Combining Animal Welfare With Experimental Rigor to Improve Reproducibility in Behavioral Neuroscience
- 1Molecular and Behavioral Neuroscience Laboratory, Departamento de Farmacologia, Universidade Federal de São Paulo, São Paulo, Brazil
- 2National Institute for Translational Medicine (INCT-TM), National Council for Scientific and Technological Development (CNPq/CAPES/FAPESP), Ribeirão Preto, Brazil
- 3Departamento de Anatomia, Instituto de Ciências Biomédicas, Universidade de São Paulo, São Paulo, Brazil
- 4Instituto de Investigação e Inovação em Saúde, Universidade do Porto, Porto, Portugal
- 5Departamento de Ciências Fisiológicas do Centro de Ciências Biológicas, Universidade Federal de Santa Catarina, Florianópolis, Brazil
- 6Independent Researcher, Mossoró, Brazil
Introduction
Reproducibility is an essential characteristic in any field of experimental sciences, this feature provides reliability to the experimentally obtained findings (for details, see Glossary). The currently available empirical estimates on the topic suggest that less than half (ranging from 49% down to 11%) of scientific results are reproducible (Prinz et al., 2011; Begley and Ellis, 2012; Freedman et al., 2015, 2017). While it can be argued that the accuracy of these estimations needs confirmation, we (as a scientific community) have to recognize that poor reproducibility is a major problem in the life sciences.
The perception of an undergoing “reproducibility crisis” has led to the establishment of crowdsourced initiatives around the world addressing reproducibility issues in sciences, such as behavioral neuroscience (Open Science, 2015; Freedman et al., 2017; Reproducibility Project and Cancer Biology, 2017; Amaral et al., 2019). Among the explanations for poor quality in published research, there is the prevalent culture of “reporting positive results” (publication bias) and the high incidence of diverse types of experimental bias, such as lack of transparency and poor description of methods, lack of predefined inclusion and exclusion criteria resulting in unlimited flexibility for deciding which experiments will be reported, insufficient knowledge of the scientific method and statistical tools when designing and analyzing experiments (Ioannidis, 2005; Cumming, 2008; Sena et al., 2010; Freedman et al., 2017; Vsevolozhskaya et al., 2017; Ramos-Hryb et al., 2018; Catillon, 2019; Neves and Amaral, 2020; Neves et al., 2020). Further discussions on the causes, consequences, and actions to overcome poor research practices and reproducibility in sciences are many (Altman, 1994; Macleod et al., 2014; Strech et al., 2020) and beyond the scope of this text. Here, we focus on the aspects relevant to the field of behavioral neuroscience, whereby poor research performance may affect not only the economic and translational aspects of science but also implies ethical issues once it involves necessarily living subjects, mostly laboratory animals (Prinz et al., 2011; Begley and Ellis, 2012; Festing, 2014; Freedman et al., 2015; Voelkl and Wurbel, 2021).
In our opinion, combining principles of animal welfare with experimental rigor may lead to improvement in the quality of studies in behavioral neuroscience. Hence, we will briefly discuss how adherence to legislations, guidelines, and ethical principles in animal research may guide more rigorous behavioral studies. Thereafter, we condense discussions on how (1) the better understanding of the conceptualization, validation, and limitations of the animal models; (2) the use of suitable statistical methods for study design and data analysis; and (3) the use of environmental enrichment in research facilities to favor welfare of animals may improve quality of studies in behavioral neuroscience (some practical tips in Table 1) and, hopefully, the reproducibility in the field.
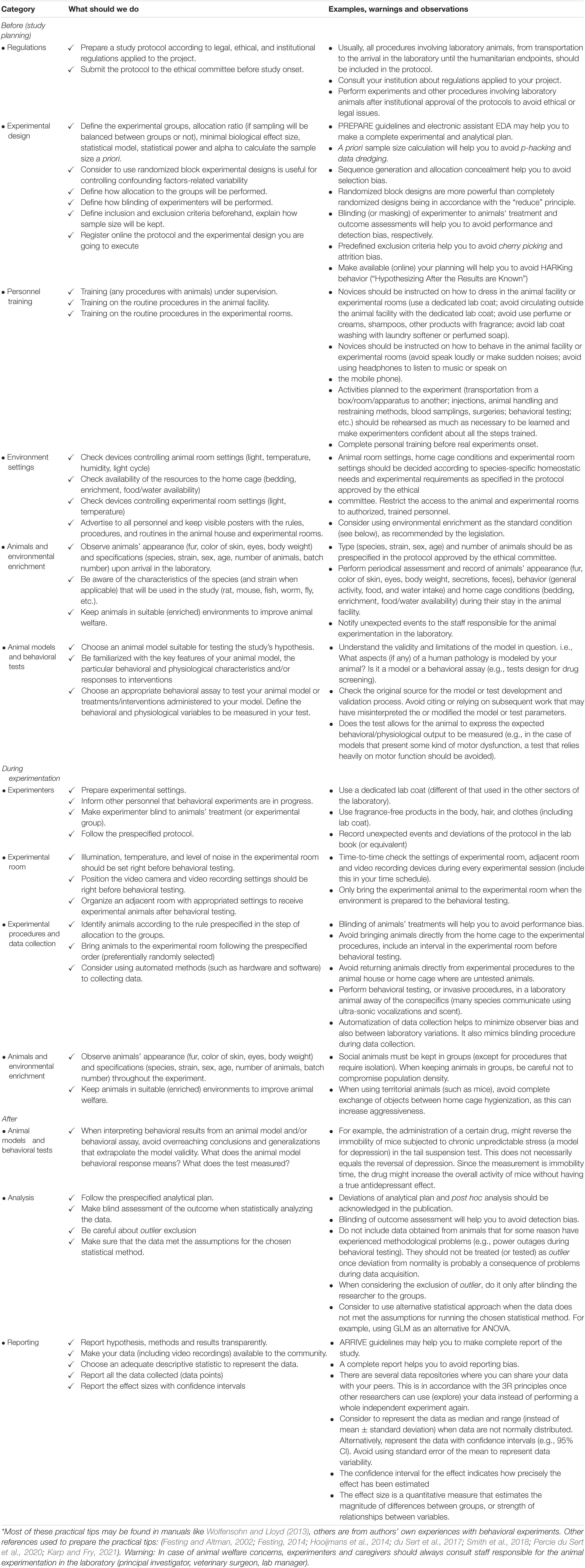
Table 1. Practical tips combining animal welfare and experimental rigor to improve reproducibility in behavioral neuroscience*.
Advantages of the Adherence to the Regulations to the Quality of Behavioral Neuroscience
Behavioral studies in laboratory animals are performed worldwide under specific guidelines conciliating the needs of science, scientists, and animal welfare (Smith et al., 2018). Regulations establish obligations and responsibilities for institutional actors involved in animal experimentation, from students to deans (please consult one’s own institution about regulations applied to a project). Here, we claim that, besides being ethical, adherence to the regulations is advantageous to the quality of behavioral studies. Why? Because, regulations in animal research consider, among other things, the 3Rs principle (replace, reduce, and refine), which are the useful frameworks to prepare good quality experiments taking animal welfare into account, as discussed by previous authors (e.g., Franco and Olsson, 2014; Bayne et al., 2015; Aske and Waugh, 2017; Strech and Dirnagl, 2019) and in the further sections. “Replace” prompts scientists to consider alternatives to behavioral studies in laboratory animals for reaching a giving aim, in the first place. Once a behavioral study in laboratory animals is considered necessary, “reduce” may guide designs using well-established rules for rigorous experimentation to extract the maximum information of a study with a minimum number of subjects. The principle “refine” assists scientists to devise better strategies guaranteeing animal welfare according to species-sex-age-specific needs. There is evidence that “happy animals make better science” (Poole, 1997; Grimm, 2018). Besides, poor welfare in laboratory settings affects the laboratory animals in unpredictable, and often deleterious ways, compromising behavioral outcomes in the experiments (e.g., Emmer et al., 2018), and increasing the number of experimental animals unnecessarily. Therefore, personnel handling animals (experimenters, technicians, and caregivers) may contribute to the efforts to minimize the risk of animal suffering during procedures improving research quality. There are many free resources for training staff in the 3Rs principle made available by international organizations, such as NC3Rs1 or Animal Research Tomorrow,2 which could be easily implemented in behavioral studies.
Suitable Animal Models and Behavioral Tests Should Improve Studies in Behavioral Neuroscience
The selection of an adequate animal model is a pivotal step in behavioral studies. Physical models (Godfrey-Smith, 2009) are central tools in neuroscientific research. Neuroscientists commonly employ in vivo animal models, aimed to simulate physiological, genetic, or anatomical features observed in humans (as is the case with studies of disease) or replicate natural situations under controlled laboratory conditions (van der Staay, 2006; Maximino and van der Staay, 2019). By definition, a model is a construct of a real physical component or property observed in nature. Therefore, a model is always imperfect and does not contemplate the full complexity of the real system that is being modeled (Garner et al., 2017). Much has been discussed about the validity and translational potential of animal models (Nestler and Hyman, 2010). Here, our aim is to consider how the misuse of animal models may affect the reproducibility and reliability of neurobiological research results. Firstly, there appears to be confusion about the definition of animal models and behavioral tests (Willner, 1986) that ultimately causes the misinterpretation of results. Animal models deliberately prompt changes in biological variables (such as behavior), while behavioral tests are paradigms in which animal models are subjected to having their behavior assessed. By this definition, a behavioral bioassay (an intact animal plus an apparatus) is not a model in a strict sense (van der Staay, 2006; Maximino and van der Staay, 2019), although useful to study normal animal behavior (e.g., exploration of a maze and immobility in forced swim test) and its underlying mechanisms (Maximino and van der Staay, 2019; de Kloet and Molendijk, 2021). Secondly, it is important to be aware of the conditions validated for the test because modifying some of them (e.g., light intensity or animal species/strain) may yield different results than those observed in the standardizations for the test (Griebel et al., 1993; Holmes et al., 2000; Garcia et al., 2005). For example, the dichotomic behavioral outcome (mobility or immobility) of mice is often registered in the tail suspension test. However, some mice (e.g., C57BL/6 strain) also present climbing behavior which may be mistaken by immobility (Mayorga and Lucki, 2001; Can et al., 2012). Third, we have to avoid the extrapolation of simple behavioral measures (those variables that we actually measure in a task) to complex multidimensional abstract behaviors (e.g., anxiety, memory, locomotor, and exploratory activities). For example, measuring only distance traveled (or the number of crossings) in an open field arena is not sufficient to fully capture the complexity of locomotor behavior (Paulus et al., 1999; Loss et al., 2014, 2015). Therefore, it alone does not provide enough information to make conclusions about locomotor activity, a multidimensional behavior that encompasses not only how much an animal moves (distance traveled and locomoting time) but also how it moves (average speed, number of stops made, among others) (Eilam et al., 2003; Loss et al., 2014, 2015). This extrapolation becomes even greater when we think about exploratory activity, which encompasses locomotor activity and other behaviors (such as time and frequency of rearing) (Loss et al., 2014, 2015). Similarly, Rubinstein et al. (1997) observed that mutant mice lacking D4 dopamine receptors moved less in the open field arena but outperformed their wild-type littermates in the rotarod test, which highlights that we cannot conclude much about motor function by measuring only the distance traveled (even if the amount of movement registered is similar between the groups). Finally, it is imperative to know whether the animal model we intend to test meets the assumptions of the behavioral paradigm (or our study hypothesis) that it will be tested. For example, animals with compromised mobility (e.g., models for spinal cord injury) will not provide meaningful results in tests that rely on preserved motor function (e.g., forced swim test, elevated plus maze). Similarly, subjecting a pigeon to the Morris water maze may lead one to conclude that pigeons have poor spatial memory. But, pigeons do not swim in the first place making the last experimental proposal not just inappropriate but absurd. Hence, knowledge about the biology of laboratory animals seems fundamental to the selection of a suitable approach for an intended behavioral study.
Rigorous Design of Studies and Analysis of Data Should Improve the Quality of Behavioral Neuroscience
Limited knowledge of the scientific method and statistics are among the reasons for the high levels of experimental bias and irreproducibility (Ioannidis, 2005; Lazic, 2018; Lazic et al., 2018) leading ones to suggest that we are actually facing an “epistemological crisis” (Park, 2020). Several guidelines for experimental design, analysis, and reporting are available (see Festing and Altman, 2002; Lazic, 2016; Percie du Sert et al., 2020), describing rigorous methods that should be adopted to avoid bias achieving high-quality data production. However, it seems that some of the most basic good practices described in these guidelines have been neglected or ignored (Goodman, 2008; Festing, 2014; Hair et al., 2019). Some frequent sources of biases are pseudoreplication (Freeberg and Lucas, 2009; Lazic, 2010; Lazic et al., 2020; Eisner, 2021; Zimmerman et al., 2021) and violations of rules for experimental design, such as a priori calculating the sample size, unbiased allocation of samples to groups (randomization), blinded assessment of outcomes, complete reporting of results, and choosing the method for data analysis beforehand (Macleod et al., 2015). The lack of a rigorous plan results in the massive production of underpowered exploratory studies (Maxwell, 2004; Button et al., 2013; Lazic, 2018), with the aggravating factor that they are often misinterpreted as confirmatory studies ones (Wagenmakers et al., 2012; Nosek et al., 2018). It is not unusual to find discussions about the so-called “statistical trend” in studies in which both biological effect sizes and sample sizes are assumed post hoc. In addition, the extensive practice of exclusively using linear models (such as Student’s t-test or ANOVA) to analyze the data, assuming that all variables present Gaussian distribution, contribute to the misinterpretation of results (Lazic, 2015; Eisner, 2021). Currently, there are alternative methods that we strongly suggest to be incorporated in research projects by the whole neuroscientific community. For example, Generalized Linear (Mixed) Models and Generalizing Estimating Equations (GLM, GLMM, and GEE, respectively) fit distinct types of distribution (such as the Gaussian distribution) and correct for confounding factors (Shkedy et al., 2005a,b; Lazic and Essioux, 2013; Lazic, 2015, 2018; Bono et al., 2021; Eisner, 2021; Zimmerman et al., 2021). Adopting randomized block experimental designs (that are more powerful, have higher external validity, and are less subject to bias than the completely randomized designs typically used in behavioral research) is also necessary for controlling confounding factor-related variability and producing more reproducible results (Festing, 2014). Considering the use of multivariate statistical tools (instead of the widely used univariate approach) is an alternative to achieve more accurate outcomes from experiments with big data, especially in behavioral studies (Sanguansat, 2012; Loss et al., 2014, 2015; Quadros et al., 2016). Among the advantages of using these alternative approaches is the increased accuracy in parameter estimation (thus avoiding making impossible predictions), resulting in reduced probability of making Type I Error (due to invalid estimation of p-values, for example) and Type II Error (due to lack of statistical power). Rigorous design of studies and analysis of data should help to extract the maximum information of a study with the adequate calculated number of subjects and prevent waste of scientific efforts in behavioral neuroscience. In addition, rigorous and systematic reporting of methods (with enough details to allow replication) and results (with complete description of effect sizes and their confidence intervals rather than uninformative p-values) are also necessary to increase transparency and, consequently, the quality of the studies (Halsey et al., 2015; Halsey, 2019; Percie du Sert et al., 2020).
Environmental Enrichment in Research Facilities May Favor Translational Neuroscience
As mentioned, “Happy animals make better science” (Poole, 1997; Grimm, 2018). It is a worldwide acknowledgment that environmental stimulus is necessary to improve the quality of life and welfare of captive animals, such as research animals. It has been more than a decade since the Directive 2010/63/EU was established (EC, 2010). However, this and other directives are far from being effectively complied with by the entire scientific community. A common non-tested argument to raise research animals in impoverished standard conditions is that the data variability among laboratories, or even within them, would increase by raising the animals in enriched non-standard conditions (Voelkl et al., 2020). This last claim has been criticized over the past two decades and suggested to be a fallacy (Wolfer et al., 2004; Kentner et al., 2021; Voelkl et al., 2021). For example, Wolfer et al. (2004) and Bailoo et al. (2018) observed that data variability did not increase after raising the animals in enriched environments when compared with raising them in standard laboratory environments. Furthermore, Richter et al. (2011) found that rearing animals in enriched environments decreased variation between experiments, strain-by-laboratory interaction on data variability. In other words, heterogenized housing designs appear to have improved data reproducibility. Therefore, it was claimed (and we agree) that we should embrace environmental variability (instead of static environmental standardization) because environmental heterogeneity better represents the wide variation (richness and complexity) of mental and physical stimulations in both human and non-human animals (Nithianantharajah and Hannan, 2006; Richter, 2017). In fact, drug development and discovery may be affected by the culture of raising animals in impoverished (extremely artificial) environments. There are studies showing that some drugs present biological effects when tested in animals raised in impoverished environments but not in animals raised in enriched environments (which is more similar to real-life conditions) (Akkerman et al., 2014; Possamai et al., 2015). Furthermore, we cannot disregard that more pronounced effects could be found whether drugs were tested in animals raised in enriched when compared to impoverished environments (Gurwitz, 2001). While one can argue that there are not enough studies strengthening this assertion, the low quality of life of captive animals, the low reproducibility of studies, and the poor translational rate of preclinical research reinforce the necessity of a paradigm shift related to the welfare of animals (Akkerman et al., 2014; Voelkl et al., 2020). This debate should not be restricted to rodents and shall include avians (Melleu et al., 2016; Campbell et al., 2018), reptiles (Burghardt et al., 1996), fishes (Turschwell and White, 2016; Fong et al., 2019; Masud et al., 2020), and even invertebrate animals (Ayub et al., 2011; Mallory et al., 2016; Bertapelle et al., 2017; Wang et al., 2018; Guisnet et al., 2021). We bring two practical examples (or recommendations) of improvements that we (the neuroscientific community) could do: (1) when using animal models we should implement environmental enrichment as the standard in the animal facilities (especially for those animal models that attempt to simulate central nervous system disorders), as raising animals in impoverished environments provides suboptimal sensory, cognitive and motor stimulation, making them too reactive to any kind of intervention (i.e., “noise amplifiers”) (Nithianantharajah and Hannan, 2006); (2) when proposing alternative organisms to study behavior (e.g., zebrafish), we should learn from past and present mistakes (mostly in rodents), keeping in mind the ethological and natural needs of the species (Branchi and Ricceri, 2004; Lee et al., 2019; Stevens et al., 2021). Importantly, when making these improvements we should carefully respect the species-specific characteristics. For example, rats and mice share some characteristics, such as nocturnal habits (which means that both species need places to hide during the light period, to provide a sense of security) (Loss et al., 2015). However, they also have some distinct characteristics, such as the need for running (which is higher in mice) (Meijer and Robbers, 2014). This means that providing running wheels for mice is really necessary, while for rats, (that run less but are more social than mice) (Kondrakiewicz et al., 2019) the space dedicated to some of the running wheels could be better used by increasing (carefully not to compromise the population density) the number of individuals in the home cage. On the other hand, zebrafish needs aquatic plants and several substrates in their environment, such as mud, gravel or sand, to represent their own eco-ethological expansions of behavior (Engeszer et al., 2007; Spence et al., 2008; Arunachalam et al., 2013; Parichy, 2015; Stevens et al., 2021). The substrates might provide some camouflage for zebrafish against the predator, which may contribute to feelings of security and improved welfare (Schroeder et al., 2014). Taking all these together, in our opinion, the scientific community must think over the long-term costs (economical and ethical ones) of keeping the culture of raising animals in impoverished environments, a condition that potentially disrupt the translation of behavioral neuroscience results into applicable benefits (Akkerman et al., 2014).
Future Directions
As previously stated, a “reproducibility crisis” is not an issue limited to the field of behavioral neuroscience, and several crowdsourced initiatives were established around the world addressing reproducibility (Open Science, 2015; Freedman et al., 2017; Reproducibility Project and Cancer Biology, 2017; Amaral et al., 2019). An essential step to confront this issue is to first recognize that there is a crisis and that it is a major problem. Secondly, the scientific communities have been developing and disseminating guidelines for good experimental practices to be implemented by themselves (more information can be found in http://www.consort-statement.org/ and also in https://www.equator-network.org/). In addition, encouraging the preregistration of the projects and experimental protocols (a practice that is essential for carrying out confirmatory studies) (Wagenmakers et al., 2012; Nosek et al., 2018) and the embracement of open research practices (open data sharing) (Ferguson et al., 2014; Steckler et al., 2015; Gilmore et al., 2017) are also alternatives to improve reproducibility. Interestingly, it seems that just encouraging good research practices is not enough to assure compliance with the proposed guidelines (Baker et al., 2014; Hair et al., 2019). This suggests that the participation of research funding agencies is necessary as well as of peer reviewers and journal editors in demanding adherence to these directives (Kilkenny et al., 2009; Baker et al., 2014; Han et al., 2017; Hair et al., 2019).
In conclusion, paraphrasing Lazic et al. (2018), “There are few ways to conduct an experiment well, but many ways to conduct it poorly.” In our opinion, we, as a scientific community, have to be worried about the rigor of the experiments we are conducting and the quality of the studies we are producing. Publishing non-reproducible results (or reproducible noise) can lead to ethical, economic, and technological consequences leading to scientific discredit. Furthermore, poor reproducibility delays discovery and development and hinders the progress of scientific knowledge. Broad adherence and advanced training to principles of animal welfare and good experimental practices may elevate the standards of behavioral neuroscience. Finally, perhaps we, as the scientific community, should strive to refine our current animal models and focus our efforts in the development of new, more robust, ethologically relevant models that could potentially improve both the description of our reality and the translational potential of our basic research.
Author Contributions
CML was responsible for the conceptualization of the opinion article. All authors were responsible for writing and revising the manuscript and read and approved the final manuscript.
Funding
Grants of Alexander von Humboldt Foundation (Germany) to CLi. CML was recipient of Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) research fellowship through the Instituto Nacional de Ciência e Tecnologia Translacional em Medicina (INCT-TM), Brazil. FM was supported by Post-doctoral fellowship grant #2018/25857-5, São Paulo Research Foundation (FAPESP), Brazil. KD was supported by Fellow BIPD/FCT Proj2020/i3S/26040705/2021, Fundação para a Ciência e Tecnologia, Portugal. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to the Alexander von Humboldt Foundation (Germany) and the Brazilian funding agencies for the financial support and fellowships granted. We are also grateful to Ann Colette Ferry (in memoriam) for providing language assistance.
Footnotes
References
Akkerman, S., Prickaerts, J., Bruder, A. K., Wolfs, K. H., De Vry, J., Vanmierlo, T., et al. (2014). PDE5 inhibition improves object memory in standard housed rats but not in rats housed in an enriched environment: implications for memory models? PLoS One 9:e111692. doi: 10.1371/journal.pone.0111692
Altman, D. G. (1994). The scandal of poor medical research. BMJ 308, 283–284. doi: 10.1136/bmj.308.6924.283
Amaral, O. B., Neves, K., Wasilewska-Sampaio, A. P., and Carneiro, C. F. (2019). The Brazilian reproducibility initiative. Elife 8:e41602.
Arunachalam, M., Raja, M., Vijayakumar, C., Malaiammal, P., and Mayden, R. L. (2013). Natural history of zebrafish (Danio rerio) in India. Zebrafish 10, 1–14. doi: 10.1089/zeb.2012.0803
Aske, K. C., and Waugh, C. A. (2017). Expanding the 3R principles: more rigour and transparency in research using animals. EMBO Rep. 18, 1490–1492. doi: 10.15252/embr.201744428
Ayub, N., Benton, J. L., Zhang, Y., and Beltz, B. S. (2011). Environmental enrichment influences neuronal stem cells in the adult crayfish brain. Dev. Neurobiol. 71, 351–361.
Bailoo, J. D., Murphy, E., Boada-Sana, M., Varholick, J. A., Hintze, S., Baussiere, C., et al. (2018). Effects of cage enrichment on behavior, welfare and outcome variability in female mice. Front. Behav. Neurosci. 12:232.
Baker, D., Lidster, K., Sottomayor, A., and Amor, S. (2014). Two years later: journals are not yet enforcing the ARRIVE guidelines on reporting standards for pre-clinical animal studies. PLoS Biol. 12:e1001756. doi: 10.1371/journal.pbio.1001756
Bayne, K., Ramachandra, G. S., Rivera, E. A., and Wang, J. (2015). The evolution of animal welfare and the 3Rs in Brazil, China, and India. J. Am. Assoc. Lab. Anim. Sci. 54, 181–191.
Begley, C. G., and Ellis, L. M. (2012). Drug development: raise standards for preclinical cancer research. Nature 483, 531–533. doi: 10.1038/483531a
Bertapelle, C., Polese, G., and Di Cosmo, A. (2017). Enriched environment increases PCNA and PARP1 levels in octopus vulgaris central nervous system: first evidence of adult neurogenesis in lophotrochozoa. J. Exp. Zool. B Mol. Dev. Evol. 328, 347–359. doi: 10.1002/jez.b.22735
Bono, R., Alarcon, R., and Blanca, M. J. (2021). Report quality of generalized linear mixed models in psychology: a systematic review. Front. Psychol. 12:666182.
Branchi, I., and Ricceri, L. (2004). Refining learning and memory assessment in laboratory rodents. an ethological perspective. Ann. Ist Super Sanita 40, 231–236.
Burghardt, G. M., Ward, B., and Rosscoe, R. (1996). Problem of reptile play: environmental enrichment and play behavior in a captive Nile soft-shelled turtle. Trionyx triunguis. Zoobiology 15, 223–238. doi: 10.1002/(sici)1098-2361(1996)15:3<223::aid-zoo3>3.0.co;2-d
Button, K. S., Ioannidis, J. P., Mokrysz, C., Nosek, B. A., Flint, J., Robinson, E. S., et al. (2013). Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14, 365–376. doi: 10.1038/nrn3475
Campbell, D. L. M., Talk, A. C., Loh, Z. A., Dyall, T. R., and Lee, C. (2018). Spatial cognition and range use in free-range laying hens. Animals 8:26. doi: 10.3390/ani8020026
Can, A., Dao, D. T., Terrillion, C. E., Piantadosi, S. C., Bhat, S., and Gould, T. D. (2012). The tail suspension test. J. Vis. Exp. 59:e3769.
Catillon, M. (2019). Trends and predictors of biomedical research quality, 1990-2015: a meta-research study. BMJ Open 9:e030342. doi: 10.1136/bmjopen-2019-030342
Cumming, G. (2008). Replication and p intervals: p values predict the future only vaguely, but confidence intervals do much better. Perspect. Psychol. Sci. 3, 286–300. doi: 10.1111/j.1745-6924.2008.00079.x
de Kloet, E. R., and Molendijk, M. L. (2021). Floating rodents and stress-coping neurobiology. Biol. Psychiatry 90, e19–e21. doi: 10.1016/j.biopsych.2021.04.003
du Sert, N. P., Bamsey, I., Bate, S. T., Berdoy, M., Clark, R. A., Cuthill, I. C., et al. (2017). The experimental design assistant. Nat. Methods 14, 1024–1025.
EC (2010). Directive 2010/63/EU of the european parliament and the council of 22 september on the protection of animals used for scientific purposes. Official J. Eur. Union 276, 33–79.
Eilam, D., Dank, M., and Maurer, R. (2003). Voles scale locomotion to the size of the open-field by adjusting the distance between stops: a possible link to path integration. Behav. Brain Res. 141, 73–81. doi: 10.1016/s0166-4328(02)00322-4
Eisner, D. A. (2021). Pseudoreplication in physiology: more means less. J. Gen. Physiol. 153:e202012826. doi: 10.1085/jgp.202012826
Emmer, K. M., Russart, K. L. G., Walker, W. H., Nelson, R. J., and DeVries, A. C. (2018). Effects of light at night on laboratory animals and research outcomes. Behav. Neurosci. 132, 302–314. doi: 10.1037/bne0000252
Engeszer, R. E., Patterson, L. B., Rao, A. A., and Parichy, D. M. (2007). Zebrafish in the wild: a review of natural history and new notes from the field. Zebrafish 4, 21–40. doi: 10.1089/zeb.2006.9997
Ferguson, A. R., Nielson, J. L., Cragin, M. H., Bandrowski, A. E., and Martone, M. E. (2014). Big data from small data: data-sharing in the ‘long tail’ of neuroscience. Nat. Neurosci. 17, 1442–1447. doi: 10.1038/nn.3838
Festing, M. F. (2014). Randomized block experimental designs can increase the power and reproducibility of laboratory animal experiments. ILAR J. 55, 472–476. doi: 10.1093/ilar/ilu045
Festing, M. F., and Altman, D. G. (2002). Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 43, 244–258. doi: 10.1093/ilar.43.4.244
Fong, S., Buechel, S. D., Boussard, A., Kotrschal, A., and Kolm, N. (2019). Plastic changes in brain morphology in relation to learning and environmental enrichment in the guppy (Poecilia reticulata). J. Exp. Biol. 222:jeb.200402. doi: 10.1242/jeb.200402
Franco, N. H., and Olsson, I. A. (2014). Scientists and the 3Rs: attitudes to animal use in biomedical research and the effect of mandatory training in laboratory animal science. Lab. Anim. 48, 50–60. doi: 10.1177/0023677213498717
Freeberg, T. M., and Lucas, J. R. (2009). Pseudoreplication is (still) a problem. J. Comp. Psychol. 123, 450–451. doi: 10.1037/a0017031
Freedman, L. P., Cockburn, I. M., and Simcoe, T. S. (2015). The economics of reproducibility in preclinical research. PLoS Biol. 13:e1002165. doi: 10.1371/journal.pbio.1002165
Freedman, L. P., Venugopalan, G., and Wisman, R. (2017). Reproducibility2020: progress and priorities. F1000Res 6:604. doi: 10.12688/f1000research.11334.1
Garcia, A. M., Cardenas, F. P., and Morato, S. (2005). Effect of different illumination levels on rat behavior in the elevated plus-maze. Physiol. Behav. 85, 265–270. doi: 10.1016/j.physbeh.2005.04.007
Garner, J. P., Gaskill, B. N., Weber, E. M., Ahloy-Dallaire, J., and Pritchett-Corning, K. R. (2017). Introducing therioepistemology: the study of how knowledge is gained from animal research. Lab. Anim. 46, 103–113. doi: 10.1038/laban.1224
Gilmore, R. O., Diaz, M. T., Wyble, B. A., and Yarkoni, T. (2017). Progress toward openness, transparency, and reproducibility in cognitive neuroscience. Ann. N. Y. Acad. Sci. 1396, 5–18. doi: 10.1111/nyas.13325
Goodman, S. (2008). A dirty dozen: twelve p-value misconceptions. Semin. Hematol. 45, 135–140. doi: 10.1053/j.seminhematol.2008.04.003
Griebel, G., Moreau, J. L., Jenck, F., Martin, J. R., and Misslin, R. (1993). Some critical determinants of the behaviour of rats in the elevated plus-maze. Behav. Process. 29, 37–47.
Grimm, D. (2018). “Are happy lab animals better for science?,” in Science Magazine. Available online at: https://www.sciencemag.org/news/2018/02/are-happy-lab-animals-better-science
Guisnet, A., Maitra, M., Pradhan, S., and Hendricks, M. (2021). A three-dimensional habitat for C. elegans environmental enrichment. PLoS One 16:e0245139. doi: 10.1371/journal.pone.0245139
Gurwitz, D. (2001). Are drug targets missed owing to lack of physical activity? Drug Discov. Today 6, 342–343. doi: 10.1016/s1359-6446(01)01747-0
Hair, K., Macleod, M. R., Sena, E. S., and Collaboration, I. I. (2019). A randomised controlled trial of an intervention to improve compliance with the ARRIVE guidelines (IICARus). Res. Integr. Peer Rev. 4:12. doi: 10.1186/s41073-019-0069-3
Halsey, L. G. (2019). The reign of the p-value is over: what alternative analyses could we employ to fill the power vacuum? Biol. Lett. 15:20190174. doi: 10.1098/rsbl.2019.0174
Halsey, L. G., Curran-Everett, D., Vowler, S. L., and Drummond, G. B. (2015). The fickle P value generates irreproducible results. Nat. Methods 12, 179–185. doi: 10.1038/nmeth.3288
Han, S., Olonisakin, T. F., Pribis, J. P., Zupetic, J., Yoon, J. H., Holleran, K. M., et al. (2017). A checklist is associated with increased quality of reporting preclinical biomedical research: a systematic review. PLoS One 12:e0183591. doi: 10.1371/journal.pone.0183591
Holmes, A., Parmigiani, S., Ferrari, P. F., Palanza, P., and Rodgers, R. J. (2000). Behavioral profile of wild mice in the elevated plus-maze test for anxiety. Physiol. Behav. 71, 509–516.
Hooijmans, C. R., Rovers, M. M., de Vries, R. B., Leenaars, M., Ritskes-Hoitinga, M., and Langendam, M. W. (2014). SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 14:43.
Karp, N. A., and Fry, D. (2021). What is the optimum design for my animal experiment? BMJ Open Sci. 5:e100126.
Kentner, A. C., Speno, A. V., Doucette, J., and Roderick, R. C. (2021). The contribution of environmental enrichment to phenotypic variation in mice and rats. eNeuro 8:ENEURO.0539-20. doi: 10.1523/ENEURO.0539-20.2021
Kilkenny, C., Parsons, N., Kadyszewski, E., Festing, M. F., Cuthill, I. C., Fry, D., et al. (2009). Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS One 4:e7824. doi: 10.1371/journal.pone.0007824
Kondrakiewicz, K., Kostecki, M., Szadzinska, W., and Knapska, E. (2019). Ecological validity of social interaction tests in rats and mice. Genes Brain Behav. 18:e12525. doi: 10.1111/gbb.12525
Landers, M. S., Knott, G. W., Lipp, H. P., Poletaeva, I., and Welker, E. (2011). Synapse formation in adult barrel cortex following naturalistic environmental enrichment. Neuroscience 199, 143–152. doi: 10.1016/j.neuroscience.2011.10.040
Lazic, S. E. (2010). The problem of pseudoreplication in neuroscientific studies: is it affecting your analysis? BMC Neurosci. 11:5. doi: 10.1186/1471-2202-11-5
Lazic, S. E. (2015). Analytical strategies for the marble burying test: avoiding impossible predictions and invalid p-values. BMC Res. Notes 8:141. doi: 10.1186/s13104-015-1062-7
Lazic, S. E. (2018). Four simple ways to increase power without increasing the sample size. Lab. Anim. 52, 621–629. doi: 10.1177/0023677218767478
Lazic, S. E. A. (2016). Experimental Design for Laboratory Biologists : Maximising Information and Improving Reproducibility. Cambridge: Cambridge University Press.
Lazic, S. E., and Essioux, L. (2013). Improving basic and translational science by accounting for litter-to-litter variation in animal models. BMC Neurosci. 14:37. doi: 10.1186/1471-2202-14-37
Lazic, S. E., Clarke-Williams, C. J., and Munafo, M. R. (2018). What exactly is ‘N’ in cell culture and animal experiments? PLoS Biol. 16:e2005282. doi: 10.1371/journal.pbio.2005282
Lazic, S. E., Mellor, J. R., Ashby, M. C., and Munafo, M. R. (2020). A Bayesian predictive approach for dealing with pseudoreplication. Sci. Rep. 10:2366. doi: 10.1038/s41598-020-59384-7
Lee, C. J., Paull, G. C., and Tyler, C. R. (2019). Effects of environmental enrichment on survivorship, growth, sex ratio and behaviour in laboratory maintained zebrafish Danio rerio. J. Fish Biol. 94, 86–95. doi: 10.1111/jfb.13865
Loss, C. M., Binder, L. B., Muccini, E., Martins, W. C., de Oliveira, P. A., Vandresen-Filho, S., et al. (2015). Influence of environmental enrichment vs. time-of-day on behavioral repertoire of male albino Swiss mice. Neurobiol. Learn. Mem. 125, 63–72. doi: 10.1016/j.nlm.2015.07.016
Loss, C. M., Córdova, S. D., Callegari-Jacques, S. M., and de Oliveira, D. L. (2014). Time-of-day influence on exploratory behaviour of rats exposed to an unfamiliar environment. Behaviour 151, 1943–1966. doi: 10.1163/1568539x-00003224
Macleod, M. R., Lawson, McLean, A., Kyriakopoulou, A., Serghiou, S., de Wilde, A., et al. (2015). Risk of bias in reports of in vivo research: a focus for improvement. PLoS Biol. 13:e1002273. doi: 10.1371/journal.pbio.1002273
Macleod, M. R., Michie, S., Roberts, I., Dirnagl, U., Chalmers, I., Ioannidis, J. P., et al. (2014). Biomedical research: increasing value, reducing waste. Lancet 383, 101–104.
Mallory, H. S., Howard, A. F., and Weiss, M. R. (2016). Timing of environmental enrichment affects memory in the house cricket, Acheta domesticus. PLoS One 11:e0152245. doi: 10.1371/journal.pone.0152245
Masud, N., Ellison, A., Pope, E. C., and Cable, J. (2020). Cost of a deprived environment - increased intraspecific aggression and susceptibility to pathogen infections. J. Exp. Biol. 223:jeb.229450. doi: 10.1242/jeb.229450
Maximino, C., and van der Staay, F. J. (2019). Behavioral models in psychopathology: epistemic and semantic considerations. Behav. Brain Funct. 15:1. doi: 10.1186/s12993-019-0152-4
Maxwell, S. E. (2004). The persistence of underpowered studies in psychological research: causes, consequences, and remedies. Psychol. Methods 9, 147–163. doi: 10.1037/1082-989X.9.2.147
Mayorga, A. J., and Lucki, I. (2001). Limitations on the use of the C57BL/6 mouse in the tail suspension test. Psychopharmacology 155, 110–112. doi: 10.1007/s002130100687
Melleu, F. F., Pinheiro, M. V., Lino-de-Oliveira, C., and Marino-Neto, J. (2016). Defensive behaviors and prosencephalic neurogenesis in pigeons (Columba livia) are affected by environmental enrichment in adulthood. Brain Struct. Funct. 221, 2287–2301. doi: 10.1007/s00429-015-1043-6
Nestler, E. J., and Hyman, S. E. (2010). Animal models of neuropsychiatric disorders. Nat. Neurosci. 13, 1161–1169.
Neves, K., and Amaral, O. B. (2020). Addressing selective reporting of experiments through predefined exclusion criteria. Elife 9:e56626. doi: 10.7554/eLife.56626
Neves, K., Carneiro, C. F., Wasilewska-Sampaio, A. P., Abreu, M., Valerio-Gomes, B., Tan, P. B., et al. (2020). Two years into the brazilian reproducibility initiative: reflections on conducting a large-scale replication of Brazilian biomedical science. Mem. Inst. Oswaldo Cruz 115:e200328. doi: 10.1590/0074-02760200328
Nithianantharajah, J., and Hannan, A. J. (2006). Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 7, 697–709. doi: 10.1038/nrn1970
Nosek, B. A., Ebersole, C. R., DeHaven, A. C., and Mellor, D. T. (2018). The preregistration revolution. Proc. Natl. Acad. Sci. U.S.A. 115, 2600–2606.
Open Science, C. (2015). Psychology. Estimating the reproducibility of psychological science. Science 349:aac4716. doi: 10.1126/science.aac4716
Parichy, D. M. (2015). Advancing biology through a deeper understanding of zebrafish ecology and evolution. Elife 4:e05635. doi: 10.7554/eLife.05635
Park, J. (2020). The epistemological (not reproducibility) crisis. Adv. Radiat. Oncol. 5, 1320–1323. doi: 10.1016/j.adro.2020.07.019
Paulus, M. P., Dulawa, S. C., Ralph, R. J., and Mark, A. G. (1999). Behavioral organization is independent of locomotor activity in 129 and C57 mouse strains. Brain Res. 835, 27–36. doi: 10.1016/s0006-8993(99)01137-3
Percie, du Sert, N., Hurst, V., Ahluwalia, A., Alam, S., Avey, M. T., et al. (2020). The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. Br. J. Pharmacol. 177, 3617–3624.
Possamai, F., dos Santos, J., Walber, T., Marcon, J. C., dos Santos, T. S., Lino, et al. (2015). Influence of enrichment on behavioral and neurogenic effects of antidepressants in Wistar rats submitted to repeated forced swim test. Prog. Neuropsychopharmacol. Biol. Psychiatry 58, 15–21. doi: 10.1016/j.pnpbp.2014.10.017
Prinz, F., Schlange, T., and Asadullah, K. (2011). Believe it or not: how much can we rely on published data on potential drug targets? Nat. Rev. Drug Discov. 10:712. doi: 10.1038/nrd3439-c1
Quadros, V. A., Silveira, A., Giuliani, G. S., Didonet, F., Silveira, A. S., Nunes, M. E., et al. (2016). Strain- and context-dependent behavioural responses of acute alarm substance exposure in zebrafish. Behav. Process. 122, 1–11. doi: 10.1016/j.beproc.2015.10.014
Ramos-Hryb, A. B., Harris, C., Aighewi, O., and Lino-de-Oliveira, C. (2018). How would publication bias distort the estimated effect size of prototypic antidepressants in the forced swim test? Neurosci. Biobehav. Rev. 92, 192–194. doi: 10.1016/j.neubiorev.2018.05.025
Richter, S. H. (2017). Systematic heterogenization for better reproducibility in animal experimentation. Lab. Anim. 46, 343–349. doi: 10.1038/laban.1330
Richter, S. H., Garner, J. P., Zipser, B., Lewejohann, L., Sachser, N., Touma, C., et al. (2011). Effect of population heterogenization on the reproducibility of mouse behavior: a multi-laboratory study. PLoS One 6:e16461. doi: 10.1371/journal.pone.0016461
Rubinstein, M., Phillips, T. J., Bunzow, J. R., Falzone, T. L., Dziewczapolski, G., Zhang, G., et al. (1997). Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell 90, 991–1001. doi: 10.1016/s0092-8674(00)80365-7
Schroeder, P., Jones, S., Young, I. S., and Sneddon, L. U. (2014). What do zebrafish want? Impact of social grouping, dominance and gender on preference for enrichment. Lab. Anim. 48, 328–337. doi: 10.1177/0023677214538239
Sena, E. S., van der Worp, H. B., Bath, P. M., Howells, D. W., and Macleod, M. R. (2010). Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 8:e1000344. doi: 10.1371/journal.pbio.1000344
Shkedy, Z., Molenberghs, G., Van Craenendonck, H., Steckler, T., and Bijnens, L. (2005a). A hierarchical binomial-poisson model for the analysis of a crossover design for correlated binary data when the number of trials is dose-dependent. J. Biopharm. Stat. 15, 225–239. doi: 10.1081/bip-200049825
Shkedy, Z., Vandersmissen, V., Molenberghs, G., Van Craenendonck, H., Aerts, N., Steckler, T., et al. (2005b). Behavioral testing of antidepressant compounds: an analysis of crossover design for correlated binary data. Biom J. 47, 286–298. doi: 10.1002/bimj.200410130
Smith, A. J., Clutton, R. E., Lilley, E., Hansen, K. E. A., and Brattelid, T. (2018). PREPARE: guidelines for planning animal research and testing. Lab. Anim. 52, 135–141. doi: 10.1177/0023677217724823
Spence, R., Gerlach, G., Lawrence, C., and Smith, C. (2008). The behaviour and ecology of the zebrafish, Danio rerio. Biol. Rev. Camb. Philos. Soc. 83, 13–34. doi: 10.1111/j.1469-185x.2007.00030.x
Steckler, T., Brose, K., Haas, M., Kas, M. J., Koustova, E., Bespalov, A., et al. (2015). The preclinical data forum network: a new ECNP initiative to improve data quality and robustness for (preclinical) neuroscience. Eur. Neuropsychopharmacol. 25, 1803–1807. doi: 10.1016/j.euroneuro.2015.05.011
Stevens, C. H., Reed, B. T., and Hawkins, P. (2021). Enrichment for laboratory zebrafish-a review of the evidence and the challenges. Animals 11:698. doi: 10.3390/ani11030698
Strech, D., and Dirnagl, U. (2019). 3Rs missing: animal research without scientific value is unethical. BMJ Open Sci. 3:e000048.
Strech, D., Weissgerber, T., Dirnagl, U., and Group, Q. (2020). Improving the trustworthiness, usefulness, and ethics of biomedical research through an innovative and comprehensive institutional initiative. PLoS Biol. 18:e3000576. doi: 10.1371/journal.pbio.3000576
Turschwell, M. P., and White, C. R. (2016). The effects of laboratory housing and spatial enrichment on brain size and metabolic rate in the eastern mosquitofish, Gambusia holbrooki. Biol. Open 5, 205–210. doi: 10.1242/bio.015024
van der Staay, F. J. (2006). Animal models of behavioral dysfunctions: basic concepts and classifications, and an evaluation strategy. Brain Res. Rev. 52, 131–159. doi: 10.1016/j.brainresrev.2006.01.006
Voelkl, B., Altman, N. S., Forsman, A., Forstmeier, W., Gurevitch, J., Jaric, I., et al. (2020). Reproducibility of animal research in light of biological variation. Nat. Rev. Neurosci. 21, 384–393.
Voelkl, B., and Wurbel, H. (2021). A reaction norm perspective on reproducibility. Theory Biosci. 140, 169–176. doi: 10.1007/s12064-021-00340-y
Voelkl, B., Wurbel, H., Krzywinski, M., and Altman, N. (2021). The standardization fallacy. Nat. Methods 18, 5–7. doi: 10.1038/s41592-020-01036-9
Vsevolozhskaya, O., Ruiz, G., and Zaykin, D. (2017). Bayesian prediction intervals for assessing P-value variability in prospective replication studies. Transl. Psychiatry 7:1271. doi: 10.1038/s41398-017-0024-3
Wagenmakers, E. J., Wetzels, R., Borsboom, D., van der Maas, H. L., and Kievit, R. A. (2012). An agenda for purely confirmatory research. Perspect. Psychol. Sci. 7, 632–638. doi: 10.1177/1745691612463078
Wang, X., Amei, A., de Belle, J. S., and Roberts, S. P. (2018). Environmental effects on Drosophila brain development and learning. J. Exp. Biol. 221:jeb.169375.
Willner, P. (1986). Validation criteria for animal models of human mental disorders: learned helplessness as a paradigm case. Prog. Neuropsychopharmacol. Biol. Psychiatry 10, 677–690. doi: 10.1016/0278-5846(86)90051-5
Wolfensohn, S., and Lloyd, M. (2013). Handbook of Laboratory Animal Management and Welfare. Chichester: Wiley-Blackwell.
Wolfer, D. P., Litvin, O., Morf, S., Nitsch, R. M., Lipp, H. P., and Wurbel, H. (2004). Laboratory animal welfare: cage enrichment and mouse behaviour. Nature 432, 821–822.
Zimmerman, K. D., Espeland, M. A., and Langefeld, C. D. (2021). A practical solution to pseudoreplication bias in single-cell studies. Nat. Commun. 12:738. doi: 10.1038/s41467-021-21038-1
Key concepts (Glossary)
• Reproducibility: Obtaining the same results (similar effect sizes) as the original study by carrying out independent experiments (in different locations, laboratories, and research groups) in which the experimental procedures were as close as possible to the original study. Importantly, there is no need for the reproduction study to have exactly the same experimental design as the original study, for its result to be considered a reproduction. Also, as stated in Reproducibility Project and Cancer Biology, 2017, “if a replication reproduces some of the key experiments in the original study and sees effects that are similar to those seen in the original in other experiments, we need to conclude that it has substantially reproduced the original study.”
• Environmental enrichment: It consists in modifying the environment of animals by increasing perceptual, cognitive, physical, and social stimulation. In captive animals, it promotes improvements in the quality of life and animal welfare. Environmental enrichment represents an opportunity for the animals to evocate their ethological behaviors. For example, nocturnal animals usually escape bright environments by entering into shelters. In a future approach, it may represent a controlled naturalistic environment, such as a forest (as described in Landers et al., 2011).
• Replace: According to NC3Rs, it is “accelerating the development and use of models and tools, based on the latest science and technologies, to address important scientific questions without the use of animals.”
• Reduce: According to NC3Rs, it is “appropriately designing and analyzing animal experiments that are robust and reproducible and truly add to the knowledge base.”
• Refine: According to NC3Rs, it is “advancing animal welfare by exploiting the latest in vivo technologies and by improving understanding of the impact of welfare on scientific outcomes.”
• Physical models: According to Godfrey-Smith (2009), they are real systems purposely built to understand another real system.
• Animal models: According to Willner (1986), they are animal manipulations designed to model certain aspects (specific symptoms, for example) of a known disease.
• Behavioral tests: Paradigms designed to assess animal behavior. Commonly, they are used to evaluate the behavior of animals that were previously subjected to genetic, pharmacological, or environmental manipulations. In addition, they can also be used to investigation of the natural behavior of “naïve” animals.
• Pseudoreplication: It occurs when the researcher artificially inflates the number of experimental units by using samples that are heavily dependent on each other without correcting for it. Example 1) measuring multiple animals in a litter (after allocating all them to the same group) and treating them as independent samples (i.e., “N” equals the multiple measurements). Example 2) measuring two experimental animals that interacted with each other in a social interaction paradigm (i.e., the way that an animal behaves is influenced by the way the other one behaves, and vice-versa) and treating them as independent samples (i.e., “N” equals two).
• Experimental unit: It is the smallest entity that can be randomly and independently assigned to a treatment condition. For experimental units to be considered as genuine replications (i.e., the real “N”) they must not influence each other and must undergo experimental treatment independently. Its biological definition can change from one experiment to another (i.e., “N equals one” can be a single animal in an experiment and a pair of animals or even a whole litter in others).
• Exploratory studies: The ones that present more flexible experimental methods and designs. Their aim is not to reach statistical conclusions, but to gather information to the postulation of experimental hypotheses that must be tested and replicated through confirmatory studies before being assumed as strong evidence.
• Confirmatory studies: The ones that present clear predefined hypotheses to be tested and rigid methods to doing so (e.g., impartial assignment of experimental units to experimental groups, blinding during data collection and analysis, complete reporting of methods and results). Experimental design cannot be changed after the experiments are running. Must be presented in advance with well-defined biological effect sizes and statistical power, in addition to the a priori calculation of sample sizes. A clear example of confirmatory study is the Phase III of clinical trials in the process of vaccine development.
• Biological effect size: The calculated minimum effect size that is considered to be biologically relevant by the researcher.
• Confounding factors: Variables that can affect the outcomes that the researcher is measuring. Usually, they are not in the interest of the researcher and may assume categorical (e.g., litter, experimental blocks, and repeated measurements) or continual nature (e.g., age and body weight). Example 1) measuring siblings (after correctly allocating each one to a distinct experimental group) and analyzing their data as if they were not relatives. If the between-litter variation is higher than within-litter variation (i.e., the difference between families is higher than differences between siblings and, in this case, between experimental groups) the high data variability between litters could mask the effect of treatments. Example 2) Measuring drug-seeking behavior in a self-administration paradigm and analyzing the data without considering the basal motivation to self-administrating the drug (even when its variability was well controlled by randomization). If the basal motivation affects self-administration behavior the high within-group data variability (as a consequence of basal motivation variability) could mask the effect of treatments.
• Impossible predictions: Incorrectly estimating of values that are impossible to be observed for some types of data. It can occur when using linear models for analyzing count data (e.g., number of visible marbles, grooming, rearing, and pressures in a lever), where negative values are impossible to be observed but they can be often estimated by the analysis when the observed mean is low and/or the standard deviations are high.
• Directive 2010/63/EU: European Union Directive about animal welfare that established, among others, that “…all animals shall be provided with space of sufficient complexity to allow expression of a wide range of normal behavior. They shall be given a degree of control and choice over their environment to reduce stress-induced behavior.”
• Impoverished standard conditions: The conditions under which laboratory animals are bred by default in research facilities around the world. In general, the cages are too limited in space and contain only bedding (e.g., sawdust) plus water and food ad libitum. Improvements were made after some directives were established, but the “new standard” remains impoverished.
• Paradigm shift: According to Kuhn (1962), it is a fundamental change of concepts and experimental practices in science. Here, we adopted a more restricted use for this term. It represents a change in the experimental practices specifically for the environmental conditions of laboratory animals.
• Ethology: According to Merriam-Webster (https://www.merriam-webster.com/dictionary/ethology), it is the scientific study of animal behavior, usually with a focus on animal behavior under natural conditions. Viewing animal behavior as an evolutionarily adaptive trait.
• Ethological needs of the species: The basic natural needs (and also behavioral phenotypes) are distinct between each species. Based on the ethology concept, the environments where laboratory animals are kept or behaviorally tested must meet the intrinsic features of each species. Even though rats and mice are both rodents, they are different species and their characteristics and basic needs are not the same. This concept should be applied to all laboratory animals. For example, for ethical reasons, researchers do not submit rats to the tail suspension test. However, they do submit mice to the forced swim test (even though mice do not swim in nature).
Keywords: replicability, reduce, refine, laboratory animals, animal models, behavior, enriched environment, ethology
Citation: Loss CM, Melleu FF, Domingues K, Lino-de-Oliveira C and Viola GG (2021) Combining Animal Welfare With Experimental Rigor to Improve Reproducibility in Behavioral Neuroscience. Front. Behav. Neurosci. 15:763428. doi: 10.3389/fnbeh.2021.763428
Received: 23 August 2021; Accepted: 18 October 2021;
Published: 30 November 2021.
Edited by:
Jess Nithianantharajah, University of Melbourne, AustraliaReviewed by:
Joshua C. Brumberg, Queens College (CUNY), United StatesAnthony Hannan, The University of Melbourne, Australia
Copyright © 2021 Loss, Melleu, Domingues, Lino-de-Oliveira and Viola. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cássio Morais Loss, cassio.m.loss@gmail.com; orcid.org/0000-0003-0552-421X
†These authors have contributed equally to this work
 Cássio Morais Loss
Cássio Morais Loss Fernando Falkenburger Melleu3†
Fernando Falkenburger Melleu3†  Karolina Domingues
Karolina Domingues Cilene Lino-de-Oliveira
Cilene Lino-de-Oliveira Giordano Gubert Viola
Giordano Gubert Viola