Coexpression analysis of nine neuropeptides in the neurosecretory preoptic area of larval zebrafish
- 1Developmental Genetics of the Nervous System, Max Planck Institute for Medical Research, Heidelberg, Germany
- 2The Hartmut Hoffmann-Berling International Graduate School of Molecular and Cellular Biology, University of Heidelberg, Heidelberg, Germany
The paraventricular nucleus (PVN) of the hypothalamus in mammals coordinates neuroendocrine, autonomic and behavioral responses pivotal for homeostasis and the stress response. A large amount of studies in rodents has documented that the PVN contains diverse neuronal cell types which can be identified by the expression of distinct secretory neuropeptides. Interestingly, PVN cell types often coexpress multiple neuropeptides whose relative coexpression levels are subject to environment-induced plasticity. Due to their small size and transparency, zebrafish larvae offer the possibility to comprehensively study the development and plasticity of the PVN in large groups of intact animals, yet important anatomical information about the larval zebrafish PVN-homologous region has been missing. Therefore we recently defined the location and borders of the larval neurosecretory preoptic area (NPO) as the PVN-homologous region in larval zebrafish based on transcription factor expression and cell type clustering. To identify distinct cell types present in the larval NPO, we also generated a comprehensive 3D map of 9 zebrafish homologs of typical neuropeptides found in the mammalian PVN (arginine vasopressin (AVP), corticotropin-releasing hormone (CRH), proenkephalin a (penka)/b (penkb), neurotensin (NTS), oxytocin (OXT), vasoactive intestinal peptide (VIP), cholecystokinin (CCK), and somatostatin (SST)). Here we extend this chemoarchitectural map to include the degrees of coexpression of two neuropeptides in the same cell by performing systematic pairwise comparisons. Our results allowed the subclassification of NPO cell types, and differences in variability of coexpression profiles suggest potential targets of biochemical plasticity. Thus, this work provides an important basis for the analysis of the development, function, and plasticity of the primary neuroendocrine brain region in larval zebrafish.
Introduction
The vertebrate neuroendocrine system is controlled by specific hypothalamic nuclei. The most extensively studied ones are the paraventricular nucleus (PVN) and supraoptic nucleus (SON) in rodents, which contain neuroendocrine cells expressing secretory neuropeptides. These peptides are transported to the pituitary gland, where they are directly released into the general circulation via the neurohypophysis, or released into the portal system of the adenohypophysis, where they trigger the release of hormones into the general circulation. The two peptides released in the neurohypophsis are arginine vasopressin (AVP), and oxytocin (OXT), and they are produced by distinct magnocellular neurons. Releasing and inhibiting factors acting on adenohypophyseal secretion are instead produced in parvocellular neurons, and include corticotropin-releasing hormone (CRH), thyrotropin-releasing hormone (TRH), methionine or leucine enkephalin (mENK/lENK), neurotensin (NTS), vasoactive intestinal peptide (VIP), cholecystokinin (CCK), and somatostatin (SST) (Swanson and Sawchenko, 1983; Mezey et al., 1985; Simmons and Swanson, 2009).
Coexpression of several of these peptides in the same cell is a common phenomenon in the mammalian PVN. For example, AVP colocalizes with mENK in neurohypophysial terminals in the rat (Martin and Voigt, 1981). Also in rats, CRH can be coexpressed with AVP, OXT, NTS, ENK, VIP, and/or CCK (Burlet et al., 1983; Hökfelt et al., 1983; Roth et al., 1983; Sawchenko et al., 1984a; Mezey et al., 1985; Piekut and Joseph, 1986; Swanson et al., 1986; Sawchenko, 1987; Whitnall and Gainer, 1988; Ceccatelli et al., 1989; Swanson and Simmons, 1989; Arima et al., 2001; Dabrowska et al., 2011). Cells producing OXT were found to also synthesize CRH, CCK, and ENK (Martin and Voigt, 1981; Vanderhaeghen et al., 1981; Rossier et al., 1983; Levin and Sawchenko, 1993). Not all peptide combinations occur, however. The populations of cells producing AVP, OXT, or SST are completely separate in the rat (Swanson and Sawchenko, 1983; Swanson et al., 1986).
The molecular development of the mammalian hypothalamus is largely conserved in zebrafish (Machluf et al., 2011). We have recently defined the location and borders of the larval neurosecretory preoptic area (NPO) as the PVN-homologous region based on transcription factor expression and cell type clustering in larval zebrafish. To identify distinct cell types present in the larval NPO, we also generated a comprehensive 3D map of 9 zebrafish homologs of typical neuropeptides found in the mammalian PVN (avp, crh, oxt, proenkephalin a (penka), proenkephalin b (penkb), nts, vip, cck, and somatostatin (sst1.1); Herget et al., 2014). The larval NPO featured 9 out of 10 peptides also found in the mammalian PVN. One exception was trh, which was found to be outside the boundaries of the larval NPO as we defined them. We found a small group of cells producing cck at the rostral border of the NPO and cells producing avp, oxt, crh, penka, nts, or sst1.1 as dense and intermingled clusters. In contrast, cells producing penkb or vip appeared to reside in separate subregions of the NPO.
Several of these neuropeptides are coexpressed in the same cells in the mammalian PVN, and extensive coexpression also in the larval NPO seemed likely based on the spatial proximity of cells after 3D registration. We reasoned that definitive classification of distinct cell types cannot be assigned in the larval NPO based on the expression of one neuropeptide alone. Therefore, we analyzed in this study the degree of coexpression of two neuropeptides in the same cell by performing systematic pairwise comparisons of coexpression of avp, crh, oxt, penka, penkb, nts, cck, vip, and sst1.1 in the larval zebrafish NPO. Our results show that many of the peptides produced by densely intermingled cells of the larval zebrafish NPO are not coexpressed, while some neuropeptide combinations show occasional, low or moderate levels of coexpression. Interestingly we observed high degrees of coexpression for certain neuropeptide combinations such as avp + crh and cck + penkb. These results illustrate that information about coexpressed peptides is essential to identify subclasses of cell types and the classification of cell types should not be based on the expression of one peptide alone within the NPO.
Plastic changes in the coexpression profile of a cell allow it to acquire additional regulatory functions. Indeed, in mammals the coexpression properties of PVN cells are subject to stress-induced plasticity, with different types of stress influencing the expression levels of different neuropeptides (Swanson et al., 1986; Harbuz and Lightman, 1989; Swanson, 1991). The larval zebrafish offers an attractive system to dissect the mechanistic basis of such environment-induced plasticity in the hypothalamus because of the possibility to study the architecture, development, and function of distinct neural circuits in intact and transparent animals. The basal characterization of coexpression profiles is a prerequisite to classify and identify distinct NPO cell types and their plasticity in response to environmental challenges.
Materials and Methods
Zebrafish Preparation
Zebrafish maintenance and breeding were carried out under standard conditions at 28.5°C (Westerfield, 2000). To avoid pigmentation, embryos were incubated in 0.2 mM 1-phenyl-2-thiourea (Sigma-Aldrich). AB/TL zebrafish larvae were fixed at 5 days post fertilization (dpf) in 4% paraformaldehyde (PFA, Merck; in phosphate buffered saline (PBS), pH 7.2–7.3) overnight. All animals were raised under constant conditions and fixed quickly to avoid chronic environmental or acute handling stress. On the following day, larvae were washed briefly with PBST (phosphate-buffered saline with 0.1% Triton X-100, Merck and Roth), then dehydrated with increasing methanol (Merck) concentrations (25%, 50%, 75%, 100%, in PBST, 5 min steps), and stored in 100% methanol at −20°C. All procedures were performed according to the guidelines of the German animal welfare law and approved by the local government.
Whole-Mount Fluorescent in Situ Hybridization
In situ hybridization (ISH) probes for avp (Eaton et al., 2008), oxt (Unger and Glasgow, 2003), sst1.1 (Devos et al., 2002), trh, crh (Löhr et al., 2009), vip (Wolf and Ryu, 2013), cck, penka, penkb, and nts (Herget et al., 2014) were previously described. Riboprobes were synthesized from linearized plasmids following the instructions provided with the digoxygenin labeling mix (Roche). Fluorescent ISH was performed based on a previously published protocol (Lauter et al., 2011).
Microscopy and Image Processing
For imaging, larval heads were cleared in 80% glycerol (Gerbu) in PBS for 1 h. Dorsal confocal stacks of larval heads were recorded using a Leica SP5 confocal microscope with a Nikon 20x glycerol objective. Each channel was recorded sequentially, using alternating excitation wavelengths specific for each tyramide, to reduce interfering signals from overlapping emission spectra. Acquisition settings were adjusted for each stack to obtain the optimal image quality of the desired volume. Stacks were evaluated using Amira 5.4 (Visualization Sciences Group) to create maximum intensity projections that were restricted to the volume of interest, excluding signals from planes above or below. Staining signal was analyzed plane by plane within the NPO. Brightness and contrast were adjusted for each channel. Any accumulation of signal with the proper shape and size of a typical cell was included in the analysis and compared to the signal in co-stained channels at the same location. Thus, coexpression was determined by the spatial overlap of cells stained for different peptide markers. Images of single planes and maximum projections were exported from Amira and arranged into figures using Adobe Illustrator. All images show dorsal views of substacks or single planes, with the rostral direction on the left side, unless indicated otherwise.
Results
To comprehensively analyze the degree of coexpression of two peptides in the same cell, we performed cell by cell comparisons of pairwise combinatorial ISH staining of nine peptide markers that we had previously identified to be expressed in the 5 dpf larval NPO. The NPO is defined by the dense clustering of cells expressing these peptides within the transcription factor orthopedia a (otpa)-positive preoptic area (Figure 1). Altogether we examined 36 pairwise combinations of neuropeptides, analyzing a minimum of 5 animals per pair, but the sample size varied and was larger for some peptide combinations. All data was obtained from 5 dpf larvae, since the original NPO cell type map was generated for that stage. We found broadly three different categories of coexpression extent: (1) Absence of coexpression in all animals; (2) Occurance of coexpression in a single, few or several cells only in some animals analyzed (“variable coexpression”); (3) Coexpression in several cells in all animals analyzed (“consistent coexpression”).
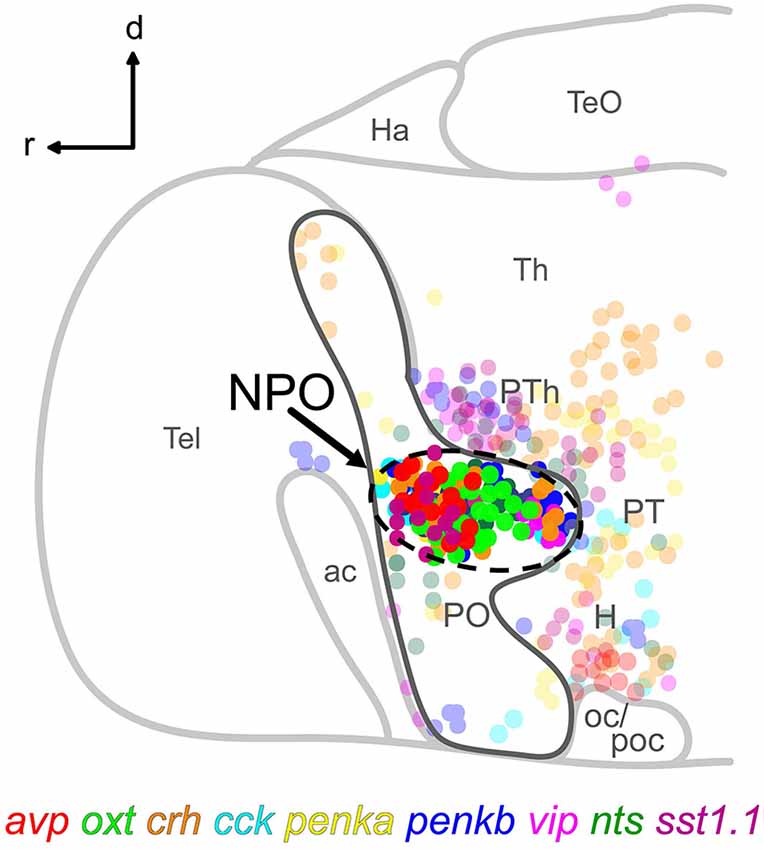
Figure 1. Schematic lateral view of a 5 dpf larval zebrafish brain showing the location of the NPO (dashed line) within the otpa-positive part (dark gray line) of the preoptic area, and the spatial distribution of nine cell types expressing the indicated neuropeptides. Cells clustering within the NPO are opaque. For more details, the reader is referred to our previously reported chemoarchitectural map (Herget et al., 2014). Abbreviations: ac, anterior commissure; d, dorsal; H, hypothalamus; Ha, habenula; NPO, neurosecretory preoptic area; oc, optic chiasm; PO, preoptic area; poc, postoptic commissure; PT, posterior tuberculum; PTh, prethalamus; r, rostral; Tel, telencephalon; TeO, optic tectum; Th, thalamus.
No Coexpression in All Animals
Many of the neuropeptide combinations (16/36) showed no coexpression in the same cell, and often these neuropeptides were expressed in spatially separate clusters. The rostralmost clusters formed by cells expressing avp, crh, cck, or sst1.1 did not show any overlap with the caudalmost cluster formed by cells expressing vip (Figures 2A–D”, 5–8 animals analyzed). Among the rostral group, crh and cck were not coexpressed in the same cells, and cck expression was also separate from the large nts-positive cluster (Figures 2E–F”, 6–7 animals analyzed). The penka-expressing cluster extended as far caudally as the small group of vip-positive cells, which however occupied a more lateral region (Figures 2G–G”, 8 animals analyzed). oxt-expressing cells formed a large central cluster, and they did not overlap with the rostrally located cck-expressing cells, nor with the caudally located vip-expressing group (Figures 2H–I”, 6–9 animals analyzed). The vip-positive cluster was also separate from cells expressing penkb (Figures 2J–J”, 7 animals analyzed). The rostral cluster of cck-positive cells was close to, but separate from the avp-positive and penka-positive clusters (Figures 2K–L”, 7 animals analyzed). penkb-positive cells surrounded the more central nts-positive cluster (Figures 2M–M”, 5 animals analyzed). Within the center of the NPO, cells expressing avp or oxt were found intermingled in the same region, but did not overlap (Figures 2N–O”, 8 animals analyzed). The central and intermingled clusters of cells expressing oxt or nts did not show coexpression of these two peptides (Figures 2P–P”, 10 animals analyzed). Similar spatial proximity without coexpression was also found for cells expressing oxt or sst1.1 (Figures 2Q–R”, 8 animals analyzed).
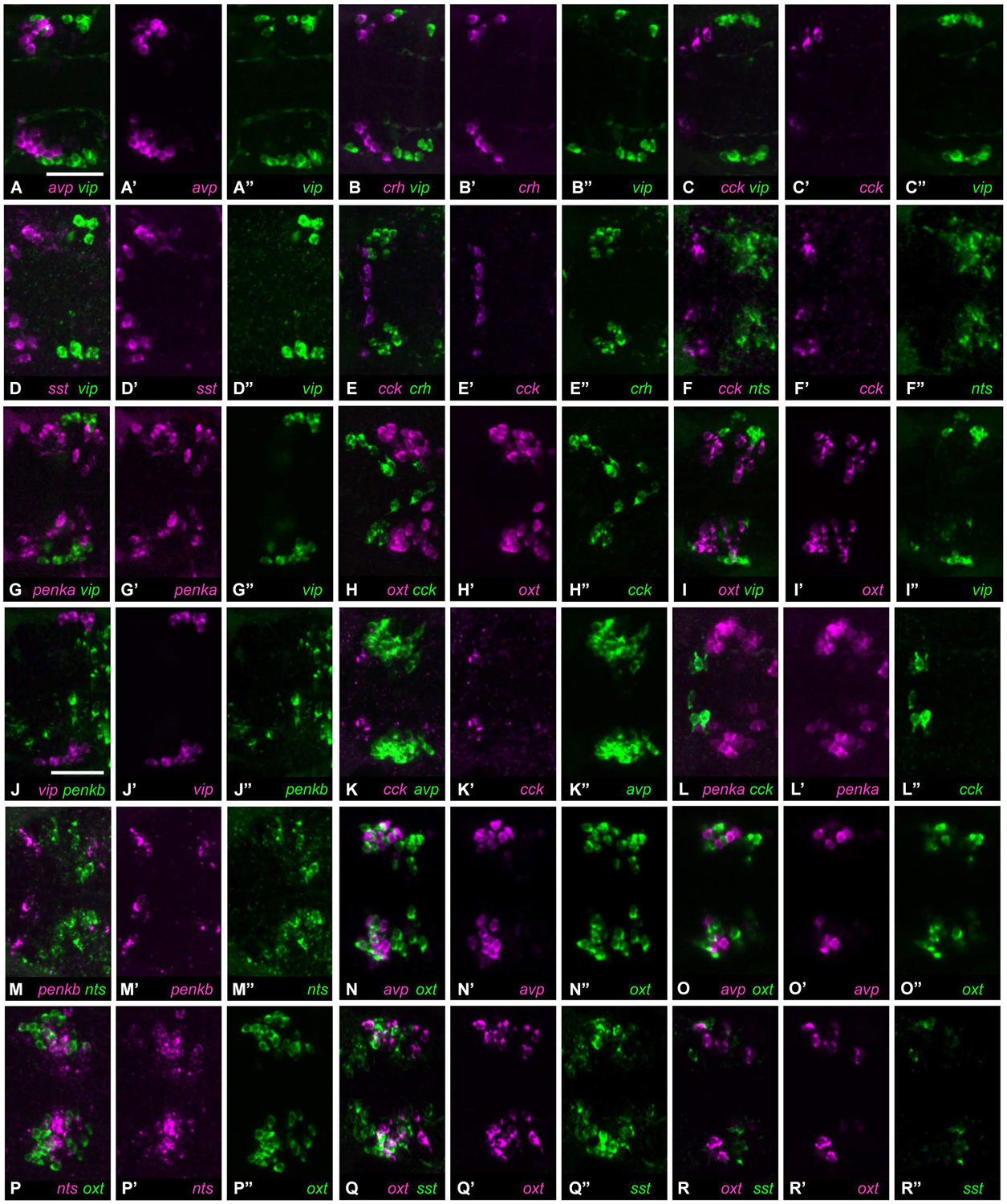
Figure 2. Cell type staining combinations showing no coexpression. (A) Cells expressing avp (A’) or vip (A”) form neighboring but separate clusters. (B) Cells expressing crh (B’) or vip (B”) are similarly separated. (C) Cells expressing cck cluster rostrally (C’), and are therefore distant from vip-positive cells (C”). (D) Cells expressing sst1.1 (D’) also cluster in a separate region from vip-positive cells (D”). (E) cck-positive cells (E’) are rostrally neighboring crh-positive cells (E”). (F) Cells expressing cck (F’) or nts (F”) are separate. (G) penka-positive cells (G’) are scattered, but do not overlap with vip expression (G”). (H) oxt-positive (H’) and cck-positive cells (H”) are also separated. (I) The clusters formed by cells expressing oxt (I’) or vip (I”) do not show coexpression. (J) vip (J’) and penkb (J”) are also not coexpressed. (K) The rostral location of cck-positive cells (K’) is separate from the neighboring avp-positive cluster (K”). (L) penka-positive cells (L’) also are close to the cck-positive population (L”), but still separate. (M) Cells positive for penkb (M’) surround the central nts-positive cluster (M”). (N) Cells expressing avp (N’) or oxt (N”) are intermingled, but the peptides are not coexpressed (single planes: O–O”). (P) Cells expressing nts (P’) or oxt (P”) have similar locations, but these peptides are not coexpressed. (Q) Cells expressing oxt (Q’) or sst1.1 (Q”) are also intermingled and do not coexpress these peptides (single planes: R–R”). All images show maximum intensity projections, unless indicated otherwise. Scale bar: 50 μm.
Variable Coexpression
Occasionally, a single cell was found coexpressing two neuropeptides in a larva, although most of the animals analyzed did not show coexpression. We observed such rarely coexpressing single cells in 10/36 neuropeptide combinations. A single avp-positive cell was occasionally found within nts-positive cells (Figure 3A, 3/11 animals). Similarly, in rare cases, single cells showed coexpression of crh and oxt (Figure 3B, 3/17 animals). The clusters of cells producing penka or penkb were spatially separate, but in one animal, coexpression could be found in a single cell (Figure 3C, 1/16 animals). The intermingled clusters of cells expressing penka or sst1.1 also in rare cases showed one cell with coexpression (Figure 3D, 2/14 animals). The crh-positive cluster occupied the rostral half of the region covered by both the penka-positive and nts-positive clusters, and in rarely occuring cells, crh was coexpressed with penka (Figure 3E, 4/14 animals) or nts (Figure 3F, 1/6 animals). While the cck-positive cluster was rostral, occasional coexpression of sst1.1 was observed (Figure 3G, 3/7 animals). The vip-producing cluster was caudal and lateral, but a cell that was more rostromedial did in one case coexpress nts (Figure 3H, 1/9 animals). The central cluster of penka-positive cells was intermingled with the clusters of cells expressing avp or oxt, and occasionally single penka-positive cells coexpressed avp (Figure 3I, 4/21 animals) or oxt (Figure 3J, 4/33 animals).
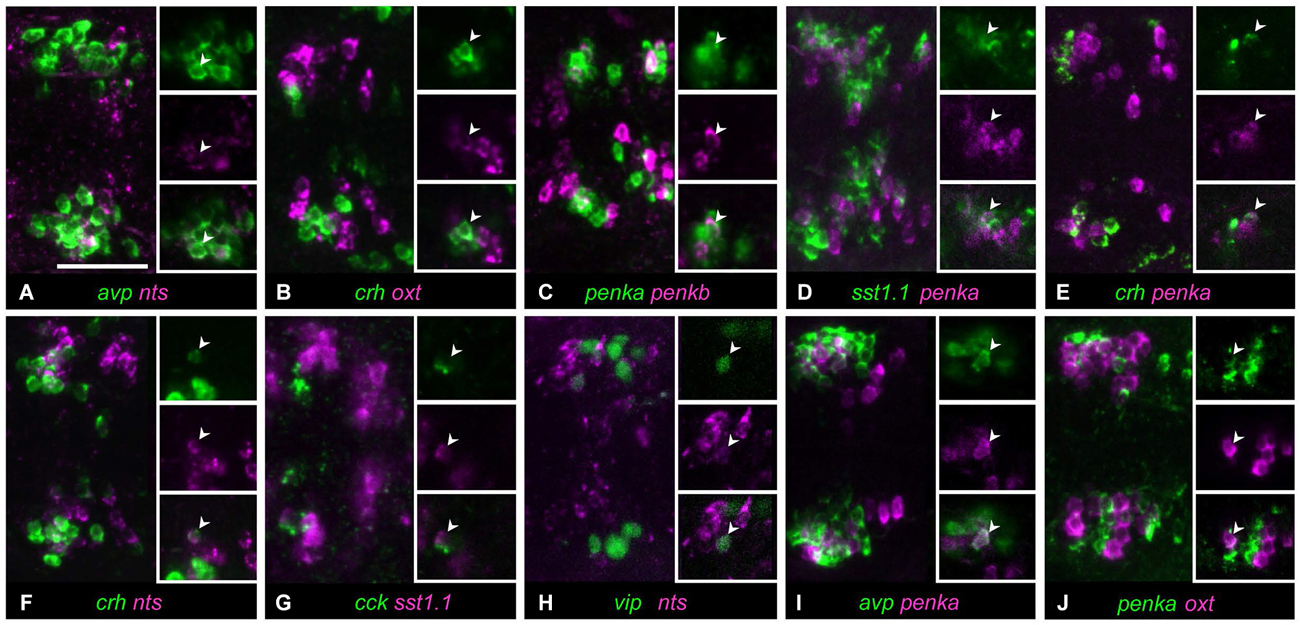
Figure 3. In some cell type staining combinations, occasional and low coexpression can be observed. (A) Cells expressing avp or nts only overlap in rare cases. (B) Cells expressing oxt or crh are usually separate, but occasially these peptides are coexpressed in a single cell. (C) Cells expressing penkb cells usually surround the penka-positive cluster, but in few animals, one cell shows coexpression. (D) penka-positive cells and sst1.1-positive cells are intermingled, and occasionally show low coexpression. (E) crh-positive cells are intermingled with penka-positive cells, but some rare occurences of coexpression were found. (F) crh can also be coexpressed with nts in rare cases. (G) cck-positive cells appear to faintly coexpress sst1.1 in few of the animals. (H) One isolated cell was sometimes found coexpressing vip and nts. (I) Cells expressing avp or penka are intermingled and usually these peptides are not coexpressed, but single coexpressing cells do occur. (J) Stainings for oxt and penka often show no coexpressing cells, but sometimes these peptides are coexpressed in few cells. Images show maximum intensity projections, insets show split channels of single confocal planes with coexpressing cells (arrowheads). Scale bar: 50 μm.
The extent of coexpression for other peptide combinations was somewhat higher where more than one cell per animal showed coexpression. In the combination of crh and penkb staining, many animals showed no coexpression, but in one animal, coexpression was observed in few cells (Figures 4A–A”’, 1/12 animals). Similarly few avp and penkb coexpressing cells were found in some animals analyzed (Figures 4B–B”’, 5/15 animals). In those cases in which crh and sst1.1 were coexpressed, we found such coexpression in several cells (Figures 4C–C”’, 2/9 animals). Few cells coexpressing penkb and sst1.1 were also found in one animal (Figures 4D–D”’, 1/5 animals). Few avp and sst1.1 coexpressing cells were also found in some animals (Figures 4E–E”’, 6/25 animals). Some animals showed few oxt and penkb coexpressing cells (Figures 4F–F”’, 4/7 animals). Although in some animals, penka and nts were not coexpressed, in most animals we found coexpression of these peptides in few cells (Figures 5A–A”’, 8/15 animals).
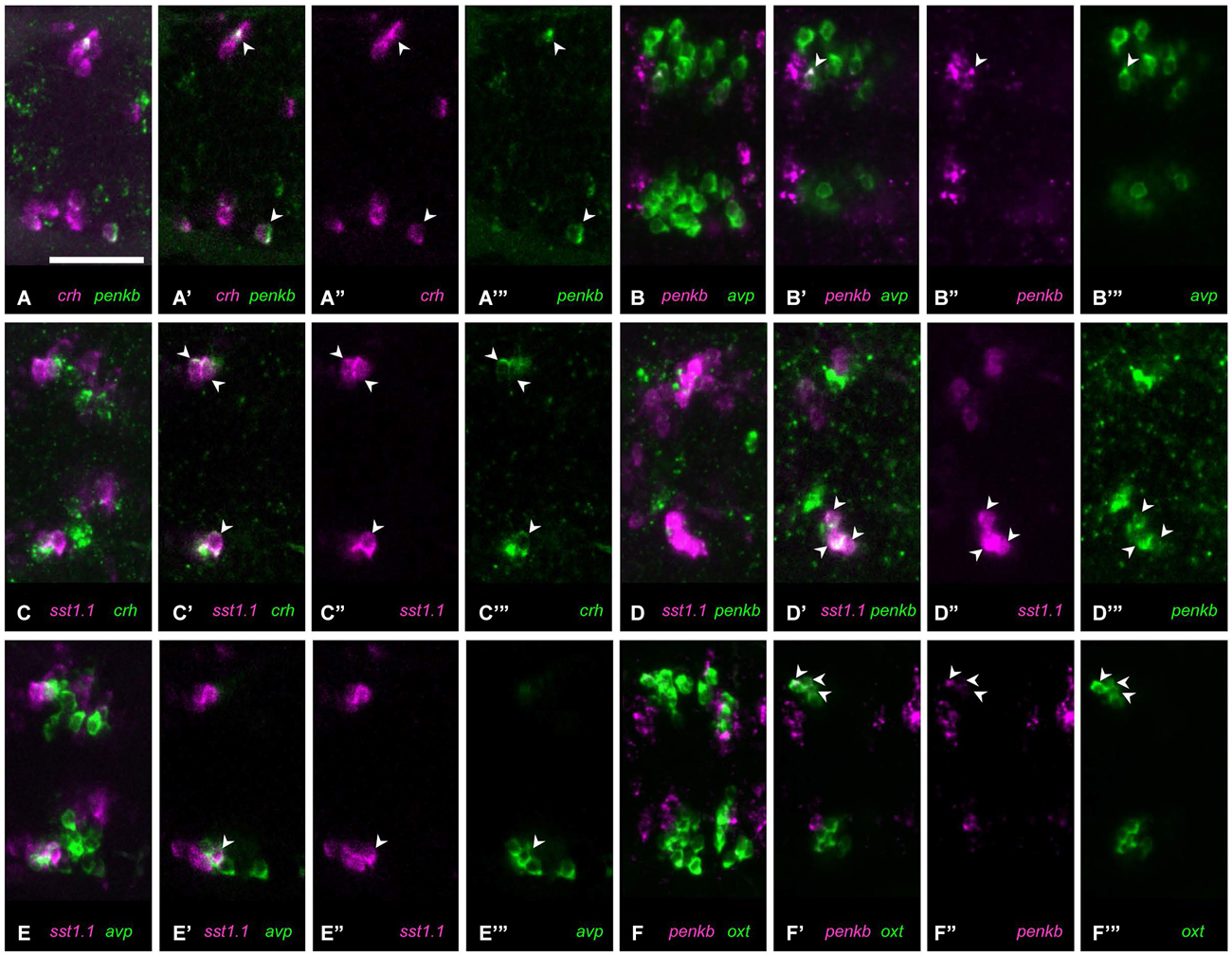
Figure 4. Some peptide combinations show low or moderate coexpression. (A) Coexpression ((A) maximum intensity projection; (A’) single plane) of crh (A”) and penkb (A”’) rarely occurs, but can be found in more than one cell. (B) In some animals, coexpression ((B) maximum intensity projection; (B’) single plane) of penkb (B”) and avp (B”’) can be found. (C) Moderate coexpression ((C) maximum intensity projection; (C’) single plane) of sst1.1 (C”) and crh (C”’) can be observed in some animals. (D) Few cells also show coexpression ((D) maximum intensity projection; (D’) single plane) of sst1.1 (D”) and penkb (D”’). (E) Coexpression is found in few cells ((E) maximum intensity projection; (E’) single plane) labeled for sst1.1 (E”) and avp (E”’). (F) In some animals, moderate coexpression ((F) maximum intensity projection; (F’) single plane) of penkb (F”) and oxt (F”’) can be found. Arrowheads mark coexpressing cells. Scale bar: 50 μm.
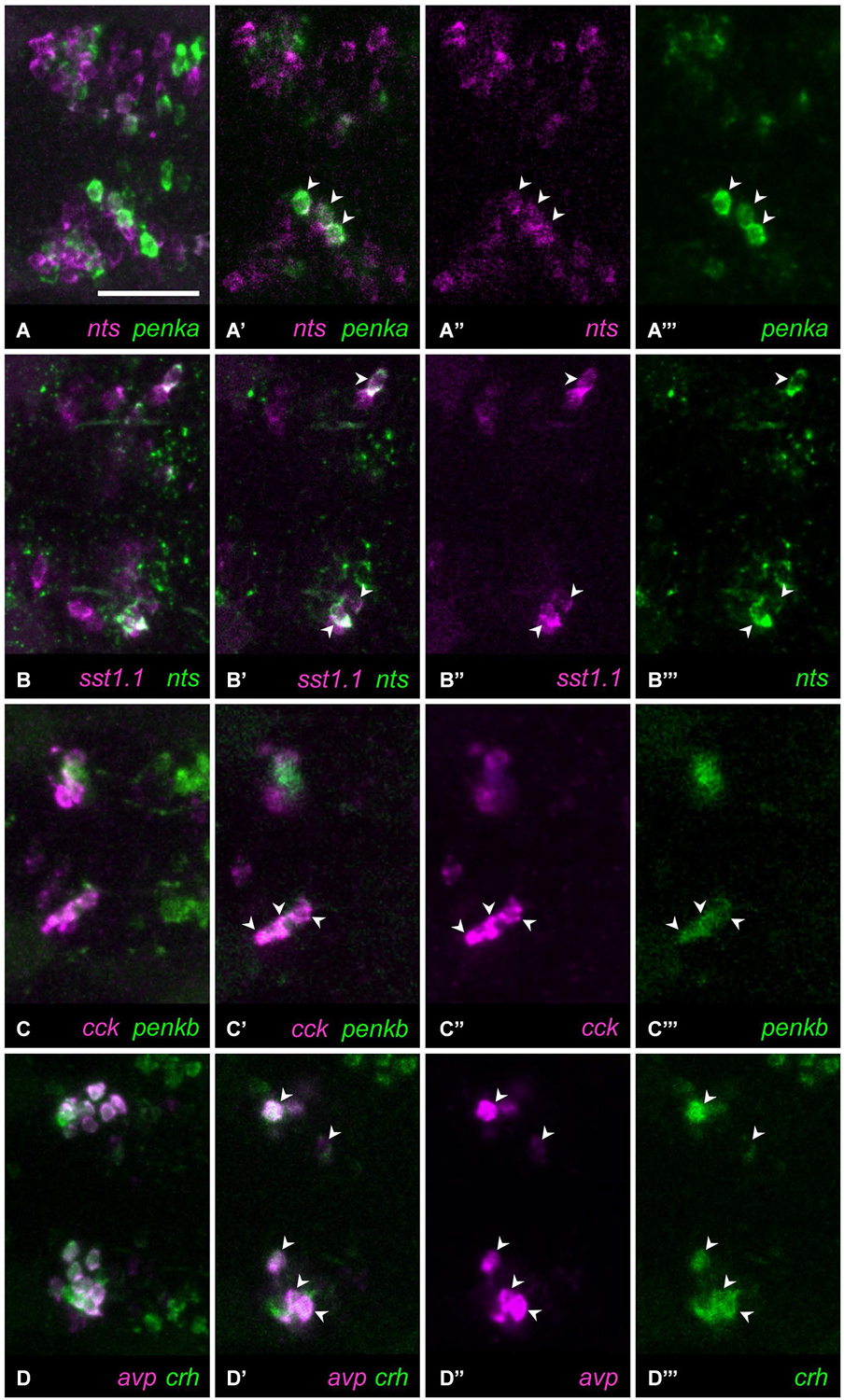
Figure 5. Four combinations of neuropeptides show higher coexpression. (A) In most animals, several cells show coexpression ((A) maximum intensity projection; (A’) single plane) of nts (A”) with penka (A”’). (B) In all animals, few cells show coexpression ((B) maximum intensity projection; (B’) single plane) of sst1.1 (B”) and nts (B”’). (C) High coexpression can be found ((C) maximum intensity projection; (C’) single plane) in the rostral clusters of cells expressing cck (C”) or penkb (C”’). (D) Consistently high coexpression ((D) maximum intensity projection; (D’) single plane) can be observed for the intermingled avp-positive (D”) and crh-positive clusters (D”’). Arrowheads mark coexpressing cells. Scale bar: 50 μm.
Consistent Coexpression in All Animals
Consistent coexpression in all animals analyzed was found for three peptide combinations. The combination of nts and sst1.1 showed consistent coexpression in few cells (Figures 5B–B”’, 5/5). High degree of coexpression was consistently found for the rostralmost clusters formed by cells producing penkb or cck (Figures 5C–C”’, 13/13 animals). Coexpression was also consistently high for the combination of avp and crh staining (Figures 5D–D”’, 19/19 animals). These results are summarized in Figure 6, in which we also indicate the range of coexpression observed (minimum and maximum degrees).
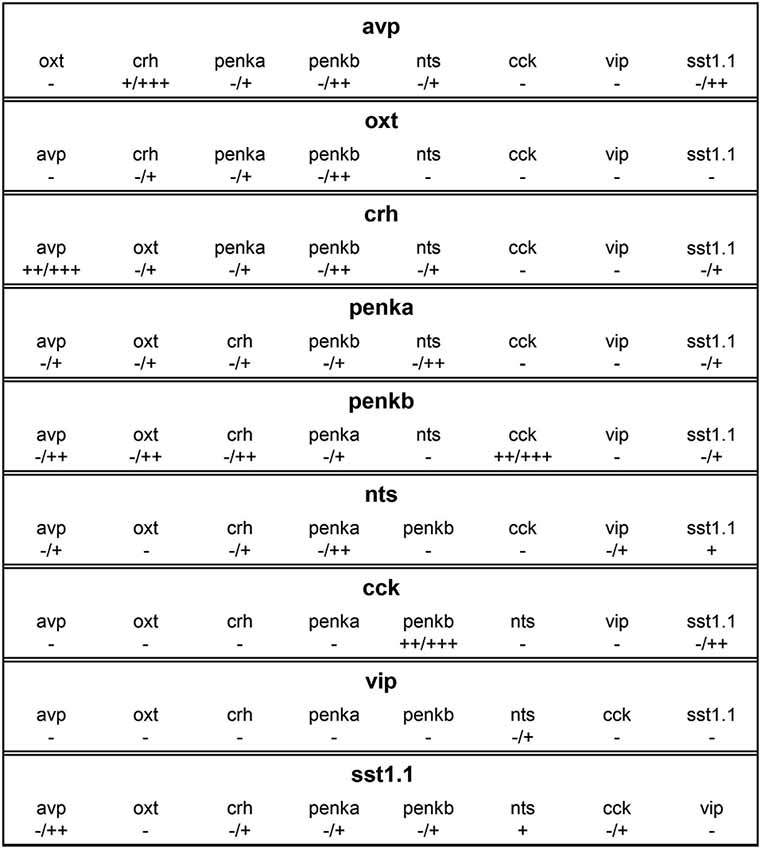
Figure 6. Overview of coexpression profiles of cells expressing avp, oxt, crh, penka, penkb, nts, cck, vip, or sst1.1. Coexpression degrees indicate complete absence (−), rare occurrence (+), low coexpression (++), or high coexpression (+++). In many combinations, coexpression was variable, and the minimum and maximum degrees are indicated (min/max). Differences between the coexpression of one peptide in one cell type and vice versa are caused by differences in cell cluster sizes.
Discussion
Our results show that many of the peptides produced by densely intermingled cells of the larval zebrafish NPO are not coexpressed. Occasional coexpression in one cell can be observed for other peptide staining combinations, and some combinations with low or moderate coexpression appear more frequently. Only three of the 36 combinations show consistent coexpression in all animals analyzed, reaching a high degree only for avp + crh and cck + penkb. It should be noted that the presence of RNA does not necessarily mean that the peptide will also be synthesized. For example, there is a distinct cluster of avp-expressing cells outside the NPO in the ventral hypothalamus, which can clearly be labeled by ISH, but not by immunohistochemistry (IHC; Eaton et al., 2008; Herget et al., 2014). Still, the information presented here about coexpressed peptide transcripts suggests the existence of subclasses of cell types which can have very different functions.
The large amount of information available on the degree of coexpression of different neuropeptides in the mammalian PVN allows comparisons of our results with those obtained in mammals. Interestingly, many peptide combinations show similar degrees of coexpression both in larval zebrafish and in mammals. The absence of coexpression of avp with oxt we observed is in line with rat data (Swanson and Sawchenko, 1983). The coexpression of avp with crh we found to vary between moderate and high levels was also shown to be low or high in the rat (Sawchenko et al., 1984b; Whitnall et al., 1985, 1987; Aubry et al., 1999; Arima et al., 2001; Simmons and Swanson, 2009), and was low in the mouse or sheep (Rivalland et al., 2005; Biag et al., 2012). Recently, it was found that only magnocellular CRH-positive cells coexpress AVP in the rat (Dabrowska et al., 2013). Coexpression of CRH and AVP was also found in other teleosts (Yulis and Lederis, 1987; Olivereau et al., 1988; Fryer, 1989). We found rare coexpression of oxt with penka, and low coexpression of oxt with penkb, and coexpression of OXT with ENK was also seen in the rat (Martin and Voigt, 1981; Rossier et al., 1983). OXT and SST do not overlap in the rat (Swanson and Sawchenko, 1983), and we saw the same absence of coexpression in the fish. We observed rare coexpression of crh with penka, and low coexpression of crh with penkb, while ENK coexpression with CRH seems to be higher in rats (Hökfelt et al., 1983; Ceccatelli et al., 1989; Pretel and Piekut, 1990), or sheep (Rivalland et al., 2005). However, one more recent study also found only low coexpression of ENK in CRH-producing cells in the rat (Dabrowska et al., 2013), which is more in line with our results. NTS and ENK are coexpressed in low degrees in the rat (Ceccatelli et al., 1989), and we also saw consistent coexpression of nts with penka, but not with penkb. It was reported that very few NTS cells coexpress VIP in the rat (Ceccatelli et al., 1989), and the coexpression of nts and vip was also rare here.
Other peptide coexpression profiles we observed in larval zebrafish deviate from previously established mammalian data. AVP and ENK are thought to be coexpressed in the rat (Martin and Voigt, 1981), and moderately coexpressed in the sheep (Rivalland et al., 2005), but penka was only rarely coexpressed with avp, and penkb showed only low coexpression with avp in our case. We did not find coexpression of avp with cck, but there are many such cells in the rat (Mezey et al., 1986). Our observation showed low coexpression of avp with sst1.1, but those are non-overlapping cell types in the rat (Swanson and Sawchenko, 1983). oxt coexpression with crh was rare here, but variably low or high in the rat (Sawchenko et al., 1984a; Dabrowska et al., 2013), and low in the mouse (Biag et al., 2012). In the zebrafish, one study reported colocalization of oxt and crh in the preoptic area (Chandrasekar et al., 2007), but with our increased resolution, we can clarify that those cells only reside in the same region, and are in fact intermingled. OXT and CCK are coexpressed in the rat (Vanderhaeghen et al., 1981; Bondy et al., 1989; Levin and Sawchenko, 1993), but we saw no such coexpression in the zebrafish. CRH coexpression with NTS was reported to be low (Sawchenko et al., 1984a) or moderate in the rat (Ceccatelli et al., 1989), but according to our results coexpression only occurs rarely. CRH and CCK are coexpressed in some cells in the rat (Mezey et al., 1986; Ceccatelli et al., 1989), but not at all in our data. CRH and VIP were reported to be coexpressed in low levels in the rat (Ceccatelli et al., 1989), but they are not coexpressed here. ENK and VIP are coexpressed in the rat (Hökfelt et al., 1987), but neither penka nor penkb overlap with vip in our results. NTS and CCK show very low coexpression in the rat (Ceccatelli et al., 1989), but no coexpression in our results. All observed differences can originate from differences in both species and age, since we compare larval zebrafish data with adult mammalian results. Changes in coexpression levels during continued development from the larval stage detailed here into adulthood can also be expected.
To our knowledge, several peptide combinations were never addressed as far as coexpression is concerned. avp coexpression with nts was not discussed before, and rarely occurs here. Also, crh coexpression with sst1.1 was not addressed before, and is rare here. We found that penka is not coexpressed with cck, which was not addressed before. Coexpression of penkb with cck was high, and is reported here for the first time. Similarly, coexpression of penka or penkb with sst1.1 was not reported before, and we found rare or low coexpression. Also the coexpression of sst1.1 and nts is shown here for the first time. Coexpression of cck and vip was never addressed before, and we found that they do not coexpress. We also report rare coexpression of cck with sst1.1 for the first time. Lastly, we also show that the expression of vip does not overlap with that of oxt or sst1.1.
The rarely observed coexpression in only few cells or few animals is a deviation from the otherwise complete absence of coexpression seen in most cells or animals for these combinations of stainings. Potential causes of such rare coexpression could be transitional stages in the development of these cells, mismapping due to lack of resolution, or stochastic variation. The confocal microscopy used allowed sufficient depth resolution to spatially separate cells in all three dimensions and therefore mismapping is unlikely.
The high degree of coexpression of avp and crh observed has immediate functional implications. Similar to CRH, AVP stimulates adrenocorticotropic hormone (ACTH) secretion (Gillies and Lowry, 1979; Rivier and Vale, 1983). A wide range of coexpression levels was reported in different fish species. Coexpression of avp and crh was very high, reported as 100%, in Catostomus, but absent in Anguilla (Yulis and Lederis, 1987; Olivereau and Olivereau, 1990). In humans, AVP/CRH coexpression increases with age, and a connection with stress has been implied (Raadsheer et al., 1993). In addition to CRH and AVP, CCK can also stimulate ACTH release, and CCK acting in concert with AVP has a similar ACTH-releasing potency as CRH alone (Mezey et al., 1986). We saw no coexpression of cck with avp or crh. In contrast to CRH, AVP, and CCK, OXT generally inhibits stress responses, while local, endogenous OXT potentiates hypothalamo-pituitary-adrenal (HPA) axis activity, suggesting a dual mechanism of OXT released within the PVN (Neumann, 2002). In our results, coexpression of oxt and crh was very rare, and only present in single cells when it occurred. The spatial proximity of the densely intermingled cells positive for oxt or crh suggests that local release of Oxt could have immediate effects on crh-producing cells. ENK, CRH, and AVP were found to be colocalized within the same secretory vesicles (Hisano et al., 1987), suggesting that coexpressed peptides are packaged into common vesicles and coreleased at the synapse. We found that either penka or penkb can be coexpressed in both crh-positive and avp-positive cells. The concerted action of coreleased CRH and ENK is thought to fine-tune stress regulation (Pretel and Piekut, 1990).
In the rat, CRH-positive innervation of the neurohypophysis was suggested by observations of CRH in neurohypophyseal terminals (Bloom et al., 1982). The coexpression of CRH in magnocellular cells innervating the neurohypophysis could allow direct CRH release to the general circulation. The stress axis is also affected by VIP (Westendorf et al., 1983; Tilders et al., 1984), and NTS (Gudelsky et al., 1989). In the parvocellular PVN, coexpression of NTS and CRH was found, and the fractions of CRH-positive cells that coexpress AVP or NTS form different discrete subsets (Sawchenko et al., 1984a). The reported segregation of cells expressing AVP or CRH in rats cannot be found in larval zebrafish, and vip is not coexpressed with crh here, but we did find coexpression of nts and crh.
The CRH-producing cells coexpressing AVP were reported to be stress-responsive, while the AVP-negative CRH-producing cells do not respond to stress (Whitnall, 1993). Under osmotic stress, ENK and CRH levels are increased, but restraint and swimming stress only elevated CRH, not ENK (Harbuz and Lightman, 1989). Such findings demonstrate the functional implications of coexpression differences. For a cell, switching on the coexpression of another peptide is an elegant strategy to change its function without the need for major structural rearrangement of input or target projections, which could impose much stronger metabolic demands. Neurochemical switching in response to alterations in environmental conditions has been suggested as a relevant biological phenomenon in PVN neurons (Kiss, 1988; Swanson, 1991). Dynamic adaptation of neuroendocrine transcription to altered supply or demand for neuropeptides has also been suggested in zebrafish larvae (Kurrasch et al., 2009). With the coexpression profiles established here for the larval zebrafish under basal conditions, alterations triggered by environmental challenges can be studied in a model organism that has important advantages for the analysis of neural structure and function in intact and genetically tractable animals.
Conflict of Interest Statement
The Guest Associate Editor Gonzalo Alvarez-Bolado and Review Editor Matthias Carl declare that, despite being affiliated to the same institution as author Ulrich Herget, the review process was handled objectively and no conflict of interest exists. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful for helpful comments and critical discussion of the data by Rodrigo De Marco, Jose Arturo Gutierrez-Triana, and Colette vom Berg. We appreciate the suggestions and critical reading of the manuscript by Mario Wullimann. We also thank Regina Singer for technical assistance, Gabi Shoeman, Christiane Brandel and Angelika Schoell for fish care, and Mona Friedrich for additional staining and imaging.
References
Arima, H., House, S. B., Gainer, H., and Aguilera, G. (2001). Direct stimulation of arginine vasopressin gene transcription by cAMP in parvocellular neurons of the paraventricular nucleus in organotypic cultures. Endocrinology 142, 5027–5030. doi: 10.1210/endo.142.11.8595
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Aubry, J. M., Bartanusz, V., Jezova, D., Belin, D., and Kiss, J. Z. (1999). Single stress induces long-lasting elevations in vasopressin mRNA levels in CRF hypophysiotrophic neurones, but repeated stress is required to modify AVP immunoreactivity. J. Neuroendocrinol. 11, 377–384. doi: 10.1046/j.1365-2826.1999.00338.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Biag, J., Huang, Y., Gou, L., Hintiryan, H., Askarinam, A., Hahn, J. D., et al. (2012). Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: a study of immunostaining and multiple fluorescent tract tracing. J. Comp. Neurol. 520, 6–33. doi: 10.1002/cne.22698
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bloom, F. E., Battenberg, E. L., Rivier, J., and Vale, W. (1982). Corticotropin releasing factor (CRF): immunoreactive neurones and fibers in rat hypothalamus. Regul. Pept. 4, 43–48. doi: 10.1016/0167-0115(82)90107-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bondy, C. A., Whitnall, M. H., Brady, L. S., and Gainer, H. (1989). Coexisting peptides in hypothalamic neuroendocrine systems: some functional implications. Cell. Mol. Neurobiol. 9, 427–446. doi: 10.1007/bf00712791
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Burlet, A., Tonon, M. C., Tankosic, P., Coy, D., and Vaudry, H. (1983). Comparative immunocytochemical localization of corticotropin releasing factor (CRF-41) and neurohypophysial peptides in the brain of Brattleboro and Long-Evans rats. Neuroendocrinology 37, 64–72. doi: 10.1159/000123517
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ceccatelli, S., Eriksson, M., and Hökfelt, T. (1989). Distribution and coexistence of corticotropin-releasing factor-, neurotensin-, enkephalin-, cholecystokinin-, galanin- and vasoactive intestinal polypeptide/peptide histidine isoleucine-like peptides in the parvocellular part of the paraventricular nucleus. Neuroendocrinology 49, 309–323. doi: 10.1159/000125133
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chandrasekar, G., Lauter, G., and Hauptmann, G. (2007). Distribution of corticotropin-releasing hormone in the developing zebrafish brain. J. Comp. Neurol. 505, 337–351. doi: 10.1002/cne.21496
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dabrowska, J., Hazra, R., Ahern, T. H., Guo, J. D., McDonald, A. J., Mascagni, F., et al. (2011). Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect. Psychoneuroendocrinology 36, 1312–1326. doi: 10.1016/j.psyneuen.2011.03.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dabrowska, J., Hazra, R., Guo, J. D., Dewitt, S., and Rainnie, D. G. (2013). Central CRF neurons are not created equal: phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Front. Neurosci. 7:156. doi: 10.3389/fnins.2013.00156
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Devos, N., Deflorian, G., Biemar, F., Bortolussi, M., Martial, J. A., Peers, B., et al. (2002). Differential expression of two somatostatin genes during zebrafish embryonic development. Mech. Dev. 115, 133–137. doi: 10.1016/s0925-4773(02)00082-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Eaton, J. L., Holmqvist, B., and Glasgow, E. (2008). Ontogeny of vasotocin-expressing cells in zebrafish: selective requirement for the transcriptional regulators orthopedia and single-minded 1 in the preoptic area. Dev. Dyn. 237, 995–1005. doi: 10.1002/dvdy.21503
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Fryer, J. N. (1989). Neuropeptides regulating the activity of goldfish corticotropes and melanotropes. Fish Physiol. Biochem. 7, 21–27. doi: 10.1007/bf00004686
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gillies, G., and Lowry, P. (1979). Corticotrophin releasing factor may be modulated vasopressin. Nature 278, 463–464. doi: 10.1038/278463a0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gudelsky, G. A., Berry, S. A., and Meltzer, H. Y. (1989). Neurotensin activates tuberoinfundibular dopamine neurons and increases serum corticosterone concentrations in the rat. Neuroendocrinology 49, 604–609. doi: 10.1159/000125176
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Harbuz, M. S., and Lightman, S. L. (1989). Responses of hypothalamic and pituitary mRNA to physical and psychological stress in the rat. J. Endocrinol. 122, 705–711. doi: 10.1677/joe.0.1220705
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Herget, U., Wolf, A., Wullimann, M. F., and Ryu, S. (2014). Molecular neuroanatomy and chemoarchitecture of the neurosecretory preoptic-hypothalamic area in zebrafish larvae. J. Comp. Neurol. 522, 1542–1564. doi: 10.1002/cne.23480
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hisano, S., Tsuruo, Y., Katoh, S., Daikoku, S., Yanaihara, N., and Shibasaki, T. (1987). Intragranular colocalization of arginine vasopressin and methionine-enkephalin-octapeptide in CRF-axons in the rat median eminence. Cell Tissue Res. 249, 497–507. doi: 10.1007/bf00217321
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hökfelt, T., Fahrenkrug, J., Ju, G., Ceccatelli, S., Tsuruo, Y., Meister, B., et al. (1987). Analysis of peptide histidine-isoleucine/vasoactive intestinal polypeptide-immunoreactive neurons in the central nervous system with special reference to their relation to corticotropin releasing factor- and enkephalin-like immunoreactivities in the paraventricular hypothalamic nucleus. Neuroscience 23, 827–857. doi: 10.1016/0306-4522(87)90162-X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hökfelt, T., Fahrenkrug, J., Tatemoto, K., Mutt, V., Werner, S., Hulting, A. L., et al. (1983). The PHI (PHI-27)/corticotropin-releasing factor/enkephalin immunoreactive hypothalamic neuron: possible morphological basis for integrated control of prolactin, corticotropin, and growth hormone secretion. Proc. Natl. Acad. Sci. U S A 80, 895–898. doi: 10.1073/pnas.80.3.895
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kiss, J. Z. (1988). Dynamism of chemoarchitecture in the hypothalamic paraventricular nucleus. Brain Res. Bull. 20, 699–708. doi: 10.1016/0361-9230(88)90080-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kurrasch, D. M., Nevin, L. M., Wong, J. S., Baier, H., and Ingraham, H. A. (2009). Neuroendocrine transcriptional programs adapt dynamically to the supply and demand for neuropeptides as revealed in NSF mutant zebrafish. Neural Dev. 4:22. doi: 10.1186/1749-8104-4-22
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lauter, G., Söll, I., and Hauptmann, G. (2011). Multicolor fluorescent in situ hybridization to define abutting and overlapping gene expression in the embryonic zebrafish brain. Neural Dev. 6:10. doi: 10.1186/1749-8104-6-10
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Levin, M. C., and Sawchenko, P. E. (1993). Neuropeptide co-expression in the magnocellular neurosecretory system of the female rat: evidence for differential modulation by estrogen. Neuroscience 54, 1001–1018. doi: 10.1016/0306-4522(93)90591-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Löhr, H., Ryu, S., and Driever, W. (2009). Zebrafish diencephalic A11-related dopaminergic neurons share a conserved transcriptional network with neuroendocrine cell lineages. Development 136, 1007–1017. doi: 10.1242/dev.033878
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Machluf, Y., Gutnick, A., and Levkowitz, G. (2011). Development of the zebrafish hypothalamus. Ann. N Y Acad. Sci. 1220, 93–105. doi: 10.1111/j.1749-6632.2010.05945.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Martin, R., and Voigt, K. H. (1981). Enkephalins co-exist with oxytocin and vasopressin in nerve terminals of rat neurohypophysis. Nature 289, 502–504. doi: 10.1038/289502a0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mezey, E., Reisine, T. D., Skirboll, L., Beinfeld, M., and Kiss, J. Z. (1985). Cholecystokinin in the medial parvocellular subdivision of the paraventricular nucleus. Co-existence with corticotropin-releasing hormone. Ann. N Y Acad. Sci. 448, 152–156. doi: 10.1111/j.1749-6632.1985.tb29915.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mezey, E., Reisine, T. D., Skirboll, L., Beinfeld, M., and Kiss, J. Z. (1986). Role of cholecystokinin in corticotropin release: coexistence with vasopressin and corticotropin-releasing factor in cells of the rat hypothalamic paraventricular nucleus. Proc. Natl. Acad. Sci. U S A 83, 3510–3512. doi: 10.1073/pnas.83.10.3510
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Neumann, I. D. (2002). Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo-pituitary-adrenal axis. Prog. Brain Res. 139, 147–162. doi: 10.1016/s0079-6123(02)39014-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Olivereau, M., Moons, L., Olivereau, J., and Vandesande, F. (1988). Coexistence of corticotropin-releasing factor-like immunoreactivity and vasotocin in perikarya of the preoptic nucleus in the eel. Gen. Comp. Endocrinol. 70, 41–48. doi: 10.1016/0016-6480(88)90092-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Olivereau, M., and Olivereau, J. (1990). Effect of pharmacological adrenalectomy on corticotropin-releasing factor-like and arginine vasotocin immunoreactivities in the brain and pituitary of the eel: immunocytochemical study. Gen. Comp. Endocrinol. 80, 199–215. doi: 10.1016/0016-6480(90)90165-i
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Piekut, D. T., and Joseph, S. A. (1986). Co-existence of CRF and vasopressin immunoreactivity in parvocellular paraventricular neurons of rat hypothalamus. Peptides 7, 891–898. doi: 10.1016/0196-9781(86)90111-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pretel, S., and Piekut, D. (1990). Coexistence of corticotropin-releasing factor and enkephalin in the paraventricular nucleus of the rat. J. Comp. Neurol. 294, 192–201. doi: 10.1002/cne.902940204
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Raadsheer, F. C., Sluiter, A. A., Ravid, R., Tilders, F. J., and Swaab, D. F. (1993). Localization of corticotropin-releasing hormone (CRH) neurons in the paraventricular nucleus of the human hypothalamus; age-dependent colocalization with vasopressin. Brain Res. 615, 50–62. doi: 10.1016/0006-8993(93)91113-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rivalland, E. T., Iqbal, J., Clarke, I. J., Turner, A. I., and Tilbrook, A. J. (2005). Co-localization and distribution of corticotrophin-releasing hormone, arginine vasopressin and enkephalin in the paraventricular nucleus of sheep: a sex comparison. Neuroscience 132, 755–766. doi: 10.1016/j.neuroscience.2005.01.045
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rivier, C., and Vale, W. (1983). Interaction of corticotropin-releasing factor and arginine vasopressin on adrenocorticotropin secretion in vivo. Endocrinology 113, 939–942. doi: 10.1210/endo-113-3-939
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rossier, J., Liston, D., Patey, G., Chaminade, M., Foutz, A. S., Cupo, A., et al. (1983). The enkephalinergic neuron: implications of a polyenkephalin precursor. Cold Spring Harb. Symp. Quant. Biol. 48(Pt. 1), 393–404. doi: 10.1101/sqb.1983.048.01.043
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Roth, K. A., Weber, E., Barchas, J. D., Chang, D., and Chang, J. K. (1983). Immunoreactive dynorphin-(1–8) and corticotropin-releasing factor in subpopulation of hypothalamic neurons. Science 219, 189–191. doi: 10.1126/science.6129700
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sawchenko, P. E. (1987). Evidence for differential regulation of corticotropin-releasing factor and vasopressin immunoreactivities in parvocellular neurosecretory and autonomic-related projections of the paraventricular nucleus. Brain Res. 437, 253–263. doi: 10.1016/0006-8993(87)91641-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sawchenko, P. E., Swanson, L. W., and Vale, W. W. (1984a). Corticotropin-releasing factor: co-expression within distinct subsets of oxytocin-, vasopressin- and neurotensin-immunoreactive neurons in the hypothalamus of the male rat. J. Neurosci. 4, 1118–1129.
Sawchenko, P. E., Swanson, L. W., and Vale, W. W. (1984b). Co-expression of corticotropin-releasing factor and vasopressin immunoreactivity in parvocellular neurosecretory neurons of the adrenalectomized rat. Proc. Natl. Acad. Sci. U S A 81, 1883–1887. doi: 10.1073/pnas.81.6.1883
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Simmons, D. M., and Swanson, L. W. (2009). Comparison of the spatial distribution of seven types of neuroendocrine neurons in the rat paraventricular nucleus: toward a global 3D model. J. Comp. Neurol. 516, 423–441. doi: 10.1002/cne.22126
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Swanson, L. W. (1991). Biochemical switching in hypothalamic circuits mediating responses to stress. Prog. Brain Res. 87, 181–200. doi: 10.1016/S0079-6123(08)63052-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Swanson, L. W., and Sawchenko, P. E. (1983). Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu. Rev. Neurosci. 6, 269–324. doi: 10.1146/annurev.ne.06.030183.001413
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Swanson, L. W., Sawchenko, P. E., and Lind, R. W. (1986). Regulation of multiple peptides in CRF parvocellular neurosecretory neurons: implications for the stress response. Prog. Brain Res. 68, 169–190. doi: 10.1016/s0079-6123(08)60238-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Swanson, L. W., and Simmons, D. M. (1989). Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: a hybridization histochemical study in the rat. J. Comp. Neurol. 285, 413–435. doi: 10.1002/cne.902850402
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tilders, F., Tatemoto, K., and Berkenbosch, F. (1984). The intestinal peptide PHI-27 potentiates the action of corticotropin-releasing factor on ACTH release from rat pituitary fragments in vitro. Endocrinology 115, 1633–1635. doi: 10.1210/endo-115-4-1633
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Unger, J. L., and Glasgow, E. (2003). Expression of isotocin-neurophysin mRNA in developing zebrafish. Gene Expr. Patterns 3, 105–108. doi: 10.1016/S1567-133X(02)00064-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Vanderhaeghen, J. J., Lotstra, F., Vandesande, F., and Dierickx, K. (1981). Coexistence of cholecystokinin and oxytocin-neurophysin in some magnocellular hypothalamo-hypophyseal neurons. Cell Tissue Res. 221, 227–231. doi: 10.1007/bf00216585
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Westendorf, J. M., Phillips, M. A., and Schonbrunn, A. (1983). Vasoactive intestinal peptide stimulates hormone release from corticotropic cells in culture. Endocrinology 112, 550–557. doi: 10.1210/endo-112-2-550
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Westerfield, M. (2000). The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio). 4th Edn. Eugene: University of Oregon Press.
Whitnall, M. H. (1993). Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog. Neurobiol. 40, 573–629. doi: 10.1016/0301-0082(93)90035-Q
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Whitnall, M. H., and Gainer, H. (1988). Major pro-vasopressin-expressing and pro-vasopressin-deficient subpopulations of corticotropin-releasing hormone neurons in normal rats. Differential distributions within the paraventricular nucleus. Neuroendocrinology 47, 176–180. doi: 10.1159/000124910
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Whitnall, M. H., Mezey, E., and Gainer, H. (1985). Co-localization of corticotropin-releasing factor and vasopressin in median eminence neurosecretory vesicles. Nature 317, 248–250. doi: 10.1038/317248a0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Whitnall, M. H., Smyth, D., and Gainer, H. (1987). Vasopressin coexists in half of the corticotropin-releasing factor axons present in the external zone of the median eminence in normal rats. Neuroendocrinology 45, 420–424. doi: 10.1159/000124768
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wolf, A., and Ryu, S. (2013). Specification of posterior hypothalamic neurons requires coordinated activities of Fezf2, Otp, Sim1a and Foxb1.2. Development 140, 1762–1773. doi: 10.1242/dev.085357
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Yulis, C. R., and Lederis, K. (1987). Co-localization of the immunoreactivities of corticotropin-releasing factor and arginine vasotocin in the brain and pituitary system of the teleost Catostomus commersoni. Cell Tissue Res. 247, 267–273. doi: 10.1007/BF00218308
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: neuroendocrine system, hypothalamus, preoptic region, paraventricular nucleus, zebrafish, coexpression
Citation: Herget U and Ryu S (2015) Coexpression analysis of nine neuropeptides in the neurosecretory preoptic area of larval zebrafish. Front. Neuroanat. 9:2. doi: 10.3389/fnana.2015.00002
Received: 09 November 2014; Accepted: 07 January 2015;
Published online: 12 February 2015.
Edited by:
Gonzalo Alvarez-Bolado, University of Heidelberg, GermanyCopyright © 2015 Herget and Ryu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Soojin Ryu, Developmental Genetics of the Nervous System, Max Planck Institute for Medical Research, Jahnstr. 29, 69120 Heidelberg, Germany e-mail: soojin.ryu@mpimf-heidelberg.mpg.de
 Ulrich Herget
Ulrich Herget Soojin Ryu
Soojin Ryu