Reduction in focal ictal activity following transplantation of MGE interneurons requires expression of the GABAA receptor α4 subunit
- 1C.V. Starr Laboratory for Molecular Neuropharmacology, Department of Anesthesiology, Weill Cornell Medical College, New York, NY, USA
- 2Department of Pediatrics, Weill Cornell Medical College, New York, NY, USA
- 3Department of Neurological Surgery, Weill Cornell Medical College, New York, NY, USA
- 4Brain and Mind Research Institute, Weill Cornell Medical College, New York, NY, USA
- 5Department of Psychiatry, Children’s Hospital of Philadelphia, University of Pennsylvania School of Medicine, Philadelphia, PA, USA
- 6Department of Anesthesiology, University of Pittsburgh, Pittsburgh, PA, USA
Despite numerous advances, treatment-resistant seizures remain an important problem. Loss of neuronal inhibition is present in a variety of epilepsy models and is suggested as a mechanism for increased excitability, leading to the proposal that grafting inhibitory interneurons into seizure foci might relieve refractory seizures. Indeed, transplanted medial ganglionic eminence interneuron progenitors (MGE-IPs) mature into GABAergic interneurons that increase GABA release onto cortical pyramidal neurons, and this inhibition is associated with reduced seizure activity. An obvious conclusion is that inhibitory coupling between the new interneurons and pyramidal cells underlies this effect. We hypothesized that the primary mechanism for the seizure-limiting effects following MGE-IP transplantation is the tonic conductance that results from activation of extrasynaptic GABAA receptors (GABAA-Rs) expressed on cortical pyramidal cells. Using in vitro and in vivo recording techniques, we demonstrate that GABAA-R α4 subunit deletion abolishes tonic currents (Itonic) in cortical pyramidal cells and leads to a failure of MGE-IP transplantation to attenuate cortical seizure propagation. These observations should influence how the field proceeds with respect to the further development of therapeutic neuronal transplants (and possibly pharmacological treatments).
Introduction
The treatment of seizures by transplantation of inhibitory interneurons into epileptic foci is an area of considerable interest (Anderson and Baraban, 2012). Various transplantation strategies have been employed to suppress epileptic activity (Raedt et al., 2007), including the use of interneuron precursors harvested from the embryonic MGE (Alvarez-Dolado et al., 2006; Baraban et al., 2009; Waldau et al., 2010; De la Cruz et al., 2011; Henderson et al., 2014). Transplanted MGE-IPs differentiate into mature GABAergic interneurons that provide inhibitory synaptic and extrasynaptic GABAergic transmission onto cortical pyramidal neurons (Sultan et al., 2013). This inhibition is associated with a reduction in seizure activity (Alvarez-Dolado et al., 2006; Baraban et al., 2009).
Extrasynaptic activity, a response based on a distinct population of GABAA receptors (GABAA-Rs), is of great significance in mediating neuronal inhibition (Lee and Maguire, 2014). Immunohistochemical (Pirker et al., 2000; Chandra et al., 2006; Hörtnagl et al., 2013) and pharmacologic (Brown et al., 2002; Krook-Magnuson and Huntsman, 2005; Drasbek and Jensen, 2006; Drasbek et al., 2007; Vardya et al., 2008) data indicate that the GABAA-R α4 subunit contributes to the formation of extrasynaptic GABAA-Rs in Layer 2/3 and Layer 4 cortical neurons in the rodent brain. In deeper neurons (Layer 5), the α5 subunit contributes to the tonic current in both neonatal (Sebe et al., 2010) and juvenile (Yamada et al., 2007) animals. Importantly, a tonic current has also been observed in Layer V-VI pyramidal cells from human neocortex, where less than a third of the tonic current results from activation of GABAA-Rs containing the α5 subunit (Scimemi et al., 2006), with the subunit-basis of the remainder of the current unknown. It is possible, however, that the non-α5 subunit-mediated current is generated by GABAA-Rs containing the α4 subunit as α4 subunit mRNA is also present in those cells (Petri et al., 2003).
GABA-mediated tonic inhibition appears to inhibit seizure activity as the anticonvulsants vigabratin and tiagabine (which inhibit GABA-transamninase and GABA reuptake, respectively) increases the concentration of GABA in the brain (Petroff et al., 1996; Richards and Bowery, 1996); importantly, this increase results, in part, from non-vesicular release of GABA (Wu et al., 2003). Deletion of the GABAA-R δ subunit, which co-assembles with the α4 subunit (Sur et al., 1999; Jia et al., 2005) and contributes to the formation of extrasynaptic GABAA-Rs (Nusser et al., 1998; Sassoè-Pognetto et al., 2000; Brickley et al., 2001; Nusser and Mody, 2002; Jia et al., 2005), is associated with a decrease in α4 expression (Peng et al., 2002) and has pro-convulsant effects (Spigelman et al., 2002). In dentate gyrus cells in a rat TLE model, there was a significant decrease in the neurosteroid-sensitive tonic current which occurred in combination with a decrease in the surface expression of the δ subunit (Peng et al., 2004; Rajasekaran et al., 2010), suggesting a close relationship between extrasynaptic GABAA-Rs and seizure activity. In a parallel fashion, there is a shift in α4 expression from the extrasynaptic to synaptic membrane in the rat TLE model (Sun et al., 2007), consistent with a loss of extrasynaptic GABAA-Rs. In a rat traumatic brain injury model, there is a significant decrease in the tonic current in semilunar granule cells (SGCs; which are excitatory neurons located in the dentate inner molecular layer), and the loss of the tonic current is associated with a significant increase in input resistance and a significant increase in SGC excitability (Gupta et al., 2012). In the epileptic mutant mouse stargazer, there is a preferential loss of extrasynaptic GABAA-Rs (Payne et al., 2006, 2007). More compellingly, mutations associated with both febrile seizures and childhood absence epilepsy compromise surface expression of GABAA-Rs and reduce tonic GABA currents but do not affect GABA-mediated synaptic events (Eugène et al., 2007). Finally, at least one clinically relevant AED, gabapentin, has been shown to enhance the tonic current in hippocampal neurons (Cheng et al., 2006). Thus, activation of extrasynaptic GABAA-Rs represents a novel approach to treating seizures arising in cortical, rather than subcortical, structures (Cope et al., 2009).
Multiple animal models exist for studying seizure activity in vivo (Pitkänen et al., 2006), including application of the transient potassium current blocker, 4-aminopyridine (4-AP; Barna et al., 2000; Bahar et al., 2006; Ma et al., 2009; Zhao et al., 2009, 2015; Medina-Ceja and Ventura-Mejía, 2010; Salazar and Tapia, 2012; Yu et al., 2014). We have chosen an acute, rather than chronic, focal seizure mouse model for several reasons. First, a model of partial onset epilepsy is required since MGE-IP transplants are intended as a focal therapy. Second, the ictal events induced with 4-AP last one to two minutes and appear electrographically exactly like partial onset human epilepsy (Avoli, 1990; Perreault and Avoli, 1991; Heinemann et al., 2006), beginning with low-voltage, fast-activity and progressing to paroxysmal spike-and-wave activity (Schwartz and Bonhoeffer, 2001). Third, ictogenesis is reliable and easy to induce. Together, these attributes make the 4-AP acute seizure model a viable and useful paradigm for examining both underlying mechanism(s) as well as potential treatment efficacy (Löscher, 2011).
We hypothesized that the observed seizure-limiting effect of MGE-IP transplantation results from activation of extrasynaptic GABAA-Rs expressed on cortical pyramidal cells. Determining whether the reduction in ictal activity following MGE-IP transplantation arises from synaptic or extrasynaptic activity will guide the development of transplant-based therapies and potential pharmacological treatments for medically refractory seizure disorders.
Methods
All animal experiments were performed in accordance with Institutional and Federal guidelines. The principles in the ARRIVE Guidelines from The National Centre for the Replacement, Refinement and Reduction of Animals in Research (London, UK)1 were considered when planning the experiments. To avoid periodic alterations in specific GABAAR subunits during the estrous cycle in mice, which are associated with cyclic changes of tonic inhibition and seizure susceptibility (Maguire et al., 2005), only male mice were used for in vitro and in vivo experiments.
In Vivo Transplantation of MGE Cells
Pan-green fluorescent protein (GFP)-expressing transgenic mice were maintained on a CD1 background. At 13 days of gestation (E13.5), dams were sacrificed, GFP+ embryos harvested, and their brains removed. MGE-IP cells were obtained from 250 µm sections by mechanical dissociation after separating the dorsal and ventral MGE from adjacent brain regions (Figure 2A). Cells were re-suspended in ice-cold neurobasal/B27 medium until transplantation (De la Cruz et al., 2011).
Post-natal GABAA-R α4 subunit deletion (α4−/−) mice or wild-type (α4+/+) male littermates at P30–40 were anesthetized with sevoflurane and mounted in a stereotaxic frame. Using a nanoinjector, 1.5 × 104 MGE-IP cells in 1.5–2.0 µl were injected into the motor cortex (coordinates relative to Bregma: anteroposterior (AP) −0.7 mm, mediolateral (ML) −1.0 mm, and dorsoventral (DV) −1.0 mm) through a small burr hole. As a sham control, in a separate group of animals, an equivalent volume and number of MGE cells killed by freezing and thawing was injected in the same location.
In Vitro Electrophysiology and Analysis
Whole-cell patch clamp recordings were made from visually identified cortical layer 2/3 neurons in acutely prepared 300-µm thick coronal brain slices (using IR/DIC microscopy) from GABAA-R α4−/− mice and α4+/+ male littermates using a Nikon Eclipse FN1 microscope equipped with a 4× objective and a 40× water immersion objective and a high-sensitivity monochrome digital camera (DS-Qi1, Nikon). Pyramidal cells were selected based on pyramidal shape, apical dendrite, and distance (200–300 µm) from pia. Standard whole-cell patch clamp recordings were made in either the voltage or current clamp configuration using a Multiclamp 700 amplifier (Molecular Devices, Sunnyvale, CA) and pClamp 10.2 software (Molecular Devices). Data were acquired at 10 kHz, filtered at 2.2 kHz, and digitized using a Digidata 1440A A/D interface (Molecular Devices). All voltage clamp experiment recordings were performed at −70 mV; neurons having resting membrane potential greater than −45 mV were omitted from the study. Liquid junction potential (+3.8 mV) was not corrected. Series resistance was less than 25 MΩ following membrane rupture and with whole-cell configuration established; data were excluded if resistance changed by more than 20% over the course of the recording. For analysis of sIPSC decay times, currents were fit with a monoexponential function and the 80–20% decay time recorded.
Drugs and Solutions
Salts and D-(-)-2-amino-5-phosphonopentanoic acid (AP5), (±)-3-piperidine carboxylic acid (nipecotic acid; NPA) were from Sigma-Aldrich (St. Louis, MO); bicuculline methochloride, 6-cyano-7-nitroquinoxaline-2, 3-dione (CNQX), 11, 12, 13, 13a-tetrahydro-7-methoxy-9-oxo-9H-imidazo [1, 5-a] pyrrolo [2, 1-c] [1, 4] benzodiazepine-1-carboxylic acid, ethyl ester (L655, 708), and (2S)-(+)-5, 5-dimethyl-2-morpholineacetic acid (SCH 50911) were from Tocris (Bristol, UK). CNQX, AP5, bicuculline, and NPA were dissolved in distilled water to a stock concentration of 10, 20, 25 and 100 mM, respectively. L655, 708 and SCH 50911 were dissolved in dimethyl sulfoxide (DMSO) to a stock concentration of 5 and 10 mM, respectively. Stock solutions were kept at −20°C and added to the extracellular recording solution prior to recording.
The various solutions contained (in mM):
Slicing solution: 250 sucrose, 2 KCl, 5 MgCl2, 0.5 CaCl2, 1.25 NaH2PO4, 26 NaHCO3 and 10 D-glucose (ice-cold) saturated with 95% O2/5% CO2.
Incubation extracellular solution: 126 NaCl, 3.6 KCl, 5 MgCl2, 0.5 CaCl2, 1.2 NaH2PO4, 26 NaHCO3 and 10 D-glucose; pH 7.4 when saturated with 95% O2/5% CO2. Incubation solution was initially at 36°C, and after 40 min the temperature of the incubation solution was gradually lowered and slices were maintained at room temperature (22–24°C); all subsequent electrophysiology experiments were performed at room temperature.
Extracellular recording solution: 126 NaCl, 3.6 KCl, 1.5 MgCl2, 2.5 CaCl2, 1.2 NaH2PO4, 26 NaHCO3, 10 D-glucose saturated with 95% O2/5% CO2. The extracellular recording solution also contained: AP5 (20 µM), CNQX (10 µM), and SCH 50911 (10 µM). When present, the final concentrations of NPA, bicuculline, and L655, 708 were, respectively: 1 mM (a concentration which completely blocks GABA reuptake (Keros and Hablitz, 2005)), 50 µM, and 250 nM.
Intracellular solution: 135 KCl, 10 HEPES, 2 Mg-ATP, 0.2 Na-GTP, 0.5 EGTA; pH 7.4.
The magnitude of the tonic GABA-mediated current was calculated by measuring the difference in the baseline whole-cell current in the absence and presence of bicuculline (50 µM; a concentration which is sufficient to block all GABAA-R-mediated currents in brain slices (Yamada et al., 2007)). The current clamp configuration was used to measure membrane potentials. Tonic current amplitude was analyzed as a function of genotype, and results were compared using either unpaired Student’s t-test or the Mann-Whitney Rank Sum Test (if the data did not pass either normality or equal variance tests) as appropriate; p < 0.05 was considered significant. Data are presented as mean ± SEM.
In Vivo Electrophysiology
Methods employed here are similar to those previously reported (De la Cruz et al., 2011). Briefly, thirty to forty days post-transplantation, adult male mice were anesthetized with isoflurane and mounted in a stereotaxic frame. Electrodes were inserted into somatosensory cortex through a small cranial window after opening the dura. A custom-designed chamber was affixed to the skull and filled with silicone oil. Ictal discharges were induced by injecting 4-AP (15 mM in 0.9% saline, 0.5 µl) through a single-barreled glass microelectrode placed 2 mm away from the site of the transplant. The local field potential (LFP) was recorded with two electrodes (see Figure 2E for general configuration). Ictal onset was recorded from the same electrode used for 4-AP injection (LFP1). Ictal propagation was recorded from the site of transplantation (LFP2), which was identified by the injection burr hole. A reference electrode (secured by one miniature stainless steel screw) was placed into the skull above the cerebellum. LFPs were amplified, filtered (0.1–1,000 Hz) and digitized at 1 kHz using two AC/DC differential amplifiers (Model 3000, A-M systems, Carlsborg, WA). Data were recorded on a PC using a CED Power 1401 and Spike2 software (Cambridge Electronic Design, Cambridge UK). The amplitude and duration of ictal activity was recorded at each of the two LFP electrodes, one in the 4-AP focus (LFP1) and the other in the region of the cell transplants (LFP2) to determine propagation efficacy.
4-AP in Vivo Data Analysis
Off-line analysis was performed using custom analysis software written in Matlab (MathWorks, Natick, MA) (De la Cruz et al., 2011). Seizure onset was determined by visual analysis of the LFP1 recording based on the morphology of the seizures. In the 4-AP seizure model, most seizures start with a large spike-and-wave, followed by a recruiting rhythm that evolves into repetitive spike-and-wave discharges that dissipate with progressive decrease in amplitude and increase in inter-spike interval (Zhao et al., 2009). Consistent with our prior experience, such activity persisted for up to 2 h (data not shown). This large initial spike was used as the marker of ictal onset; termination of ictal activity (offset) was determined by the cessation of the spike-and-wave discharges and the return to baseline, pre-ictal activity. The time windows between ictal onset and offset was used for the further analysis. The average seizure duration at the 4-AP injection electrode (LFP1 recording site) was 33.6 ± 10.8 s (315 seizures, 20 mice). After setting a threshold 2 × SD above baseline activity, total LFP (ΣLFP) power was calculated as the integral of the LFP power during each period of ictal activity (as demarcated by ictal onset and offset as defined above). Baseline activity was measured over a 2 s epoch prior to the initial “onset” spike.
The ratios of ΣLFP power and duration from LFP1 were compared to LFP2 to establish the effect of MGE cells transplantation on each epileptic event. The power and duration calculations were performed using custom-made analysis program based on Matlab. The averaged ratio result of all ictal discharges from an individual mouse was used for the further group analysis. For fast Fourier transform (FFT) analysis between LFP1 and LFP2, cross power spectrum and coherence calculations were performed using “cpsd” and “mscohere” functions in Matlab (Bronzino, 1984; Davey et al., 2000). Each computation used Welch’s averaged periodogram method (Welch, 1967) with NFFT of 256 and a Hanning window of the same size. Comparisons of the effects of transplantation on the power ratio between the two groups, α4+/+ and α4−/−, as a percentage of the power ratio for control killed cell injections were performed with two-way ANOVA with Tukey-Kramer post hoc analysis for multiple comparisons (SPSS Statistics, IBM). Repeated-measures ANOVA was used for analysis of both the cross spectral density estimates and magnitude-squared coherence (MSC).
4-AP injected at site 1 (LFP1) simultaneously induces ictal activity at both LFP1 and LFP2 recording sites under control conditions (Figures 3A,C); this is in contrast to the situation following MGE-IP transplantation in wild-type mice, where ictal activity is significantly reduced at LFP2, but is preserved at the LFP1 site of 4-AP injection (Figure 3B). Thus, ictal activity induced by 4-AP injection reflects ictal propagation by cortical spread rather than 4-AP diffusion.
Immunohistochemistry
Mice transplanted with MGE-IPs were intracardially perfused with phosphate buffered saline (PBS) followed by 4% paraformaldehyde prepared in PBS immediately after 4-AP induced seizure experiments. Brains were stored in 4% paraformaldehyde for at least 48 h to allow for proper fixation, after which 50 µm-thick coronal sections were prepared. Free floating sections were placed in blocking solution (5% bovine serum albumin (BSA) in PBS with 3% triton) for 1 h followed by incubation in primary antibody (chicken anti-GFP; 1:1000; Abcam, Cambridge, MA) in blocking solution for 72 h at 4°C. Sections were then incubated in secondary antibody (Alexa-488 goat anti-chicken; 1:250; Life Technologies, Grand Island, NY) for 2 h at room temperature, washed, mounted on a glass slide with aqueous mounting medium (Fluoromount; Southern Biotech, Birmingham, AL) and visualized using epifluorescent microscopy.
Results
GABA activates synaptic GABAA-Rs, which underlie “phasic” inhibitory postsynaptic currents (IPSCs), as well as extrasynaptic GABAA-Rs, which mediate persistent “tonic” currents (Lee and Maguire, 2014). A limited repertoire of GABAA-Rs generate Itonic, including those containing the α4 subunit (Lee and Maguire, 2014). Evidence suggests that the GABAA-R α4 subunit contributes to Itonic in cortical neurons (Chandra et al., 2006; Drasbek et al., 2007). Electrophysiological confirmation of this hypothesis is provided in Figure 1; the average tonic current in α4+/+ pyramidal neurons was 9.0 ± 2.8 pA (n = 21). Deletion of the α4 subunit abolishes Itonic (Figures 1A,B); the average tonic current in α4−/− pyramidal neurons was 0.2 ± 1.0 pA (n = 11). Spontaneous IPSCs (sIPSCs) were unaffected [average (from >2,000 events/genotype) amplitude, decay time, and frequency for α4+/+ and α4−/− neurons are, respectively: −48.2 ± 3.2 and −44.4 ± 2.6 pA, 11.5 ± 0.6 and 10.2 ± 0.5 ms, and 4.2 ± 0.3 and 4.4 ± 0.3 Hz; Figure 1A inset]. Deletion of the α4 subunit results in neuronal hyper-excitability (Figure 1C), consistent with a lower threshold for chemically-induced seizures (Chandra et al., 2008). Interestingly, however, the input resistance (Rin) was not significantly different between the two genotypes (α4+/+, 125.8 ± 11.9 MΩ, n = 20 and α4−/−, 118.1 ± 6.1 MΩ, n = 20; p = 0.735, Mann-Whitney Rank Sum test as data did not pass normality test). Preservation of Rin despite the loss of the tonic GABAA-R α4 subunit-dependent Cl− conductance could result from compensatory changes in a voltage-independent K+ conductance as is seen in response to loss of the tonic current generated by GABAA-Rs containing the α6 subunit (Brickley et al., 2001).
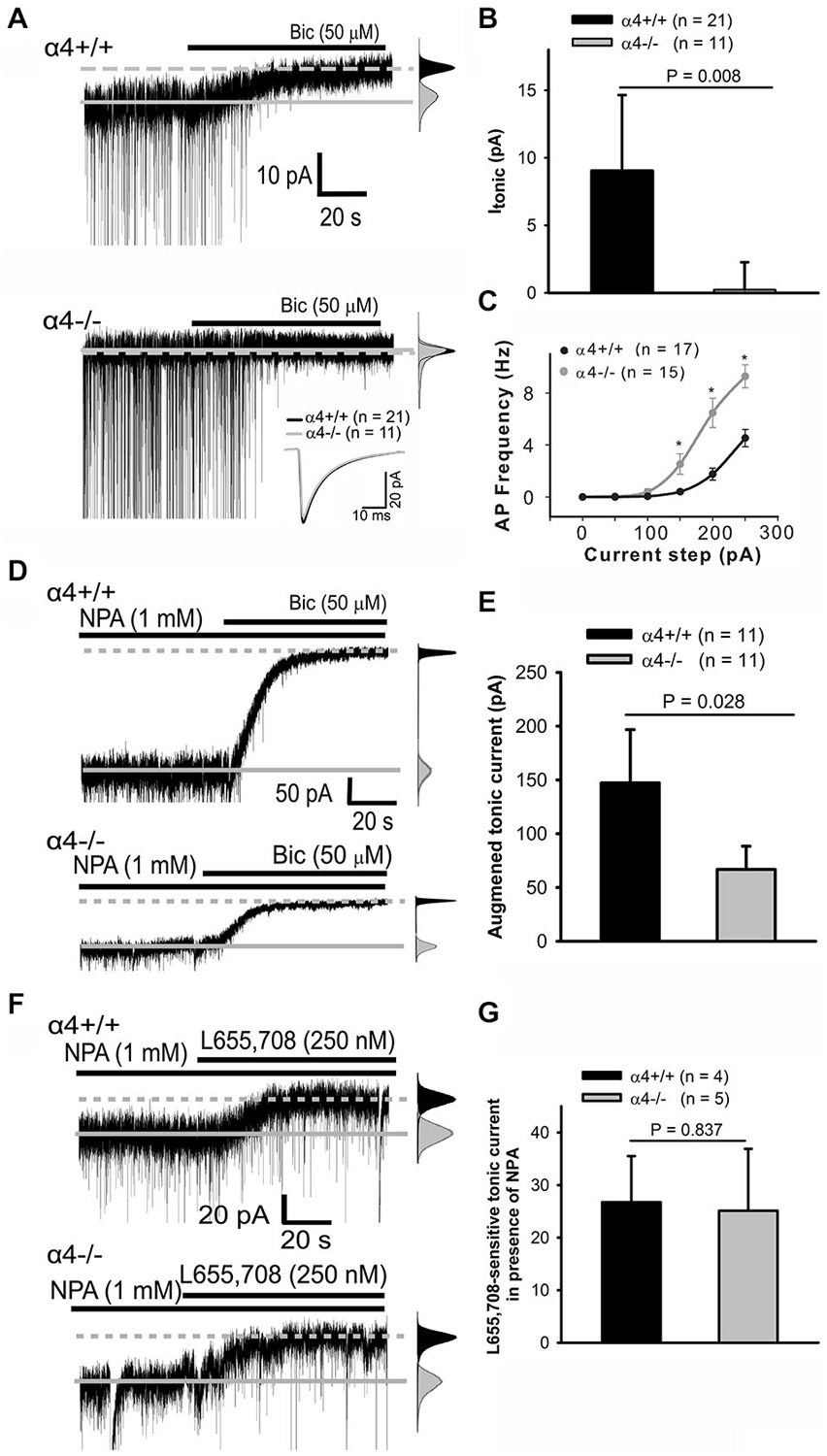
Figure 1. α4 subunit-containing GABAA-Rs are the basis of Itonic in cortical pyramidal neurons. (A) Whole-cell currents from α4+/+ (top trace) and α4−/− (bottom trace) layer II/III pyramidal cells in the absence and presence of bicuculline (bic); traces truncated here and in d top for clarity. sIPSCs are readily visible; inset shows overlay of averaged ensemble sIPSCs. The solid line indicates average baseline current without bicuculline while the dashed line indicates average baseline current in the presence of bicuculline. All-points histograms (gray: control; black, bicuculline) highlight the shift in the baseline current in the α4+/+, but not α4−/−, neuron. (A, right) (B) Bar graph summarizing Itonic data. (C) Input-output curves for evoked spike firing in neurons from α4+/+ and α4−/− mice. * p < 0.05 (Mann-Whitney Rank Sum test). (D, left) Representative traces demonstrating that nipecotic acid (NPA) enhances the persistent current in both α4+/+ (top trace) and α4−/− (bottom trace) neurons. All-points histograms here (and in F) were constructed as in Panel (A). (E) The effects of NPA are significantly different between genotypes (Mann-Whitney Rank Sum Test). (F, left) Representative traces demonstrating that superfusion of L655, 708 in the presence of NPA has a comparable effect on persistent currents in α4+/+ (top trace) and α4−/− neurons (bottom trace); this effect is not significant between genotypes (unpaired t-test) (G).
The GABAA-R α5 (but not α1) subunit has been proposed to contribute to Itonic in cortical neurons (Drasbek et al., 2007; Sebe et al., 2010; Lee and Maguire, 2014). This was tested pharmacologically by increasing extracellular GABA concentrations with nipecotic acid (NPA) in order to maximize the detection of all possible GABAA-Rs to Itonic. Inhibition of GABA reuptake with NPA significantly (Mann-Whitney Rank Sum test) enhanced persistent currents in layer 2/3 pyramidal cells from α4+/+ (147.3 ± 24.8 pA, n = 11) as compared to α4−/− mice (66.7 ± 10.8 pA, n = 11; Figures 1D,E). The NPA-induced “tonic” current in α4−/− neurons could result from either α5 up-regulation and/or persistent activation of low affinity synaptic GABAA-Rs. To evaluate the role of the α5 subunit in generating this current, the GABAA-R α5 subunit-selective inverse agonist L655, 709 was co-applied with NPA and found to have the same effect on Itonic in both α4+/+ (26.8 ± 4.4 pA, n = 4) and α4−/− (25.1 ± 5.9 pA, n = 5) mice (Figures 1F,G), indicating that there is no compensatory up-regulation of the α5 subunit in α4−/− mice. The NPA-induced “tonic” current observed in α4−/− neurons after α5 subunit blockade likely reflects persistent activation of incompletely desensitized synaptic receptors (Keros and Hablitz, 2005).
We have previously demonstrated that transplantation of medial ganglionic eminence interneuron precursors (MGE-IPs) harvested from embryonic (E13.5) brain (Figure 2A) and transplanted into the adult cortex results in long-term survival, migration, and differentiation into interneurons (De la Cruz et al., 2011). A population of transplanted MGE-IPs remains at the site of injection (Figure 2B), and consistent with our earlier reported results, MGE-IPs survive and migrate following transplantation in α4+/+ (Figure 2C) and α4−/− (Figure 2D) mice. In α4+/+ and α4−/− mice, the transplanted MGE-IPs readily migrated throughout the cortex as far as 1 mm from the injection site without apparent differences between the two genotypes. These migratory cells display a pattern of arborization consistent with mature neurons (Figures 2Cii,Dii).
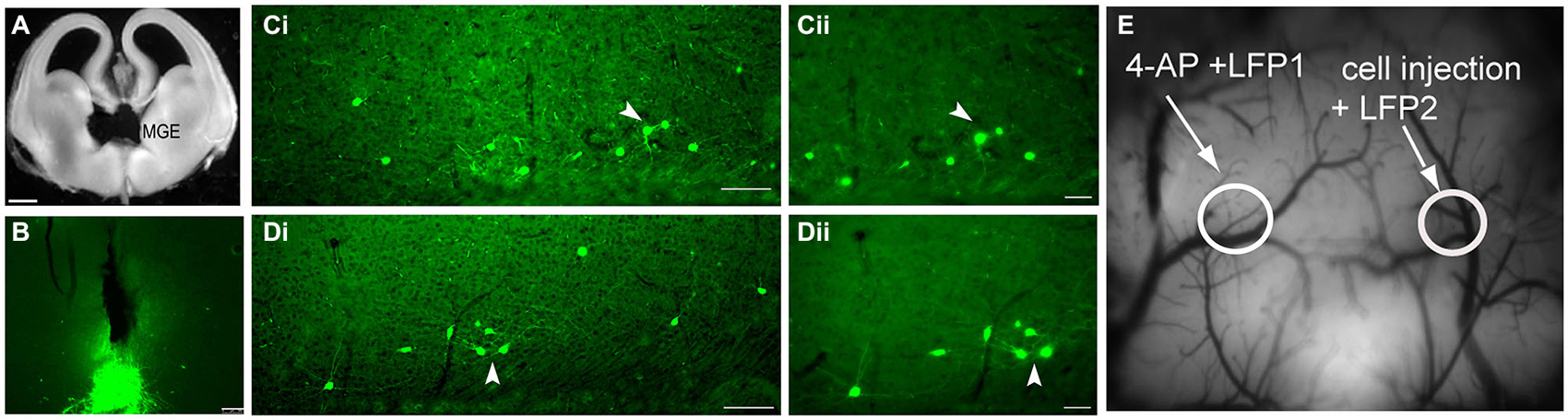
Figure 2. Transplantation of green fluorescent protein (GFP)-positive embryonic medial ganglionic eminence (MGE) cells into the adult cortex results in long-term survival, migration, and arborization. (A) MGE dissection in an E13.5 embryo. (B) Photomicrograph of a coronal brain section from an α4+/+ mouse demonstrating residual clustering of MGE neurons 30 days post-transplantation at the site of injection (see Methods for stereotaxic coordinates). Scale bar: 100 µm. (C,D) Low-power photomicrographs (scale bar: 100 µm) of coronal brain sections from α4+/+ (Ci) and α4−/− (Di) mice demonstrating that transplanted GFP+ neurons migrate from the site of injection and form comparable arborization patterns. The transplanted MGE progenitors migrated throughout the cortex, and as far as 1 mm from the injection site. (Cii–Dii) Higher magnification images (scale bar: 50 µm) of the area indicated by the white arrowhead in (Ci,Di) shows that the transplanted interneurons have processes similar to mature neurons. (E) Image of the cortical surface indicating the locations of the LFP1 electrode, the 4-AP injection site, the LFP2 electrode, and the MGE/control cell injection site.
As shown above (Figure 1C), deletion of the α4 subunit results in neuronal hyper-excitability. Focal neocortical ictal activity was evoked using 4-aminopyridine (4-AP) and measured (Figures 2E, 3A–D) as described (De la Cruz et al., 2011). Focal injection of 4-AP into somatosensory cortex 2 mm away from the site of MGE-IP transplant (same site as LFP1 as shown in Figure 2E) readily evoked ictal activity in α4+/+ (Figure 3A) and α4−/− (Figure 3C) mice under control (dead cell) conditions. Ictal activity occurred simultaneously at LFP1 and LFP2, indicating that 4-AP-induced ictal activity resulted from electrical propagation by cortical spread rather than 4-AP diffusion. 4-AP-induced seizure activity was not significantly different between genotypes (Figure 3E). MGE-IP transplantation significantly reduced seizure power in α4+/+ (F3,16 = 9.368, p < 0.01; Figures 3B,E), but not in α4−/− animals (Figures 3D,E), while sham injections had no effect (Figures 3A,C,E).
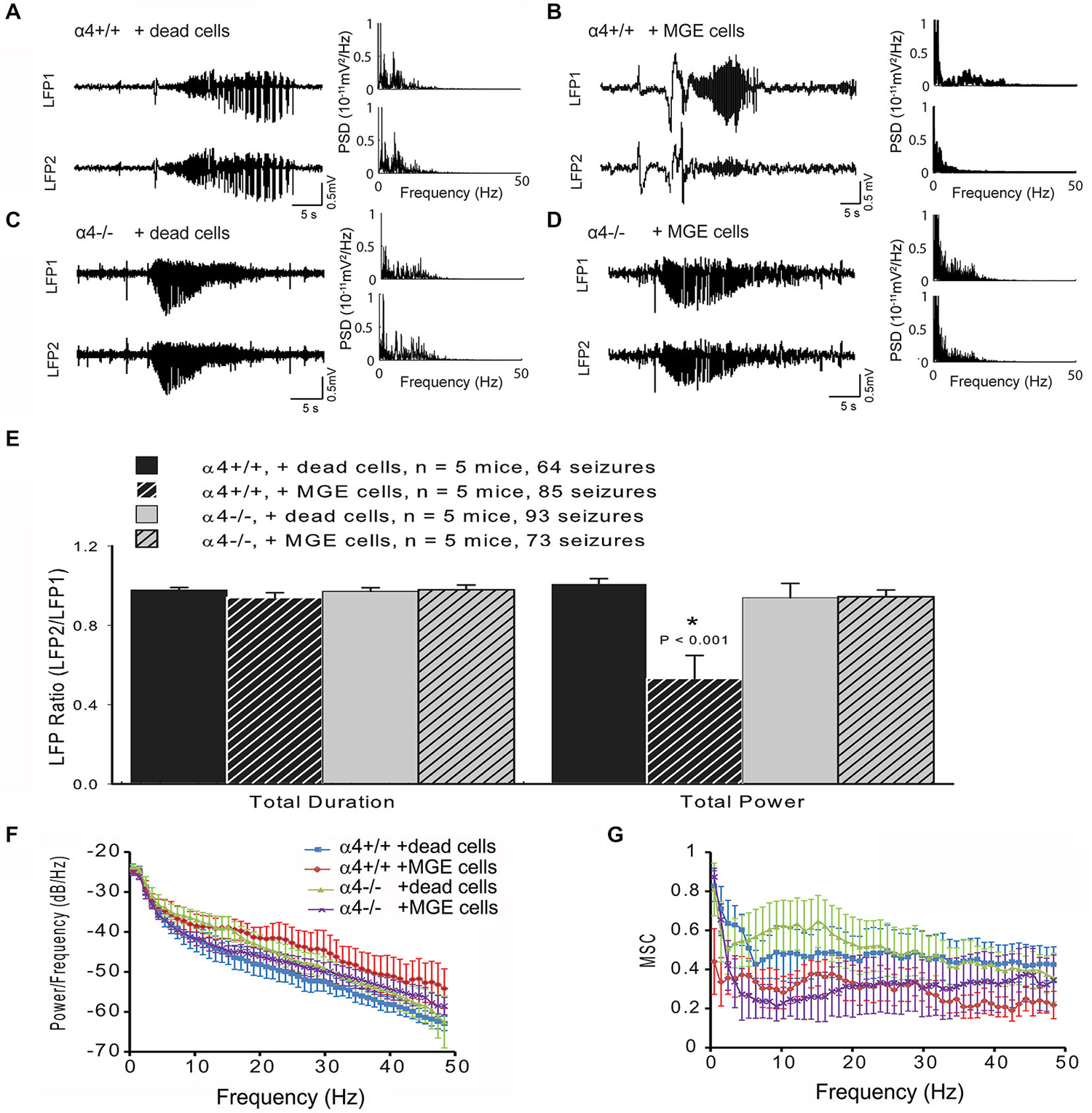
Figure 3. MGE-IP transplants fail to attenuate seizure propagation in GABAA-R α4−/− mice. (A–D) local field potential (LFP) recording (left panel) from an α4+/+ mouse under control conditions (A) and following MGE-transplantation (B). (C,D) 4-AP-induced seizure-like electrical activity recorded from α4−/− mice under control (C) and 30-days post-MGE transplant (D) conditions. Right panel (A–D) displays the power spectral density (PSD) of the LFP recording. (E) Summary data for ictal duration and power for each condition by genotype. (F) Averaged cross spectral density estimate and (G) averaged magnitude-squared coherence (MSC) for each condition by genotype.
The raw LFP signal is a complex waveform which shows temporal variations in multiple domains, including amplitude, frequency, and phase. Fourier analysis isolates individual components of a compound waveform, and has been used to describe various aspects of the electrical seizure signal (Bronzino, 1984; Hilfiker and Egli, 1992; Blanke et al., 2000; Onoe and Nishigaki, 2004; Tsuchiya and Kogure, 2011). To analyze ictal activity beyond the standard measures of duration and total power, we also performed FFT analyses for cross power spectrum and coherence, which are additional means of analyzing the relationship between Fourier-transformed domains of the underlying electrical waveform (Bronzino, 1984; Davey et al., 2000). FFT analysis showed that cross spectral density estimate (F3,16 = 1.017, p > 0.05; Figure 3F) and MSC (F3,16 = 76.116, p > 0.05; Figure 3G) of paired LFP did not significantly differ between the four groups, indicating that absence of the α4 subunit did not fundamentally change seizure domain frequency. These data indicate that the Itonic generated by GABAA-R α4 subunit expression is required for the ictal power reduction following MGE-IP transplantation.
Discussion
Synaptic receptors mediate phasic inhibition whereas extrasynaptic receptors mediate tonic inhibition (Stell and Mody, 2002; Mody and Pearce, 2004; Farrant and Nusser, 2005; Belelli et al., 2009). Identification of the GABAA-R α4 subunit as sufficient for the reduction in ictal activity following MGE-IP transplantation suggests that extrasynaptic α4 subunit-containing GABAA-Rs are important targets for treating seizures arising in cortical, and perhaps subcortical, structures (Cope et al., 2009). MGE-IP transplants were shown to increase both phasic and tonic currents in cortical pyramidal neurons (Baraban et al., 2009). Augmentation of tonic currents was reported to be highly-efficient in enhancing inhibition (Bai et al., 2001). Our data emphasize the importance of extrasynaptic GABAA-Rs which contain the α4 subunit in constraining cellular and system level excitability. Our findings are also consistent with the observation that mutations associated with both febrile seizures and childhood absence epilepsy compromise surface expression of GABAA-Rs and reduce tonic GABA currents but do not affect GABA-mediated synaptic events (Eugène et al., 2007).
Is it possible that other GABAA-R configurations might contribute to the observed effect? δ subunit-containing GABAA-Rs are exclusively extrasynaptic (Nusser et al., 1998; Wei et al., 2003; Sun et al., 2004), and mediate tonic inhibition in dentate gyrus granule cells (Nusser and Mody, 2002; Stell et al., 2003; Wei et al., 2004; Maguire et al., 2005), cerebellar granule cells (Watts and Thomson, 2005), thalamic neurons (Belelli et al., 2005; Cope et al., 2005; Jia et al., 2005), and cortical pyramidal neurons (Scimemi et al., 2006). α4 and δ subunits show overlapping patterns of distribution (Peng et al., 2002), and the δ subunit normally partners with either α4 or α6 subunits (Jones et al., 1997; Sur et al., 1999; Jia et al., 2005), and while α1-δ subunits co-assemble in heterologous expression systems (Feng and Macdonald, 2004; Feng et al., 2004; Kaur et al., 2009; Baur et al., 2010), the data for such expression in neurons, while extant, is less extensive (Glykys et al., 2007).
Although the α1 subunit is well expressed in cortical neurons (Fritschy and Möhler, 1995; Araujo et al., 1996; Dunning et al., 1999; Pirker et al., 2000; Hörtnagl et al., 2013), it does not appear to contribute to the formation of α1-δ extrasynaptic GABAA-Rs in cortical neurons for the following reasons: (1) this current is strongly enhanced by THIP (Drasbek and Jensen, 2006; Drasbek et al., 2007; Vardya et al., 2008), and we have previously shown that α1-δ GABAA-Rs are relatively unaffected by the low concentrations of THIP used in those experiments (Jia et al., 2005), and more importantly; (2) there is no detectable tonic current in cortical neurons from α4−/− mice (Figure 1).
Of note, there is no evidence of compensatory up-regulation in α5 or α1 subunit expression in α4−/− mice, and as might be predicted, there is a significant concomitant decrease in δ subunit expression (Suryanarayanan et al., 2011); the latter effect is consistent with the decrease in δ subunit expression that is seen in α6−/− mice (Jones et al., 1997), which is the other major α subunit to contribute to extrasynaptic GABAA-Rs. Together, those data emphasize the importance of α4 subunits in the formation of extrasynaptic GABAA-Rs which also contain the δ subunit. While the potential contribution of other subunits might be worth pursuing using additional pharmacologic tools, those results would not change the fundamental conclusion that the α4 subunit contributes to the efficacy of MGE transplants in constraining seizure power.
Consistent with in vivo data demonstrating an enhanced sensitivity to the pro-convulsant pentylentetrazole (Chandra et al., 2008), Layer 2/3 pyramidal cells from α4−/− were hyperexcitable when compared to similar neurons from α4+/+ mice (Figure 1C). Surprisingly, Rin was unchanged despite the loss the tonic current, although there is precedent for such homeostatic compensation (Brickley et al., 2001). What then, could account for the change in excitability? Action potentials primarily arise in the axon initial segment (AIS; Bender and Trussell, 2012). GABAA-Rs have been shown to cluster at the AIS (Christie and De Blas, 2003; Muir and Kittler, 2014), even in the absence of GABAergic innervation (Christie and De Blas, 2003). Recently, it has been shown that inhibitory input to the AIS constrains both action potential formation and epileptiform activity in vitro, even more robustly than perisomatic inhibition (Wang et al., 2014). Thus, if extrasynaptic GABAA-Rs are lost at the AIS while there is a concomitant increase in an outward somatic K+ conductance, there will be an increase in excitability even though the overall input resistance of the cell would be unchanged. Future additional experiments will be required to test this hypothesis.
There are limitations to the present study. The implicit assumption is that efficacy of MGE transplantation in reducing ictal activity arises from an increase in GABAergic inhibition; such inhibition can be phasic, tonic (or both). Indeed, MGE transplantation has been shown to increase both phasic and tonic inhibition onto host cortical pyramidal cells, but not host interneurons, in Kv1.1 null mice (Baraban et al., 2009). Whether such an increase occurs following MGE transplantation in our animal model remains to be determined. Similarly, it is possible than the enhancement of phasic currents might also be sufficient for the observed efficacy. The α1 subunit contributes to GABAA-Rs at cortical pyramidal neuron synapses in adult animals (Bosman et al., 2005), and as α1 gene deletion mice have been generated (Sur et al., 2001; Vicini et al., 2001) this hypothesis could be tested; however, phasic activity is well preserved in multiple brain regions, including the cortex, despite the loss of the α1 subunit (Vicini et al., 2001; Goldstein et al., 2002; Bosman et al., 2005). This suggests that there is a strong compensatory mechanism to preserve synaptically driven GABAergic inhibition, and so multiple mouse models would appear to be required to adequately test the role of MGE-mediated enhanced phasic inhibition. Finally, differences in cell survival and/or patterns of arborization might also account for the observed lack of transplant efficacy in α4−/− mice, but the degree to which survival and arborization appear to be comparable (Figures 2C,D) suggests that this is not the case. Our results are consistent with the recently reported observation that expression of the GABAB1 receptor subunit is not required for the migration and laminar distribution of MGE-IPs in the neocortex following transplantation (Sebe et al., 2014).
Interestingly, merely increasing overall GABA concentration, and activating all GABAA-Rs as with vigabatrin or tiagabine, to treat acquired seizures is not sufficient given the relatively poor side-effect profile associated with this approach. Our data confirm that activation of extrasynaptic, rather than synaptic, GABAA-Rs is the basis for the reduction in seizure activity following MGE transplants, and should allow us to refine the overall approach and facilitate the translation of a promising experimental observation into a clinically valuable therapy that can be used to treat patients. Cortical interneurons, including those originating in the MGE, are heterogeneous, and are classified by their morphology, neurochemical content, and electrophysiological properties (Sultan et al., 2013). Selection of an interneuron population that preferentially enhances extrasynaptic GABAA-R activity may improve the efficacy of cell-based therapies for the treatment of seizures arising in the cortex. Neurogliaform-type cells provide inhibitory input to cortical neurons (Tamás et al., 2003; Miyoshi et al., 2010) in a non-synaptic, spatially non-specific manner that can activate extrasynaptic GABAA-Rs (Oláh et al., 2009). Whether transplantation of neurogliaform interneurons can reduce seizure activity more effectively than a mixed population of interneurons remains to be tested once methods to obtain a pure population of neurogliaform progenitors is developed. Lastly, our data reveal a potentially novel target for drug development, which could be exploited as an alternative and/or complimentary approach to cell-based therapies.
Author Contributions
MKJ: experimental design, data collection/analysis, manuscript preparation/editing, approved final approval. SK: experimental design, data collection/analysis, manuscript preparation/editing, approved final manuscript. MZ: experimental design, data collection/analysis, manuscript preparation/editing, approved final manuscript. MI: experimental design, data collection/analysis, manuscript preparation/editing, approved final manuscript. THS: experimental design, manuscript editing, approved final manuscript. SAA: experimental design, manuscript editing, approved final manuscript. GEH: experimental design, manuscript editing, approved final manuscript. PAG: experimental design, manuscript preparation/editing, approved final manuscript. Collectively the Authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. We confirm that the all authors adhere to the International Committee of Medical Journal Editor’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
References
Alvarez-Dolado, M., Calcagnotto, M. E., Karkar, K. M., Southwell, D. G., Jones-Davis, D. M., Estrada, R. C., et al. (2006). Cortical inhibition modified by embryonic neural precursors grafted into the postnatal brain. J. Neurosci. 26, 7380–7389. doi: 10.1523/jneurosci.1540-06.2006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Anderson, S. A., and Baraban, S. C. (2012). “Cell therapy using GABAergic neural progenitors,” in Jasper’s Basic Mechanisms of the Epilepsies, eds J. L. Noebels, M. Avoli, M. A. Rogawski, R. W. Olsen and A. V. Delgado-Escueta 4th Edn. (Bethesda, MD: National Center for Biotechnology Information (US)). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK98214/
Araujo, F., Tan, S., Ruano, D., Schoemaker, H., Benavides, J., and Vitorica, J. (1996). Molecular and pharmacological characterization of native cortical γ-aminobutyric acidA receptors containing both α1 and α3 subunits. J. Biol. Chem. 271, 27902–27911. doi: 10.1074/jbc.271.44.27902
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Avoli, M. (1990). Epileptiform discharges and a synchronous GABAergic potential induced by 4-aminopyridine in the rat immature hippocampus. Neurosci. Lett. 117, 93–98. doi: 10.1016/0304-3940(90)90125-s
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bahar, S., Suh, M., Zhao, M., and Schwartz, T. H. (2006). Intrinsic optical signal imaging of neocortical seizures: the ‘epileptic dip’. Neuroreport 17, 499–503. doi: 10.1097/01.wnr.0000209010.78599.f5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bai, D., Zhu, G., Pennefather, P., Jackson, M. F., Macdonald, J. F., and Orser, B. A. (2001). Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acidA receptors in hippocampal neurons. Mol. Pharmacol. 59, 814–824. doi: 10.1124/mol.59.4.814
Baraban, S. C., Southwell, D. G., Estrada, R. C., Jones, D. L., Sebe, J. Y., Alfaro-Cervello, C., et al. (2009). Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proc. Natl. Acad. Sci. U S A 106, 15472–15477. doi: 10.1073/pnas.0900141106
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Barna, B., Szász, A., Világi, I., and Szente, M. (2000). Anticonvulsive effect of AMPA receptor antagonist GYKI 52466 on 4-aminopyridine-induced cortical ictal activity in rat. Brain Res. Bull. 51, 241–248. doi: 10.1016/s0361-9230(99)00224-5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Baur, R., Kaur, K. H., and Sigel, E. (2010). Diversity of structure and function of α1α6β3δ GABAA receptors: comparison with α1β3δ and α6β3δ receptors. J. Biol. Chem. 285, 17398–17405. doi: 10.1074/jbc.m110.108670
Belelli, D., Harrison, N. L., Maguire, J., Macdonald, R. L., Walker, M. C., and Cope, D. W. (2009). Extrasynaptic GABAA receptors: form, pharmacology and function. J. Neurosci. 29, 12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Belelli, D., Peden, D. R., Rosahl, T. W., Wafford, K. A., and Lambert, J. J. (2005). Extrasynaptic GABAA receptors of thalamocortical neurons: a molecular target for hypnotics. J. Neurosci. 25, 11513–11520. doi: 10.1523/jneurosci.2679-05.2005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bender, K. J., and Trussell, L. O. (2012). The physiology of the axon initial segment. Annu. Rev. Neurosci. 35, 249–265. doi: 10.1146/annurev-neuro-062111-150339
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Blanke, O., Lantz, G., Seeck, M., Spinelli, L., Grave de Peralta, R., Thut, G., et al. (2000). Temporal and spatial determination of EEG-seizure onset in the frequency domain. Clin. Neurophysiol. 111, 763–772. doi: 10.1016/s1388-2457(00)00251-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bosman, L. W., Heinen, K., Spijker, S., and Brussaard, A. B. (2005). Mice lacking the major adult GABAA receptor subtype have normal number of synapses, but retain juvenile IPSC kinetics until adulthood. J. Neurophysiol. 94, 338–346. doi: 10.1152/jn.00084.2005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Brickley, S. G., Revilla, V., Cull-Candy, S. G., Wisden, W., and Farrant, M. (2001). Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature 409, 88–92. doi: 10.1038/35051086
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bronzino, J. D. (1984). Quantitative analysis of the EEG–general concepts and animal studies. IEEE Trans. Biomed. Eng. 31, 850–856. doi: 10.1109/tbme.1984.325247
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Brown, N., Kerby, J., Bonnert, T. P., Whiting, P. J., and Wafford, K. A. (2002). Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br. J. Pharmacol. 136, 965–974. doi: 10.1038/sj.bjp.0704795
Chandra, D., Jia, F., Liang, J., Peng, Z., Suryanarayanan, A., Werner, D. F., et al. (2006). GABAA receptor α4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc. Natl. Acad. Sci. U S A 103, 15230–15235. doi: 10.1073/pnas.0604304103
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chandra, D., Werner, D. F., Liang, J., Suryanarayanan, A., Harrison, N. L., Spigelman, I., et al. (2008). Normal acute behavioral responses to moderate/high dose ethanol in GABAA receptor α4 subunit knockout mice. Alcohol. Clin. Exp. Res. 32, 10–18. doi: 10.1111/j.1530-0277.2007.00563.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cheng, V. Y., Bonin, R. P., Chiu, M. W., Newell, J. G., MacDonald, J. F., and Orser, B. A. (2006). Gabapentin increases a tonic inhibitory conductance in hippocampal pyramidal neurons. Anesthesiology 105, 325–333. doi: 10.1097/00000542-200608000-00015
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Christie, S. B., and De Blas, A. L. (2003). GABAergic and glutamatergic axons innervate the axon initial segment and organize GABAA receptor clusters of cultured hippocampal pyramidal cells. J. Comp. Neurol. 456, 361–374. doi: 10.1002/cne.10535
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cope, D. W., Di Giovanni, G., Fyson, S. J., Orbán, G., Errington, A. C., Lorincz, M. L., et al. (2009). Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat. Med. 15, 1392–1398. doi: 10.1038/nm.2058
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cope, D. W., Hughes, S. W., and Crunelli, V. (2005). GABAA receptor-mediated tonic inhibition in thalamic neurons. J. Neurosci. 25, 11553–11563. doi: 10.1523/jneurosci.3362-05.2005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Davey, M. P., Victor, J. D., and Schiff, N. D. (2000). Power spectra and coherence in the EEG of a vegetative patient with severe asymmetric brain damage. Clin. Neurophysiol. 111, 1949–1954. doi: 10.1016/s1388-2457(00)00435-1
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
De la Cruz, E., Zhao, M., Guo, L., Ma, H., Anderson, S. A., and Schwartz, T. H. (2011). Interneuron progenitors attenuate the power of acute focal ictal discharges. Neurotherapeutics 8, 763–773. doi: 10.1007/s13311-011-0058-9
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Drasbek, K. R., Hoestgaard-Jensen, K., and Jensen, K. (2007). Modulation of extrasynaptic THIP conductances by GABAA-receptor modulators in mouse neocortex. J. Neurophysiol. 97, 2293–2300. doi: 10.1152/jn.00651.2006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Drasbek, K. R., and Jensen, K. (2006). THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cereb. Cortex 16, 1134–1141. doi: 10.1093/cercor/bhj055
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dunning, D. D., Hoover, C. L., Soltesz, I., Smith, M. A., and O’Dowd, D. K. (1999). GABAA receptor-mediated miniature postsynaptic currents and α-subunit expression in developing cortical neurons. J. Neurophysiol. 82, 3286–3297.
Eugène, E., Depienne, C., Baulac, S., Baulac, M., Fritschy, J. M., Le Guern, E., et al. (2007). GABAA receptor γ2 subunit mutations linked to human epileptic syndromes differentially affect phasic and tonic inhibition. J. Neurosci. 27, 14108–14116. doi: 10.1523/JNEUROSCI.2618-07.2007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Farrant, M., and Nusser, Z. (2005). Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat. Rev. Neurosci. 6, 215–229. doi: 10.1038/nrn1625
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Feng, H. J., Bianchi, M. T., and Macdonald, R. L. (2004). Pentobarbital differentially modulates α1β3δ and α1β3γ2L GABAA receptor currents. Mol. Pharmacol. 66, 988–1003. doi: 10.1124/mol.104.002543
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Feng, H. J., and Macdonald, R. L. (2004). Proton modulation of α1β3δ GABAA receptor channel gating and desensitization. J. Neurophysiol. 92, 1577–1585. doi: 10.1152/jn.00285.2004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fritschy, J. M., and Möhler, H. (1995). GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J. Comp. Neurol. 359, 154–194. doi: 10.1002/cne.903590111
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Glykys, J., Peng, Z., Chandra, D., Homanics, G. E., Houser, C. R., and Mody, I. (2007). A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat. Neurosci. 10, 40–48. doi: 10.1038/nn1813
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Goldstein, P. A., Elsen, F. P., Ying, S. W., Ferguson, C., Homanics, G. E., and Harrison, N. L. (2002). Prolongation of hippocampal miniature inhibitory postsynaptic currents in mice lacking the GABAA receptor α1 subunit. J. Neurophysiol. 88, 3208–3217. doi: 10.1152/jn.00885.2001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gupta, A., Elgammal, F. S., Proddutur, A., Shah, S., and Santhakumar, V. (2012). Decrease in tonic inhibition contributes to increase in dentate semilunar granule cell excitability after brain injury. J. Neurosci. 32, 2523–2537. doi: 10.1523/JNEUROSCI.4141-11.2012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Heinemann, U., Kann, O., and Schuchmann, S. (2006). “An overview of in vitro seizure models in acute and organotypic slices,” in Models of Seizures and Epilepsy, eds A. Pitkänen, P. A. Schwartzkroin and S. L. Moshé (Amsterdam: Elsevier Academic Press), 35–44.
Henderson, K. W., Gupta, J., Tagliatela, S., Litvina, E., Zheng, X., Van Zandt, M. A., et al. (2014). Long-term seizure suppression and optogenetic analyses of synaptic connectivity in epileptic mice with hippocampal grafts of GABAergic interneurons. J. Neurosci. 34, 13492–13504. doi: 10.1523/JNEUROSCI.0005-14.2014
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hilfiker, P., and Egli, M. (1992). Detection and evolution of rhythmic components in ictal EEG using short segment spectra and discriminant analysis. Electroencephalogr. Clin. Neurophysiol. 82, 255–265. doi: 10.1016/0013-4694(92)90106-r
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hörtnagl, H., Tasan, R. O., Wieselthaler, A., Kirchmair, E., Sieghart, W., and Sperk, G. (2013). Patterns of mRNA and protein expression for 12 GABAA receptor subunits in the mouse brain. Neuroscience 236, 345–372. doi: 10.1016/j.neuroscience.2013.01.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jia, F., Pignataro, L., Schofield, C. M., Yue, M., Harrison, N. L., and Goldstein, P. A. (2005). An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J. Neurophysiol. 94, 4491–4501. doi: 10.1152/jn.00421.2005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jones, A., Korpi, E. R., McKernan, R. M., Pelz, R., Nusser, Z., Makela, R., et al. (1997). Ligand-gated ion channel subunit partnerships: GABAA receptor α6 subunit gene inactivation inhibits δ subunit expression. J. Neurosci. 17, 1350–1362.
Kaur, K. H., Baur, R., and Sigel, E. (2009). Unanticipated structural and functional properties of δ-subunit-containing GABAA receptors. J. Biol. Chem. 284, 7889–7896. doi: 10.1074/jbc.M806484200
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keros, S., and Hablitz, J. J. (2005). Subtype-specific GABA transporter antagonists synergistically modulate phasic and tonic GABAA conductances in rat neocortex. J. Neurophysiol. 94, 2073–2085. doi: 10.1152/jn.00520.2005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Krook-Magnuson, E. I., and Huntsman, M. M. (2005). Excitability of cortical neurons depends upon a powerful tonic conductance in inhibitory networks. Thalamus Relat. Syst. 3, 115–120. doi: 10.1017/s1472928807000192
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lee, V., and Maguire, J. (2014). The impact of tonic GABAA receptor-mediated inhibition on neuronal excitability varies across brain region and cell type. Front. Neural Circuits 8:3. doi: 10.3389/fncir.2014.00003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Löscher, W. (2011). Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure 20, 359–368. doi: 10.1016/j.seizure.2011.01.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ma, H., Zhao, M., Suh, M., and Schwartz, T. H. (2009). Hemodynamic surrogates for excitatory membrane potential change during interictal epileptiform events in rat neocortex. J. Neurophysiol. 101, 2550–2562. doi: 10.1152/jn.90694.2008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Maguire, J. L., Stell, B. M., Rafizadeh, M., and Mody, I. (2005). Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat. Neurosci. 8, 797–804. doi: 10.1038/nn1469
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Medina-Ceja, L., and Ventura-Mejía, C. (2010). Differential effects of trimethylamine and quinine on seizures induced by 4-aminopyridine administration in the entorhinal cortex of vigilant rats. Seizure 19, 507–513. doi: 10.1016/j.seizure.2010.07.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Miyoshi, G., Hjerling-Leffler, J., Karayannis, T., Sousa, V. H., Butt, S. J., Battiste, J., et al. (2010). Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J. Neurosci. 30, 1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mody, I., and Pearce, R. A. (2004). Diversity of inhibitory neurotransmission through GABAA receptors. Trends Neurosci. 27, 569–575. doi: 10.1016/j.tins.2004.07.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Muir, J., and Kittler, J. T. (2014). Plasticity of GABAA receptor diffusion dynamics at the axon initial segment. Front. Cell. Neurosci. 8:151. doi: 10.3389/fncel.2014.00151
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nusser, Z., and Mody, I. (2002). Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J. Neurophysiol. 87, 2624–2628.
Nusser, Z., Sieghart, W., and Somogyi, P. (1998). Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J. Neurosci. 18, 1693–1703.
Oláh, S., Füle, M., Komlósi, G., Varga, C., Báldi, R., Barzó, P., et al. (2009). Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature 461, 1278–1281. doi: 10.1038/nature08503
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Onoe, S., and Nishigaki, T. (2004). EEG spectral analysis in children with febrile delirium. Brain Dev. 26, 513–518. doi: 10.1016/j.braindev.2004.02.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Payne, H. L., Connelly, W. M., Ives, J. H., Lehner, R., Furtmuller, B., Sieghart, W., et al. (2007). GABAA α6-containing receptors are selectively compromised in cerebellar granule cells of the ataxic mouse, stargazer. J. Biol. Chem. 282, 29130–29143. doi: 10.1074/jbc.m700111200
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Payne, H. L., Donoghue, P. S., Connelly, W. M., Hinterreiter, S., Tiwari, P., Ives, J. H., et al. (2006). Aberrant GABAA receptor expression in the dentate gyrus of the epileptic mutant mouse stargazer. J. Neurosci. 26, 8600–8608. doi: 10.1523/jneurosci.1088-06.2006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Peng, Z., Hauer, B., Mihalek, R. M., Homanics, G. E., Sieghart, W., Olsen, R. W., et al. (2002). GABAA receptor changes in δ subunit-deficient mice: altered expression of α4 and γ2 subunits in the forebrain. J. Comp. Neurol. 446, 179–197. doi: 10.1002/cne.10210
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Peng, Z., Huang, C. S., Stell, B. M., Mody, I., and Houser, C. R. (2004). Altered expression of the δ subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J. Neurosci. 24, 8629–8639. doi: 10.1523/jneurosci.2877-04.2004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Perreault, P., and Avoli, M. (1991). Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J. Neurophysiol. 65, 771–785.
Petri, S., Krampfl, K., Hashemi, F., Grothe, C., Hori, A., Dengler, R., et al. (2003). Distribution of GABAA receptor mRNA in the motor cortex of ALS patients. J. Neuropathol. Exp. Neurol. 62, 1041–1051.
Petroff, O. A., Behar, K. L., Mattson, R. H., and Rothman, D. L. (1996). Human brain γ-aminobutyric acid levels and seizure control following initiation of vigabatrin therapy. J. Neurochem. 67, 2399–2404. doi: 10.1046/j.1471-4159.1996.67062399.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pirker, S., Schwarzer, C., Wieselthaler, A., Sieghart, W., and Sperk, G. (2000). GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101, 815–850. doi: 10.1016/s0306-4522(00)00442-5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pitkänen, A., Schwartzkroin, P. A., and Moshé, S. L. (2006). Models of Seizures and Epilepsy. Burlington, MA: Elsevier Academic Press.
Raedt, R., Van Dycke, A., Vonck, K., and Boon, P. (2007). Cell therapy in models for temporal lobe epilepsy. Seizure 16, 565–578. doi: 10.1016/j.seizure.2007.05.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rajasekaran, K., Joshi, S., Sun, C., Mtchedlishvilli, Z., and Kapur, J. (2010). Receptors with low affinity for neurosteroids and GABA contribute to tonic inhibition of granule cells in epileptic animals. Neurobiol. Dis. 40, 490–501. doi: 10.1016/j.nbd.2010.07.016
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Richards, D. A., and Bowery, N. G. (1996). Comparative effects of the GABA uptake inhibitors, tiagabine and NNC-711, on extracellular GABA levels in the rat ventrolateral thalamus. Neurochem. Res. 21, 135–140. doi: 10.1007/bf02529130
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Salazar, P., and Tapia, R. (2012). Allopregnanolone potentiates the glutamate-mediated seizures induced by 4-aminopyridine in rat hippocampus in vivo. Neurochem. Res. 37, 596–603. doi: 10.1007/s11064-011-0649-1
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sassoè-Pognetto, M., Panzanelli, P., Sieghart, W., and Fritschy, J. M. (2000). Colocalization of multiple GABAA receptor subtypes with gephyrin at postsynaptic sites. J. Comp. Neurol. 420, 481–498. doi: 10.1002/(sici)1096-9861(20000515)420:4<481::aid-cne6>3.0.co;2-5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schwartz, T. H., and Bonhoeffer, T. (2001). In vivo optical mapping of epileptic foci and surround inhibition in ferret cerebral cortex. Nat. Med. 7, 1063–1067. doi: 10.1038/nm0901-1063
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Scimemi, A., Andersson, A., Heeroma, J. H., Strandberg, J., Rydenhag, B., Mcevoy, A. W., et al. (2006). Tonic GABAA receptor-mediated currents in human brain. Eur. J. Neurosci. 24, 1157–1160. doi: 10.1111/j.1460-9568.2006.04989.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sebe, J. Y., Looke-Stewart, E., Dinday, M. T., Alvarez-Buylla, A., and Baraban, S. C. (2014). Neocortical integration of transplanted GABA progenitor cells from wild type and GABAB receptor knockout mouse donors. Neurosci. Lett. 561, 52–57. doi: 10.1016/j.neulet.2013.11.012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sebe, J. Y., Looke-Stewart, E. C., Estrada, R. C., and Baraban, S. C. (2010). Robust tonic GABA currents can inhibit cell firing in mouse newborn neocortical pyramidal cells. Eur. J. Neurosci. 32, 1310–1318. doi: 10.1111/j.1460-9568.2010.07373.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Spigelman, I., Li, Z., Banerjee, P. K., Mihalek, R. M., Homanics, G. E., and Olsen, R. W. (2002). Behavior and physiology of mice lacking the GABAA-receptor δ subunit. Epilepsia 43(Suppl. 5), 3–8. doi: 10.1046/j.1528-1157.43.s.5.8.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stell, B. M., Brickley, S. G., Tang, C. Y., Farrant, M., and Mody, I. (2003). Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc. Natl. Acad. Sci. U S A 100, 14439–14444. doi: 10.1073/pnas.2435457100
Stell, B. M., and Mody, I. (2002). Receptors with different affinities mediate phasic and tonic GABAA conductances in hippocampal neurons. J. Neurosci. 22:RC223.
Sultan, K. T., Brown, K. N., and Shi, S. H. (2013). Production and organization of neocortical interneurons. Front. Cell. Neurosci. 7:221. doi: 10.3389/fncel.2013.00221
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sun, C., Mtchedlishvili, Z., Erisir, A., and Kapur, J. (2007). Diminished neurosteroid sensitivity of synaptic inhibition and altered location of the α4 subunit of GABAA receptors in an animal model of epilepsy. J. Neurosci. 27, 12641–12650. doi: 10.1523/jneurosci.4141-07.2007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sun, C., Sieghart, W., and Kapur, J. (2004). Distribution of α1, α4, γ2 and δ subunits of GABAA receptors in hippocampal granule cells. Brain Res. 1029, 207–216. doi: 10.1016/j.brainres.2004.09.056
Sur, C., Farrar, S. J., Kerby, J., Whiting, P. J., Atack, J. R., and Mckernan, R. M. (1999). Preferential coassembly of α4 and δ subunits of the γ-aminobutyric acidA receptor in rat thalamus. Mol. Pharmacol. 56, 110–115.
Sur, C., Wafford, K. A., Reynolds, D. S., Hadingham, K. L., Bromidge, F., Macaulay, A., et al. (2001). Loss of the major GABAA receptor subtype in the brain is not lethal in mice. J. Neurosci. 21, 3409–3418.
Suryanarayanan, A., Liang, J., Meyer, E. M., Lindemeyer, A. K., Chandra, D., Homanics, G. E., et al. (2011). Subunit compensation and plasticity of synaptic GABAA receptors induced by ethanol in α4 subunit knockout mice. Front. Neurosci. 5:110. doi: 10.3389/fnins.2011.00110
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tamás, G., Lőrincz, A., Simon, A., and Szabadics, J. (2003). Identified sources and targets of slow inhibition in the neocortex. Science 299, 1902–1905. doi: 10.1126/science.1082053
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tsuchiya, K., and Kogure, S. (2011). Fast fourier transformation analysis of kindling-induced afterdischarge in the rabbit hippocampus. Epilepsy Res. 95, 144–151. doi: 10.1016/j.eplepsyres.2011.03.015
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vardya, I., Drasbek, K. R., Dósa, Z., and Jensen, K. (2008). Cell type-specific GABAA receptor-mediated tonic inhibition in mouse neocortex. J. Neurophysiol. 100, 526–532. doi: 10.1152/jn.01224.2007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vicini, S., Ferguson, C., Prybylowski, K., Kralic, J., Morrow, A. L., and Homanics, G. E. (2001). GABAA receptor α1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J. Neurosci. 21, 3009–3016.
Waldau, B., Hattiangady, B., Kuruba, R., and Shetty, A. K. (2010). Medial ganglionic eminence-derived neural stem cell grafts ease spontaneous seizures and restore GDNF expression in a rat model of chronic temporal lobe epilepsy. Stem Cells 28, 1153–1164. doi: 10.1002/stem.446
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, X., Hooks, B. M., and Sun, Q. Q. (2014). Thorough GABAergic innervation of the entire axon initial segment revealed by an optogenetic ‘laserspritzer’. J. Physiol. 592, 4257–4276. doi: 10.1113/jphysiol.2014.275719
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Watts, J., and Thomson, A. M. (2005). Excitatory and inhibitory connections show selectivity in the neocortex. J. Physiol. 562, 89–97. doi: 10.1113/jphysiol.2004.076984
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wei, W., Faria, L. C., and Mody, I. (2004). Low ethanol concentrations selectively augment the tonic inhibition mediated by δ subunit-containing GABAA receptors in hippocampal neurons. J. Neurosci. 24, 8379–8382. doi: 10.1523/jneurosci.2040-04.2004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wei, W., Zhang, N., Peng, Z., Houser, C. R., and Mody, I. (2003). Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J. Neurosci. 23, 10650–10661.
Welch, P. D. (1967). The use of fast fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoustics 15, 70–73. doi: 10.1109/tau.1967.1161901
Wu, Y., Wang, W., and Richerson, G. B. (2003). Vigabatrin induces tonic inhibition via GABA transporter reversal without increasing vesicular GABA release. J. Neurophysiol. 89, 2021–2034. doi: 10.1152/jn.00856.2002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yamada, J., Furukawa, T., Ueno, S., Yamamoto, S., and Fukuda, A. (2007). Molecular basis for the GABAA receptor-mediated tonic inhibition in rat somatosensory cortex. Cereb. Cortex 17, 1782–1787. doi: 10.1093/cercor/bhl087
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yu, W., Smith, A. B., Pilitsis, J., and Shin, D. S. (2014). Isovaline attenuates epileptiform activity and seizure behavior in 4-aminopyridine treated rats. Epilepsy Res. 108, 331–335. doi: 10.1016/j.eplepsyres.2013.11.020
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhao, M., Ma, H., Suh, M., and Schwartz, T. H. (2009). Spatiotemporal dynamics of perfusion and oximetry during ictal discharges in the rat neocortex. J. Neurosci. 29, 2814–2823. doi: 10.1523/JNEUROSCI.4667-08.2009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhao, M., McGarry, L. M., Ma, H., Harris, S., Berwick, J., Yuste, R., et al. (2015). Optical triggered seizures using a caged 4-Aminopyridine. Front. Neurosci. 9:25. doi: 10.3389/fnins.2015.00025
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: GABAA receptor, extrasynaptic, α4 subunit, interneuron, epilepsy, cortex
Citation: Jaiswal MK, Keros S, Zhao M, Inan M, Schwartz TH, Anderson SA, Homanics GE and Goldstein PA (2015) Reduction in focal ictal activity following transplantation of MGE interneurons requires expression of the GABAA receptor α4 subunit. Front. Cell. Neurosci. 9:127. doi: 10.3389/fncel.2015.00127
Received: 16 January 2015; Accepted: 18 March 2015;
Published online: 09 April 2015.
Edited by:
Maria Cristina D’Adamo, University of Perugia, ItalyReviewed by:
Elizabeth M. Powell, University of Maryland, USAWilliam Martin Connelly, Cardiff University, UK
Copyright © 2015 Jaiswal, Keros, Zhao, Inan, Schwartz, Anderson, Homanics and Goldstein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter A. Goldstein, C.V. Starr Laboratory for Molecular Neuropharmacology, Department of Anesthesiology, Weill Cornell Medical College, 1300 York Avenue, Room A-1050 New York, NY 10065, USA pag2014@med.cornell.edu
 Manoj K. Jaiswal
Manoj K. Jaiswal Sotirios Keros
Sotirios Keros Mingrui Zhao
Mingrui Zhao Melis Inan
Melis Inan Theodore H. Schwartz
Theodore H. Schwartz Stewart A. Anderson
Stewart A. Anderson Gregg E. Homanics
Gregg E. Homanics Peter A. Goldstein
Peter A. Goldstein