sci. 257, 45–62. doi: 10.1098/rstb.1970.0007
Jouve, L., Salin, P., Melon, C., and Kerkerian-Le Goff, L. (2010). Deep brain stimulation of the center median-parafascicular complex of the thalamus has efficient anti-parkinsonian action associated with widespread cellular responses in the basal ganglia network in a rat model of parkinson’s disease. J. Neurosci. 30, 9919–9928. doi: 10.1523/JNEUROSCI.1404-10.2010
Killackey, H., and Ebner, F. (1973). Convergent projection of three separate thalamic nuclei on to a single cortical area. Science 179, 283–285. doi: 10.1126/science.179.4070.283
Kleim, J. A., Hogg, T. M., Vandenberg, P. M., Cooper, N. R., Bruneau, R., and Remple, M. (2004). Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J. Neurosci. 24, 628–633. doi: 10.1523/jneurosci.3440-03.2004
Kleim, J. A., Lussnig, E., Schwarz, E. R., Comery, T. A., and Greenough, W. T. (1996). Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J. Neurosci. 16, 4529–4535.
Komal, P., Estakhr, J., Kamran, M., Renda, A., and Nashmi, R. (2015). cAMP-dependent protein kinase inhibits alpha7 nicotinic receptor activity in layer 1 cortical interneurons through activation of D1/D5 dopamine receptors. J. Physiol. 593, 3513–3532. doi: 10.1113/jp270469
Komiyama, T., Sato, T. R., O’Connor, D. H., Zhang, Y. X., Huber, D., Hooks, B. M., et al. (2010). Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature 464, 1182–1186. doi: 10.1038/nature08897
Kristt, D. A., McGowan, R. A., Jr., Martin-Mackinnon, N., and Solomon, J. (1985). Basal forebrain innervation of rodent neocortex: studies using acetylcholinesterase histochemistry, Golgi and lesion strategies. Brain Res. 337, 19–39. doi: 10.1016/0006-8993(85)91606-3
Kubota, Y., Shigematsu, N., Karube, F., Sekigawa, A., Kato, S., Yamaguchi, N., et al. (2011). Selective coexpression of multiple chemical markers defines discrete populations of neocortical GABAergic neurons. Cereb. Cortex 21, 1803–1817. doi: 10.1093/cercor/bhq252
Kultas-Ilinsky, K., and Ilinsky, I. A. (1990). Fine structure of the magnocellular subdivision of the ventral anterior thalamic nucleus (VAmc) of Macaca mulatta: II. Organization of nigrothalamic afferents as revealed with EM autoradiography. J. Comp. Neurol. 294, 479–489. doi: 10.1002/cne.902940314
Kuramoto, E., Fujiyama, F., Nakamura, K. C., Tanaka, Y., Hioki, H., and Kaneko, T. (2011). Complementary distribution of glutamatergic cerebellar and GABAergic basal ganglia afferents to the rat motor thalamic nuclei. Eur. J. Neurosci. 33, 95–109. doi: 10.1111/j.1460-9568.2010.07481.x
Kuramoto, E., Furuta, T., Nakamura, K. C., Unzai, T., Hioki, H., and Kaneko, T. (2009). Two types of thalamocortical projections from the motor thalamic nuclei of the rat: a single neuron-tracing study using viral vectors. Cereb. Cortex 19, 2065–2077. doi: 10.1093/cercor/bhn231
Kuramoto, E., Ohno, S., Furuta, T., Unzai, T., Tanaka, Y. R., Hioki, H., et al. (2015). Ventral medial nucleus neurons send thalamocortical afferents more widely and more preferentially to layer 1 than neurons of the ventral anterior-ventral lateral nuclear complex in the rat. Cereb. Cortex 25, 221–235. doi: 10.1093/cercor/bht216
Kuroda, M., and Price, J. L. (1991). Ultrastructure and synaptic organization of axon terminals from brainstem structures to the mediodorsal thalamic nucleus of the rat. J. Comp. Neurol. 313, 539–552. doi: 10.1002/cne.903130313
Lanciego, J. L., and Vázquez, A. (2012). The basal ganglia and thalamus of the long-tailed macaque in stereotaxic coordinates. A template atlas based on coronal, sagittal and horizontal brain sections. Brain Struct. Funct. 217, 613–666. doi: 10.1007/s00429-011-0370-5
Larkum, M. E., and Zhu, J. J. (2002). Signaling of layer 1 and whisker-evoked Ca2+ and Na+ action potentials in distal and terminal dendrites of rat neocortical pyramidal neurons in vitro and in vivo. J. Neurosci. 22, 6991–7005.
Larkum, M. E., Nevian, T., Sandler, M., Polsky, A., and Schiller, J. (2009). Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: a new unifying principle. Science 325, 756–760. doi: 10.1126/science.1171958
Larkum, M. E., Senn, W., and Lüscher, H. R. (2004). Top-down dendritic input increases the gain of layer 5 pyramidal neurons. Cereb. Cortex 14, 1059–1070. doi: 10.1093/cercor/bhh065
Larsen, D. D., and Callaway, E. M. (2006). Development of layer-specific axonal arborizations in mouse primary somatosensory cortex. J. Comp. Neurol. 494, 398–414. doi: 10.1002/cne.20754
Letzkus, J. J., Wolff, S. B., Meyer, E. M., Tovote, P., Courtin, J., Herry, C., et al. (2011). A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480, 331–335. doi: 10.1038/nature10674
Levitt, P., and Moore, R. Y. (1978). Noradrenaline neuron innervation of the neocortex in the rat. Brain Res. 139, 219–231. doi: 10.1016/0006-8993(78)90925-3
Levitt, P., and Moore, R. Y. (1979). Development of the noradrenergic innervation of neocortex. Brain Res. 162, 243–259. doi: 10.1016/0006-8993(79)90287-7
Li, X. Y., Chen, T., Descalzi, G., Koga, K., Qiu, S., and Zhuo, M. (2012). Characterization of neuronal intrinsic properties and synaptic transmission in layer I of anterior cingulate cortex from adult mice. Mol. Pain 8:53. doi: 10.1186/1744-8069-8-53
Lorente De Nó, R. (1922). La corteza cerebral del ratón. Trabajo de Investigación Biologica Madrid 20, 41–78.
Lu, S. M., and Lin, R. C. (1993). Thalamic afferents of the rat barrel cortex: a light- and electron-microscopic study using Phaseolus vulgaris leucoagglutinin as an anterograde tracer. Somatosens. Mot. Res. 10, 1–16. doi: 10.3109/08990229309028819
Ma, J., Yao, X. H., Fu, Y., and Yu, Y. C. (2014). Development of layer 1 neurons in the mouse neocortex. Cereb. Cortex 24, 2604–2618. doi: 10.1093/cercor/bht114
Ma, L., Qiao, Q., Tsai, J. W., Yang, G., Li, W., and Gan, W. B. (2015). Experience-dependent plasticity of dendritic spines of layer 2/3 pyramidal neurons in the mouse cortex. Dev. Neurobiol. doi: 10.1002/dneu.22313 [Epub ahead of print].
Macchi, G., Bentivoglio, M., Molinari, M., and Minciacchi, D. (1984). The thalamo-caudate versus thalamo-cortical projections as studied in the cat with fluorescent retrograde double labeling. Exp. Brain Res. 54, 225–239. doi: 10.1007/bf00236222
MacLeod, N. K., James, T. A., Kilpatrick, I. C., and Starr, M. S. (1980). Evidence for a GABAergic nigrothalamic pathway in the rat. II. Electrophysiological studies. Exp. Brain Res. 40, 55–61. doi: 10.1007/bf00236662
Magill, P. J., Sharott, A., Bolam, J. P., and Brown, P. (2004). Brain state-dependency of coherent oscillatory activity in the cerebral cortex and basal ganglia of the rat. J. Neurophysiol. 92, 2122–2136. doi: 10.1152/jn.00333.2004
Marin-Padilla, M., and Marin-Padilla, T. M. (1982). Origin, prenatal development and structural organization of layer I of the human cerebral (motor) cortex. A Golgi study. Anat. Embryol. (Berl) 164, 161–206. doi: 10.1007/bf00318504
Masamizu, Y., Tanaka, Y. R., Tanaka, Y. H., Hira, R., Ohkubo, F., Kitamura, K., et al. (2014). Two distinct layer-specific dynamics of cortical ensembles during learning of a motor task. Nat. Neurosci. 17, 987–994. doi: 10.1038/nn.3739
Matsuda, Y., and Nakamura, M. (1982). Mode of activation of thalamic neurons by stimulation of the cerebellar fastigial nuclei in the cat. Brain Res. 241, 370–373. doi: 10.1016/0006-8993(82)91081-2
Middleton, F. A., and Strick, P. L. (1994). Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science 266, 458–461. doi: 10.1126/science.7939688
Mitchell, B. D., and Cauller, L. J. (2001). Corticocortical and thalamocortical projections to layer I of the frontal neocortex in rats. Brain Res. 921, 68–77. doi: 10.1016/s0006-8993(01)03084-0
Mohammed, H., and Jain, N. (2014). Two whisker motor areas in the rat cortex: evidence from thalamocortical connections. J. Comp. Neurol. 522, 528–545. doi: 10.1002/cne.23424
Monconduit, L., and Villanueva, L. (2005). The lateral ventromedial thalamic nucleus spreads nociceptive signals from the whole body surface to layer I of the frontal cortex. Eur. J. Neurosci. 21, 3395–3402. doi: 10.1111/j.1460-9568.2005.04160.x
Muralidhar, S., Wang, Y., and Markram, H. (2013). Synaptic and cellular organization of layer 1 of the developing rat somatosensory cortex. Front. Neuroanat. 7:52. doi: 10.3389/fnana.2013.00052
Nakamura, K. C., Sharott, A., and Magill, P. J. (2014). Temporal coupling with cortex distinguishes spontaneous neuronal activities in identified basal ganglia-recipient and cerebellar-recipient zones of the motor thalamus. Cereb. Cortex 24, 81–97. doi: 10.1093/cercor/bhs287
Nakano, K., Hasegawa, Y., Tokushige, A., Nakagawa, S., Kayahara, T., and Mizuno, N. (1990). Topographical projections from the thalamus, subthalamic nucleus and pedunculopontine tegmental nucleus to the striatum in the Japanese monkey, macaca fuscata. Brain Res. 537, 54–68. doi: 10.1016/0006-8993(90)90339-d
Ohtake, T., and Yamada, H. (1989). Efferent connections of the nucleus reuniens and the rhomboid nucleus in the rat: an anterograde PHA-L tracing study. Neurosci. Res. 6, 556–568. doi: 10.1016/0168-0102(89)90044-8
Patino, P., and Garcia-Munoz, M. (1985). Electrophysiological thalamic responses evoked by dopamine-receptor stimulation into the striatum. Brain Res. 361, 1–9. doi: 10.1016/0006-8993(85)91268-5
Paz, J. T., Chavez, M., Saillet, S., Deniau, J. M., and Charpier, S. (2007). Activity of ventral medial thalamic neurons during absence seizures and modulation of cortical paroxysms by the nigrothalamic pathway. J. Neurosci. 27, 929–941. doi: 10.1523/jneurosci.4677-06.2007
Peters, A. J., Chen, S. X., and Komiyama, T. (2014). Emergence of reproducible spatiotemporal activity during motor learning. Nature 510, 263–267. doi: 10.1038/nature13235
Plowman, E. K., and Kleim, J. A. (2010). Motor cortex reorganization across the lifespan. J. Commun. Disord. 43, 286–294. doi: 10.1016/j.jcomdis.2010.04.005
Reig, R., and Silberberg, G. (2014). Multisensory integration in the mouse striatum. Neuron 83, 1200–1212. doi: 10.1016/j.neuron.2014.07.033
Royce, G. J. (1983). Single thalamic neurons which project to both the rostral cortex and caudate nucleus studied with the fluorescent double labeling method. Exp. Neurol. 79, 773–784. doi: 10.1016/0014-4886(83)90041-9
Royce, G. J., and Mourey, R. J. (1985). Efferent connections of the centromedian and parafascicular thalamic nuclei: an autoradiographic investigation in the cat. J. Comp. Neurol. 235, 277–300. doi: 10.1002/cne.902350302
Royce, G. J., Bromley, S., Gracco, C., and Beckstead, R. M. (1989). Thalamocortical connections of the rostral intralaminar nuclei: an autoradiographic analysis in the cat. J. Comp. Neurol. 288, 555–582. doi: 10.1002/cne.902880404
Rubenstein, J. L. (1998). Development of serotonergic neurons and their projections. Biol. Psychiatry 44, 145–150. doi: 10.1016/s0006-3223(98)00133-4
Rubio-Garrido, P., Pérez-de-Manzo, F., Porrero, C., Galazo, M. J., and Clascá, F. (2009). Thalamic input to distal apical dendrites in neocortical layer 1 is massive and highly convergent. Cereb. Cortex 19, 2380–2395. doi: 10.1093/cercor/bhn259
Sadikot, A. F., Parent, A., and François, C. (1992). Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a PHA-L study of subcortical projections. J. Comp. Neurol. 315, 137–159. doi: 10.1002/cne.903150203
Sakai, S. T., Grofova, I., and Bruce, K. (1998). Nigrothalamic projections and nigrothalamocortical pathway to the medial agranular cortex in the rat: single- and double-labeling light and electron microscopic studies. J. Comp. Neurol. 391, 506–525. doi: 10.1002/(sici)1096-9861(19980222)391:4<506::aid-cne7>3.0.co;2-4
Sanes, J. N., and Donoghue, J. P. (2000). Plasticity and primary motor cortex. Annu. Rev. Neurosci. 23, 393–415. doi: 10.1146/annurev.neuro.23.1.393
Sato, M., Itoh, K., and Mizuno, N. (1979). Distribution of thalamo-caudate neurons in the cat as demonstrated by horseradish peroxidase. Exp. Brain Res. 34, 143–153. doi: 10.1007/bf00238347
Scheibel, M. E., and Scheibel, A. B. (1967). Structural organization of nonspecific thalamic nuclei and their projection toward cortex. Brain Res. 6, 60–94. doi: 10.1016/0006-8993(67)90183-7
Sheets, P. L., Suter, B. A., Kiritani, T., Chan, C. S., Surmeier, D. J., and Shepherd, G. M. (2011). Corticospinal-specific HCN expression in mouse motor cortex: I(h)-dependent synaptic integration as a candidate microcircuit mechanism involved in motor control. J. Neurophysiol. 106, 2216–2231. doi: 10.1152/jn.00232.2011
Soda, T., Nakashima, R., Watanabe, D., Nakajima, K., Pastan, I., and Nakanishi, S. (2003). Segregation and coactivation of developing neocortical layer 1 neurons. J. Neurosci. 23, 6272–6279.
Strick, P. L. (1973). Light-microscopic-analysis-of-the-cortical-projection-of-the-thalamic-ventrolateral-nucleus-in-the-cat. Brain Res. 55, 1–24. doi: 10.1016/0006-8993(73)90485-x
Strick, P. L., and Sterling, P. (1974). Synaptic termination of afferents from the ventrolateral nucleus of the thalamus in the cat motor cortex. A light and electron microscopy study. J. Comp. Neurol. 153, 77–106. doi: 10.1002/cne.901530107
Takada, M., Ng, G., and Hattori, T. (1986). Single pallidal neurons project both to the striatum and thalamus in the rat. Neurosci. Lett. 69, 217–220. doi: 10.1016/0304-3940(86)90482-9
Tanibuchi, I., Kitano, H., and Jinnai, K. (2009). Substantia nigra output to prefrontal cortex via thalamus in monkeys. I. Electrophysiological identification of thalamic relay neurons. J. Neurophysiol. 102, 2933–2945. doi: 10.1152/jn.91287.2008
Tennant, K. A., Adkins, D. L., Donlan, N. A., Asay, A. L., Thomas, N., Kleim, J. A., et al. (2011). The organization of the forelimb representation of the C57BL/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture. Cereb. Cortex 21, 865–876. doi: 10.1093/cercor/bhq159
Thomson, A. M., and Lamy, C. (2007). Functional maps of neocortical local circuitry. Front. Neurosci. 1, 19–42. doi: 10.3389/neuro.01.1.1.002.2007
Tsumori, T., Yokota, S., Ono, K., and Yasui, Y. (2002). Synaptic organization of GABAergic projections from the substantia nigra pars reticulata and the reticular thalamic nucleus to the parafascicular thalamic nucleus in the rat. Brain Res. 957, 231–241. doi: 10.1016/s0006-8993(02)03554-0
Ueki, A. (1983). The mode of nigro-thalamic transmission investigated with intracellular recording in the cat. Exp. Brain Res. 49, 116–124. doi: 10.1007/bf00235546
Uno, M., Ozawa, N., and Yoshida, M. (1978). The mode of pallido-thalamic transmission investigated with intracellular recording from cat thalamus. Exp. Brain Res. 33, 493–507. doi: 10.1007/bf00235570
Van Der Werf, Y. D., Witter, M. P., and Groenewegen, H. J. (2002). The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res. Brain Res. Rev. 39, 107–140. doi: 10.1016/s0165-0173(02)00181-9
Vertes, R. P., Linley, S. B., and Hoover, W. B. (2015). Limbic circuitry of the midline thalamus. Neurosci. Biobehav. Rev. 54, 89–107. doi: 10.1016/j.neubiorev.2015.01.014
Walcott, E. C., and Langdon, R. B. (2001). Short-term plasticity of extrinsic excitatory inputs to neocortical layer 1. Exp. Brain Res. 136, 143–151. doi: 10.1007/s002210000582
Weiler, N., Wood, L., Yu, J., Solla, S. A., and Shepherd, G. M. (2008). Top-down laminar organization of the excitatory network in motor cortex. Nat. Neurosci. 11, 360–366. doi: 10.1038/nn2049
Williams, S. R. (2005). Encoding and decoding of dendritic excitation during active states in pyramidal neurons. J. Neurosci. 25, 5894–5902. doi: 10.1523/jneurosci.0502-05.2005
Wozny, C., and Williams, S. R. (2011). Specificity of synaptic connectivity between layer 1 inhibitory interneurons and layer 2/3 pyramidal neurons in the rat neocortex. Cereb. Cortex 21, 1818–1826. doi: 10.1093/cercor/bhq257
Wu, J., and Hablitz, J. J. (2005). Cooperative activation of D1 and D2 dopamine receptors enhances a hyperpolarization-activated inward current in layer I interneurons. J. Neurosci. 25, 6322–6328. doi: 10.1523/jneurosci.1405-05.2005
Xu, N. L., Harnett, M. T., Williams, S. R., Huber, D., O’connor, D. H., Svoboda, K., et al. (2012). Nonlinear dendritic integration of sensory and motor input during an active sensing task. Nature 492, 247–251. doi: 10.1038/nature11601
Xu, T., Yu, X., Perlik, A. J., Tobin, W. F., Zweig, J. A., Tennant, K., et al. (2009). Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462, 915–919. doi: 10.1038/nature08389
Yang, G., Pan, F., and Gan, W. B. (2009). Stably maintained dendritic spines are associated with lifelong memories. Nature 462, 920–924. doi: 10.1038/nature08577
Ye, Z., McGee, T. P., Houston, C. M., and Brickley, S. G. (2013). The contribution of delta subunit-containing GABAA receptors to phasic and tonic conductance changes in cerebellum, thalamus and neocortex. Front. Neural Circuits 7:203. doi: 10.3389/fncir.2013.00203
Yu, X., and Zuo, Y. (2011). Spine plasticity in the motor cortex. Curr. Opin. Neurobiol. 21, 169–174. doi: 10.1016/j.conb.2010.07.010
Yuste, R., and Bonhoeffer, T. (2001). Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu. Rev. Neurosci. 24, 1071–1089. doi: 10.1146/annurev.neuro.24.1.1071
Zecevic, N., and Rakic, P. (2001). Development of layer I neurons in the primate cerebral cortex. J. Neurosci. 21, 5607–5619.
Zhou, F. M., and Hablitz, J. J. (1996). Layer I neurons of rat neocortex. I. Action potential and repetitive firing properties. J. Neurophysiol. 76, 651–667.
Zhou, W. L., Oikonomou, K. D., Short, S. M., and Antic, S. D. (2013). Dopaminergic regulation of dendritic calcium: fast multisite calcium imaging. Methods Mol. Biol. 964, 123–138. doi: 10.1007/978-1-62703-251-3_9
Keywords: ventromedial, ventrolateral, ventral anterior, midline-intralaminar, basal ganglia, motor cortex
Citation: Garcia-Munoz M and Arbuthnott GW (2015) Basal ganglia—thalamus and the “crowning enigma”. Front. Neural Circuits 9:71. doi: 10.3389/fncir.2015.00071
Received: 11 June 2015; Accepted: 22 October 2015;
Published: 04 November 2015.
Edited by:
Vincenzo Crunelli, Cardiff University, UKReviewed by:
Giuseppe Di Giovanni, University of Malta, MaltaMasahiko Takada, Kyoto University, Japan
Copyright © 2015 Garcia-Munoz and Arbuthnott. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gordon W. Arbuthnott, gordon@oist.jp
 Marianela Garcia-Munoz
Marianela Garcia-Munoz Gordon W. Arbuthnott
Gordon W. Arbuthnott