-
PDF
- Split View
-
Views
-
Cite
Cite
Zdenek Berger, Evangelia K. Ttofi, Claire H. Michel, Matthieu Y. Pasco, Sean Tenant, David C. Rubinsztein, Cahir J. O'Kane, Lithium rescues toxicity of aggregate-prone proteins in Drosophila by perturbing Wnt pathway, Human Molecular Genetics, Volume 14, Issue 20, 15 October 2005, Pages 3003–3011, https://doi.org/10.1093/hmg/ddi331
Close - Share Icon Share
Abstract
We have previously shown that lithium can protect against the polyglutamine toxicity of the Huntington's disease mutation in cell models. Here, we demonstrate for the first time in vivo that lithium can protect against the toxicity caused by aggregate-prone proteins with either polyglutamine or polyalanine expansions in Drosophila. We also show that these protective effects can be partly accounted for by lithium acting through the Wnt/Wg pathway, as a GSK3β-specific inhibitor and overexpression of dTCF also mediate protective effects. Our data suggest that lithium deserves serious consideration for further studies as a therapeutic for polyglutamine diseases, particularly as it is an established drug that has been used for several decades for chronic treatment of affective disorders.
INTRODUCTION
Codon reiteration diseases are a large group of human conditions caused by abnormally long polyglutamine or polyalanine tracts in different proteins, often resulting in aggregate formation. Polyglutamine expansions are seen in Huntington's disease (HD) and eight other conditions including spinocerebellar ataxias types 1, 2, 3, 6, 7 and 17 (1). Expansions or duplications of polyalanine tracts leading to tracts of up to 29 repeats cause nine known diseases (2), including oculopharyngeal muscular dystrophy (3).
Previously, lithium has been shown to protect against toxicity in cell models of HD (4). However, lithium treatment of a mouse model of HD resulted only in very modest and equivocal benefit (5). This might be explained by inappropriate dose as lithium has a very narrow therapeutic window (6), a short half-life in mice compared with humans (7) and lithium levels were not monitored in this mouse trial (5).
Given the promising cell model data, we felt that further studies of this well-characterized drug were warranted. We used Drosophila for these studies as they are cheaper than mice, there is a large resource of mutant and transgenic stocks that can allow mechanisms of phenotypes to be dissected, they allow the potential of analysing compounds at various doses easily and we can readily confirm therapeutic effects across a panel of related disease models. Furthermore, positive therapeutic effects in Drosophila polyglutamine diseases have been highly predictive of success in subsequent mouse studies (8,9).
RESULTS
Lithium treatment protects against polyglutamine-mediated toxicity
We first treated a Drosophila model of HD (10) (gmr-httQ120) expressing the N-terminal part of mutant huntingtin with expanded polyglutamines, which is characterized by adult-onset time-dependent loss of visible rhabdomeres (light gathering parts of photoreceptor cells).
We carefully considered the most appropriate read-out to analyse Q120-induced degeneration in this model. We analysed toluidine blue-stained plastic sections of gmr-httQ120 Drosophila eyes and characterized the rhabdomere loss in detail. Consistent with a previous report (10), we observed loss of rhabdomeres followed by degeneration of the eyes, which manifested as structural disorganization but only subtle and low levels of photoreceptor loss. As we have observed some variability in the eye disorganization and it is difficult to quantify structural changes, we have used the pseudopupil technique as a quantifiable read-out. This method assesses the number of visible rhabdomeres by light microscopy and has been widely used to quantify the toxicity of proteins with long polyglutamines in the fly eye (8–12). The loss of visible rhabdomeres in this model preceded photoreceptor death/loss assessed by toluidine blue staining of plastic sections and is a progressive degenerative phenotype seen only in flies expressing the mutant transgene (not wild-type) and was not present at eclosion (10) (data not shown). The photoreceptor dropout assessed by pseudopupil analysis is much more dramatic and far easier to quantify reliably in large numbers of flies than the minor loss seen by toluidine blue-stained sections. This pseudopupil phenotype is therefore probably a sign of dysfunction rather than death—this may be particularly valuable, because neuronal dysfunction may be very important in HD (13). In addition, degeneration/loss of rhabdomeres in this model does not result in any visible external abnormalities, such as rough eye, even at late stages. For all of the aforementioned reasons, we selected pseudopupil analysis for subsequent experiments in the gmr-httQ120 flies.
Lithium treatment with concentrations in fly food similar to those observed in lithium-treated patients (0.5–1.5 mm) (14) resulted in a dramatic increase of visible rhabdomeres at 2 days after eclosion (Fig. 1A and B) (Supplementary Material, Fig. S1) and a similar effect was observed at a later time point (Fig. 1C).
To test whether lithium could also protect against toxicity in Drosophila expressing other polyglutamine expansion constructs, we studied flies expressing an isolated 108 residue polyglutamine stretch tagged with a myc/flag epitope (15) (Q108) in neurons. Flies homozygous for the neuronal driver elav-GAL4 were crossed to flies of genotype UAS-Q108/TM3 and the percentage of flies with the UAS-Q108 transgene was evaluated. Expression of Q108 in neurons caused dramatic pre-adult lethality, which was partially rescued by lithium treatment (Fig. 1D). Expression of a similar Q22 protein, which carried a glutamine repeat typical of wild-type alleles of aggregate-prone proteins, did not have any effect on survival to adulthood. The percentage of flies carrying the Q22 transgene in this cross is higher than 50% due to deleterious effects of the TM3 balancer (Fig. 1E). Also, the lithium rescue of the Q108 lethality (measuring ratio Q108:TM3 flies) is a specific effect on Q108, as the ratio of Q22:TM3 flies remains unchanged in the presence of lithium (Fig. 1E).
We have generated a fly model expressing enhanced green fluorescent protein tagged to 97 glutamines and a nuclear localization signal (NLS) (NLS-Q97). All the NLS-Q97 lines examined exhibited complete lethality when expressed using a gmr-GAL4 driver at 29°C, whereas no phenotype was seen in flies expressing an otherwise identical construct with a 25-glutamine repeat (NLS-Q25). Expression of NLS-Q97 using an ey-GAL4 driver resulted in the formation of abnormal eyes, and occasionally eyes were completely absent (Fig. 1F). Some Q97 lines exhibited pre-adult lethality with ey-GAL4. We observed this phenomenon with different Q97 insertions, whereas no obvious pre-adult lethality or abnormal eyes were observed with any NLS-Q25 insertions (see Materials and Methods) (Fig. 1F). Note that the lethality of NLS-Q97 when expressed with these two GAL4 drivers in the eyes is similar to the previously described toxicity of Q108, probably because of the expression in tissues other than eyes (15). The higher toxicity of our NLS-polyQ model (compared with Q108 flies) is consistent with the idea that nuclear localized polyQs are more toxic (16).
We then tested whether lithium was effective against toxicity in the NLS-Q97 fly model. Lithium significantly reduced the proportion of NLS-Q97 flies with rough eyes (Fig. 1G). Therefore, lithium can rescue toxicity in different Drosophila polyglutamine models.
Inhibition of GSK3β/shaggy and activation of TCF-responsive genes protect against polyglutamine-mediated toxicity
Lithium acts on various pathways in the cell, including the Wnt/Wg pathway by inhibiting GSK3β/shaggy at therapeutically relevant doses (14,17). Accordingly, we first used the GSK3β-specific inhibitor (AR-A014418), which moderately but consistently increased the number of visible rhabdomeres in the gmr-httQ120 HD fly model (Fig. 2A). Similarly, AR-A014418 decreased polyglutamine toxicity in two other models, Q127 (18) and NLS-Q97, as assessed by the percentage of flies with rough eyes (Fig. 2B and C). Thus, at least part of the lithium effect can be accounted for by GSK3β/shaggy inhibition.
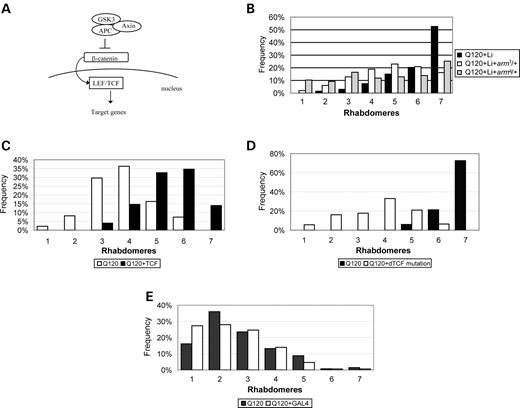
GSK3β/shaggy inhibition leads to increased levels of β-catenin/Armadillo (Arm) (Fig. 3A). Overexpression of Arm without any polyQ transgene disrupts Drosophila eye morphology (19) (even using weak GAL4 drivers, data not shown), precluding experiments testing whether Arm overexpression could suppress polyglutamine toxicity. Accordingly, we tested the effects of two different loss-of-function arm alleles on polyglutamine toxicity. Although a heterozygous loss-of- function arm allele (i.e. absence of one functional copy of arm+) had no effect on polyQ-mediated neurodegeneration (without any drug, data not shown), it significantly decreased the lithium protective effect (Fig. 3B). Similar results were seen with two different loss-of-function arm alleles. Thus, partial loss of Arm may not influence polyglutamine toxicity under basal conditions but does limit the ability of lithium to protect against polyglutamine toxicity, suggesting that part of the lithium effect is mediated through the Wnt pathway.
Inhibition of GSK3β/shaggy increases levels of β-catenin/Arm, which translocates to the nucleus, where it then enhances the activity of TCF/LEF transcription factors (20). In agreement with the model that upregulation of the Wnt/Wg pathway is protective against polyglutamine toxicity overexpression of the Drosophila TCF homologue, dTCF, decreased the toxicity of gmr-httQ120 (Fig. 3C) (Supplementary Material, Fig. S1), whereas a heterozygous dTCF mutation enhanced huntingtin-mediated toxicity (Fig. 3D) (Supplementary Material, Fig. S1). The heterozygous dTCF mutation did not cause any degeneration in flies expressing N-terminal fragment of wild-type huntingtin (data not shown).
Lithium and GSK3β protect against toxicity caused by long polyalanines
To test whether lithium treatment and manipulation of the Wnt pathway could also rescue the toxicity caused by long poly alanines, we used a Drosophila model expressing NLS-A37 (Z. Berger et al., submitted for publication). This model expresses a construct identical to the NLS-Q97 flies, except that the stretch of 97 glutamines is replaced by 37 uninterrupted alanines. Independent insertions of NLS-A37 cause toxicity in a variety of tissues when expressed at similar levels to NLS-A7 constructs, which are not toxic (data not shown). Lithium and AR-A014418 treatments and dTCF overexpression, each dramatically decreased polyalanine-induced toxicity, as assessed by the frequencies of flies with rough eyes and survival to adulthood (Fig. 4A–E), suggesting that manipulation of the Wnt pathway can also be beneficial in other diseases caused by aggregate-prone proteins.
DISCUSSION
We show that lithium attenuates toxicity of two aggregate-prone proteins, polyglutamines and polyalanines, in vivo in Drosophila. Thus, lithium may protect against a range of codon reiteration mutations. Lithium is known to inhibit both GSK3β and inositol monophosphatase (14,21–26). Here, we explored whether at least some of the protective effect seen with lithium could be attributed to Wnt pathway perturbation and whether perturbation of the Wnt pathway alone could lead to decreased toxicity in vivo.
Using both pharmacological and genetic approaches, we showed that the lithium protective effect was mediated at least partly through the inhibition of GSK3β. This was supported by a reduction in the effect of lithium when one of the two functional copies of armadillo+ (arm) was lacking. We saw a consistent reduction in the lithium effect in our experiments with two different arm alleles. Although there were minor differences between the different arm alleles, these were very small compared with the differences between the arm alleles and the HD flies on wild-type arm backgrounds. The small differences between the arm alleles were likely due to the fact that these are different alleles. In addition, polyglutamine and polyalanine toxicity was decreased by treatment with a specific GSK3β inhibitor, AR-014418. We used AR-014418 as one of the most specific GSK3β inhibitors (27) and does not inhibit other kinases (such as CDKs), compared with other commercially available drugs such as SB 216763 and SB 415286 (28). The effects of the Wingless/Wnt pathway have been extensively studied in flies and it is well accepted that the inhibition of shaggy (GSK3β) (which can be mediated in flies by lithium) (23–26) leads to elevated levels of armadillo, resulting in turn in increased levels of TCF signalling (29–36). Consistent with these pathway predictions, we showed that up-regulation of TCF transcription by overexpression of TCF also decreased toxicity of both long polyglutamines and polyalanines. Thus, perturbation of the Wnt pathway protects against toxicity of two aggregate-prone proteins. However, our data do not rule out a contribution of other pathways (for example, inositol monophosphatase) towards the lithium protective effect. Indeed, we have recently shown that the inhibition of inositol monosphatase may also be beneficial in the context of aggregate-prone proteins (Sarkar et al., submitted for publication).
We used Drosophila eyes as a model system because this enables easy quantification of neurodegeneration. We used two different read-outs in Drosophila eyes, pseudopupil in the HD model (gmr-httQ120) and rough/small eyes in flies expressing other polyglutamine and polyalanine constructs and in addition, we assessed survival. Thus, we have supported our findings in various models and using different read-outs, which represent a more rigorous approach than often previously reported (8,11,12).
Our results are consistent with our previously published data in cell models (4), where we reported the effect of lithium and a GSK3β-specific inhibitor in cell models of HD and characterized changes in aggregation (4). These studies confirmed that GSK3β inhibition did not act by reducing aggregation. We did not quantify the effects on aggregation in our Drosophila models because previous studies have shown that the suppression of polyglutamine toxicity can occur even in the absence of detectable changes in the levels of aggregation in Drosophila models (37,38), Furthermore, small changes in aggregation are not easily quantifiable in Drosophila models compared with mammalian cell culture (39–41) (data not shown).
Suppression or enhancement of a phenotype by heterozygous mutations is most likely to occur when the levels of the affected gene product are limiting for that phenotype. Loss of one of the two functional copies of arm+ reduced the rescue of gmr-Q120 toxicity by lithium but did not enhance the severity of gmr-Q120 toxicity in the absence of lithium. This implies that Arm is limiting only during lithium treatment (when GSK3β inhibition will reduce Arm degradation), and also that at least part of the lithium effect requires Arm. The opposite effects on gmr-Q120 toxicity, of either increasing TCF expression or by loss of one of the two functional copies of TCF+, imply that TCF counteracts the toxic effects of gmr-Q120 and that TCF levels are limiting for this effect even in the absence of lithium.
Wood and Morton (5) described effects of lithium in the R6/2 HD mouse model. Unfortunately, their paper does not allow definitive conclusions about the possible therapeutic efficacy of lithium in HD. They tested effects of lithium on survival, weight gain and rotarod performance. When lithium treatment was initiated pre-symptomatically, no beneficial effects were seen on any of these parameters. Indeed, in this group, lithium enhanced the weight loss and resulted in a tendency towards decreased survival. Post-symptomatic treatment had no clear effect on weight and reduced life expectancy in a certain window in the post-symptomatic group. They reported improvement in the rotarod performance, suggesting beneficial effects of lithium treatment. However, the likely reason for the apparent improvement in rotarod performance in the lithium-treated post-symptomatic group is the large numbers of mice that would have died fairly soon after the trial was initiated in this group. After 3 weeks of dosing, the mice were around 14 weeks, a time when 75% of the mice were dead. Thus, the apparent improvement in the lithium-treated mice may simply reflect early death of the sicker mice in this treated group (which also have a lower life expectancy until about 14 weeks). However, it is not possible to rule out the possible therapeutic effect of lithium in HD on the basis of this mouse trial. This is particularly important because lithium has a narrow therapeutic window (6,14), short half life in mice (7), different mouse inbred strains have different LD50 (42) and the mode of administration used by Wood and Morton is standardly used for rats rather than mice (43–46).
Previous studies have tested lithium in excitotoxicity models (44). However, these cannot be considered as valid HD models—HD is caused by a defined genetic lesion and results in progressive chronic damage, in contrast to excitotoxins or mitochondrial toxins, which are much more acute, and, at best, may only resemble a small portion of the HD pathogenic process.
The fly model allows testing of various doses of compounds (for instance by experimentally determining sublethal doses for treatment trials) and genetic manipulations to analyse the specific pathway. In contrast to the apparently equivocal results of Wood and Morton (5), we provide compelling support for the efficacy of this drug and for the modulating role of the Wnt pathway in polyglutamine and polyalanine diseases. It is interesting to note that the lithium concentrations we used in fly food were similar to the optimal safe plasma concentrations aimed for in humans with bipolar disorder (14,47).
The possibility that lithium may have a therapeutic effect in HD and related diseases are very important, given that lithium has been used in humans for 50 years and has well-characterized safety profiles (48). Our data also suggest that lithium might be beneficial in the context of the polyalanine expansion mutation. Although we have used a model protein with a polyalanine expansion independent of protein context, our results suggest that lithium or GSK3β inhibition should be considered as a new therapeutic intervention for oculopharyngeal muscular dystrophy, a late-onset muscle disorder caused by the polyalanine expansion mutation (3).
In summary, we provide evidence that lithium protects against toxicity caused by polyglutamines and polyalanines and that at least part of this effect is due to the effects on the Wnt pathway. Further studies in mouse models of these disorders will be necessary to confirm our findings.
MATERIALS AND METHODS
Drosophila stocks and crosses
Flies were grown on standard cornmeal molasses medium supplemented with dry yeast (1.2 l water, 12.5 g agar, 105 g dextrose, 105 g maize, 21 g yeast, 35 g Nipagin) at 25 or 29°C with 40–70% humidity, with 12/12 h light/dark cycle. In order to generate NLS-Q97 and NLS-Q25 flies, DNA fragments encoding either 25 or 97 glutamines fused to EGFP and the NLS from SV40 T-antigen (49) were subcloned into the pPUAST vector (50). Eight independent lines of NLS-Q97 and nine independent lines of NLS-Q25 were generated. Presence of the relevant transgene was verified by PCR in all transgenic lines.
Flies containing gmr-httQ120 (10) were a kind gift from Dr George Jackson, UAS-Q108 (15) from Dr Larry Marsh and UAS-127Q (18) from Dr Parsa Kazemi Esfarjani. The following strains were obtained from Bloomington stock centre: elav-GAL4C155 (51), gmr-GAL4 (52), ey-GAL4 (53), sev-GAL4 (with sevenless-derived promoter) (54), arm1 (55), arm4 (56), dTCF2 and UAS-dTCF (32).
Drug treatment
Flies were allowed to mate on normal fly food for 2–3 days and then transferred to instant fly food (Philip Harris Ltd, UK) containing the appropriate drug. Flies were treated with lithium chloride or water as a control or with either AR-A014418 (Calbiochem) or DMSO as a control. For testing the effects of mutants or drug treatments, crosses were always done at the same time under exactly the same conditions. Analysis was performed with the observer blinded to the identity of the flies. In case of gmr-Q120, flies were treated with the same concentration of the drug during adult stage and new fly food was prepared daily. For gmr-httQ120 flies (HD flies), flies were treated both during larval and adult stage. Other flies were treated only during the pre-adult stage as they were analysed shortly after eclosion.
Pseudopupil analysis
Pseudopupil analysis allows visualization of the rhabdomeres in the ommatidia of the compound eye (10–12,57). Flies were raised at 25°C and rhabdomeres were counted at the indicated times after eclosion. Eyes were analysed with an optical miscroscope (Nikon) using a 60× objective. Values for each genotype were obtained from at least 100 ommatidia counted in approximately 10 flies and this was done at least twice (see text and figure legend for more details).
The numbers of visible rhabdomeres were compared in control and treated flies (or mutants), determined on the same day under identical conditions. Mann–Whitney U-tests were used to compare raw data, and paired T-tests were used to compare averages based on multiple experiments (control was arbitrarily set at 100% to enable comparison of multiple independent experiments). Statview for Windows version 4.5 was used for statistical analysis.
Survival to adulthood
Lines with polyglutamine and polyalanine insertions (Q108 and A37) on the third chromosome heterozygous with a TM3 or TM6B balancer chromosome were used. Flies eclosing as adults with either the transgene or the balancer were counted. The transgene/balancer ratio in control (untreated) flies was arbitrarily taken as one and the transgene/balancer ratio in experimental flies (treated, mutants) was compared to that value.
To confirm the effect of drug treatment was specific to the survival of flies with the mutant transgene, identical treatments/genetic experiments were done with wild-type constructs (Q22 or A7) on the third chromosome heterozygous with the appropriate balancer (TM3 and TM6B, respectively). Unconditional logistical regression analysis with the general log linear analysis option of SPSS version 6.1 was used (SPSS, Chicago, IL, USA). Odds ratios (OR, the ratios of the proportion of flies with transgene/balancer in different experimental conditions with 95% confidence intervals) and P-values can be found in the text. The same statistical test was used to compare the effects of various treatments on the frequency of rough eyes.
Western blotting
Fly lysates were prepared in RIPA buffer with protease inhibitor cocktail (Complete; Roche Diagnostics) and were separated on 15% SDS–polyacrylamide gels. Flies were homogenized in a 1.5 ml microtube containing RIPA buffer. Proteins were quantified using a Bradford assay. SDS–PAGE sample buffer [0.0625 m Tris pH 6.8, 2% SDS, 10% glycerol, 5% β-mercaptoethanol, 0.01% bromophenol blue, 1× cocktail of protease inhibitors (Roche Diagnostics)] was added to the sample, and the homogenate was boiled at 95°C for 5 min, centrifuged at 17 000 g, and the supernatant was used for western blotting. Approximately one fly-equivalent was used for each lane. Lysates were subjected to SDS–PAGE (12%). Proteins were transferred onto nitrocellulose membranes (Hybond ECL membrane; Amersham Biosciences), which were blocked by incubation in 5% dried milk in 0.1 m PBS, 0.1% Tween-20. Membranes were probed with primary antibodies raised against GFP (Clontech 1:2000) and, as a loading control α-tubulin (Sigma, 1:2000). HRP- conjugated antibodies (Amersham Biosciences; 1:2000) were then added to the blots. Immunoreactive bands were detected with enhanced chemiluminescence reagent (ECL; Amersham Biosciences) and the signal was visualized by exposing the membrane to the ECL Hyperfilm (Amersham Biosciences).
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG Online.
ACKNOWLEDGEMENTS
We thank Drs L. Marsh, P. Kazemi-Esfarjani, G. Jackson and the Bloomington Drosophila Stock Centre for fly stocks. We are grateful for ORS Award (Z.B.), Wellcome Trust Senior Clinical Research Fellowship (D.C.R.) and Wellcome Trust Prize Studentship (Z.B.), BBSRC Career Development Award (C.J.O'K.) and an MRC programme grant to D.C.R. and S. Brown.
Conflict of Interest statement: The authors declare that they have no competing financial interests.
These authors are joint senior authors.
Figure 1. Lithium rescues polyglutamine-mediated toxicity. (A) Photographs of visible rhabdomeres in Drosophila eyes 2 days after eclosion in gmr-httQ120 flies. Expression of the N-terminal fragment of huntingtin with 120Q driven by the gmr promoter leads to progressive rhabdomere loss, which is partially rescued by rearing on lithium (0.18 mg/ml in fly food, ∼4.2 mm—also used for other panels). (B) Quantification of visible rhabdomeres in gmr-httQ120 flies reared in the absence or presence of lithium, 2 days after eclosion. About 360 ommatidia (24 flies) were analysed in each experiment (P<0.0001). A representative experiment is shown (four experiments with similar results were performed at the same time point). (C) Pseudopupil analysis of gmr-httQ120 flies reared in the presence or absence of lithium, 10 days after eclosion. Flies were treated both during larval and adult stages. The experiment was done as in (B). Frequencies of ommatidia with different numbers of rhabdomeres are shown (P<0.001). (D) Lethality of flies expressing a Q108 transgene is partially rescued by lithium. Females carrying the neuronal driver elav-GAL4C155 were crossed to Q108/TM3 and the percentage of adult flies carrying the Q108 transgene was determined (P=0.003). Error bars: SEM. We evaluated 24 bottles (∼900 flies). (E) Lithium does not affect the relative survival of flies carrying a TM3 balancer. Females of genotype elav-GAL4c155 were crossed to Q22/TM3 and the percentage of adult flies containing Q22 transgene was determined. The ratio of flies with Q22 and TM3 was compared in control and lithium-containing fly food (P=0.781). We evaluated eight bottles (∼1000 flies). (F) Flies expressing NLS-Q97 under the control of ey-GAL4 exhibit abnormal eyes characterized by abnormal shape (arrow in middle panel points to the irregular shape of the eye) and occasionally the eyes are absent (left panel). Expression of NLS-Q25 under control of ey-GAL4 (right panel) does not lead to any abnormalities. (G) Lithium reduces the percentage of NLS-Q97 flies with rough/small eyes. Female ey-GAL4 flies were crossed to males of genotype UAS-NLS-Q97/CyO. To facilitate comparison across multiple experiments, untreated flies were taken as 100%. [Odds ratios—see Materials and Methods, OR=0.232; 95% CI (0.118–0.457); P<0.001]. Error bars represent 95% confidence intervals. We evaluated 10 bottles (∼350 flies, fewer than in most other experiments as there is some lethality caused by the expression of long polyglutamines in other parts of the fly than eyes, data not shown).
Figure 2. GSK3β-specific inhibitor AR-A014418 rescues polyglutamine-mediated toxicity. (A) Quantification of visible rhabdomeres in gmr-httQ120 flies treated with DMSO (vehicle) or with 20 µm AR-A014418 (GSK3β inhibitor). Rhabdomeres were scored 2 days after eclosion. Graph shows summary data from seven experiments (∼1600 ommatidia from 100 flies per group; P<0.001). (B) AR-A014418 reduces the percentage of Q127 flies with abnormal eyes. Females carrying ey-GAL4 were crossed to flies carrying UAS-127Q. To facilitate comparison across multiple experiments, untreated flies were taken as 100%. [OR=0.291; 95% CI (0.145–0.586); P=0.001]. Error bars: 95% confidence intervals. We evaluated 16 bottles (∼150 flies, as there is some lethality caused by the expression of long polyglutamines in other parts of the fly than eyes, data not shown). (C) AR-A014418 reduces the percentage of NLS-Q97 flies with abnormal eyes. Females carrying ey-GAL4 were crossed to flies of genotype UAS-NLS-Q97/CyO. To facilitate comparison across multiple experiments, untreated flies were taken as 100%. (OR=0.555; 95% CI (0.379–0.812); P=0.002). Error bars: 95% confidence intervals. We evaluated 12 bottles (∼500 flies).
Figure 3. Perturbation of the Wnt pathway rescues polyglutamine-mediated toxicity. (A) Scheme of Wnt pathway. Under normal conditions, GSK3 (shaggy) phosphorylates β-catenin (armadillo) in a complex with APC and Axin, leading to its proteasome-dependent degradation. Inhibition of GSK3 (shaggy) activity results in increased levels of β-catenin, leading to its translocation to the nucleus and activation of LEF/TCF transcription factors. This leads to the transcription of Wnt-target genes. (B) Heterozygous armadillo mutations (arm1 and arm4) partially abolish the protective effect of lithium. Quantification of visible rhabdomeres in gmr-httQ120 flies with or without arm mutations in the presence or absence of lithium 2 days after eclosion. Approximately 180 ommatidia analysed per group (12 flies). One representative experiment is shown (two experiments with similar results were performed at the same time point). (P<0.001 for Q120+Li+arm1 and Q120+Li+arm4 compared with Q120+Li). (C) Quantification of visible rhabdomeres in flies expressing gmr-httQ120 either alone or together with UAS-dTCF. Rhabdomeres were scored 2 days after eclosion. One representative experiment is shown (eight experiments were performed, see Supplementary Material, Fig. S1). Genotypes are Q120: UAS-dTCF/+; gmr-httQ120/+, Q120+TCF: sev-GAL4/UAS-dTCF; gmr-httQ120/+. Note that dTCF is driven by sev-GAL4 and therefore not expressed in Q120 flies (P<0.001). (D) Quantification of visible rhabdomeres in flies expressing gmr-httQ120 either alone or in the presence of a heterozygous dTCF mutation. Rhabdomeres were scored 1 day after eclosion. One representative experiment is shown (six experiments were performed, see Supplementary Material, Fig. S1). Genotypes: Q120: w; gmr-httQ120/+; +, Q120+dTCF mutation: w; gmr-httQ120/+; +; dTCF2/+ (P<0.001). (E) Quantification of visible rhabdomeres in gmr-httQ120 flies in the absence or presence of sev-GAL4. Rhabdomeres were scored 2 days after eclosion. Representative experiment from (D) showing the frequency of ommatidia with different numbers of rhabdomeres. Q120 and Q120+GAL4 show almost identical distributions (P=0.126). A representative experiment is shown (four experiment were performed, see Supplementary material, Fig. S1).
Figure 4. Lithium and GSK3β-specific inhibitor rescue polyalanine-induced toxicity. (A) Lithium decreases long polyalanine toxicity. Flies of genotype w; +; UAS-NLS-A37/TM6B were crossed to ey-GAL4 flies and the percentage of flies with abnormal eyes was evaluated in their progeny that carried both UAS-NLS-A37 and ey-GAL4. Two different lithium concentrations were used 0.18 mg/ml (lithium low, 4.2 mm) and 0.25 mg/ml (lithium high, 5.9 mm). Flies were raised at 28°C. [0.18 mg/ml: OR (used for Odds ratio)=0.091; 95% CI (0.059–0.140); P<0.0001 and 0.25 mg/ml: OR=0.030; 95% CI (0.001–0.061); P<0.001]. We evaluated 16 bottles, ∼1100 flies. Error bars: 95% confidence intervals. (B) AR-A014418 decreases toxicity of long polyalanines. Experiment was done as in (A) but flies were treated either with DMSO (vehicle) or with 100 µm AR-A014418. Flies were raised at 25°C (OR=0.059; 95% CI (0.008–0.444); P=0.006). We evaluated 18 bottles (∼1100 flies). Error bars: 95% confidence intervals. (C) AR-A014418 increases survival to adult stage of flies with long polyalanines. Flies were crossed as in (A) and the percentage of flies with the A37 transgene was calculated. Flies were treated either with DMSO (vehicle) or with 100 µm AR-A014418. Flies were raised at 29°C [OR=1.838; 95% CI (1.274–2.652); P=0.001]. We evaluated 12 bottles (∼800 flies). (D) AR-A014418 does not decrease survival of TM6B flies. Flies of genotype w; +; UAS-EGFP-NLS-A7/TM6B were crossed to ey-GAL4 flies and the percentage of progeny with the UAS-EGFP-NLS-A7 transgene was calculated. Flies were treated either with DMSO (vehicle) or with 100 µm AR-A014418. Flies were raised at 29°C. We evaluated 12 bottles (∼600 flies). (C and D) Error bars: SEM. (E) Overexpression of dTCF decreases the percentage of flies with rough eyes. Females of genotype ey-GAL4/CyO; UAS-NLS-A37/TM6B were crossed to UAS-EGFP (control) or UAS-dTCF (TCF). Progeny was raised at 28°C, and the percentages of ey-GAL4 UAS-NLS-A37 flies with rough eyes were scored. We evaluated 14 bottles (∼300 flies) [OR=0.047; 95% CI (0.020–0.107); P<0.001]. Error bars: 95% confidence intervals.
References
Gusella, J.F. and MacDonald, M.E. (
Brown, L.Y. and Brown, S.A. (
Brais, B., Bouchard, J.P., Xie, Y.G., Rochefort, D.L., Chretien, N., Tome, F.M., Lafreniere, R.G., Rommens, J.M., Uyama, E., Nohira, O. et al. (
Carmichael, J., Sugars, K.L., Bao, Y.P. and Rubinsztein, D.C. (
Wood, N.I. and Morton, A.J. (
Wood, A.J., Goodwin, G.M., De Souza, R. and Green, A.R. (
Ravikumar, B., Vacher, C., Berger, Z., Davies, J.E., Luo, S., Oroz, L.G., Scaravilli, F., Easton, D.F., Duden, R., O'Kane, C.J. et al. (
Marsh, J.L. and Thompson, L.M. (
Jackson, G.R., Salecker, I., Dong, X., Yao, X., Arnheim, N., Faber, P.W., MacDonald, M.E. and Zipursky, S.L. (
Steffan, J.S., Agrawal, N., Pallos, J., Rockabrand, E., Trotman, L.C., Slepko, N., Illes, K., Lukacsovich, T., Zhu, Y.Z., Cattaneo, E. et al. (
Steffan, J.S., Bodai, L., Pallos, J., Poelman, M., McCampbell, A., Apostol, B.L., Kazantsev, A., Schmidt, E., Zhu, Y.Z., Greenwald, M. et al. (
Rubinsztein, D.C. and Carmichael, J. (
Phiel, C.J. and Klein, P.S. (
Marsh, J.L., Walker, H., Theisen, H., Zhu, Y.Z., Fielder, T., Purcell, J. and Thompson, L.M. (
Peters, M.F., Nucifora, F.C., Jr, Kushi, J., Seaman, H.C., Cooper, J.K., Herring, W.J., Dawson, V.L., Dawson, T.M. and Ross, C.A. (
Gould, T.D., Chen, G. and Manji, H.K. (
Kazemi-Esfarjani, P. and Benzer, S. (
Greaves, S., Sanson, B., White, P. and Vincent, J.P. (
Moon, R.T., Bowerman, B., Boutros, M. and Perrimon, N. (
Klein, P.S. and Melton, D.A. (
Hedgepeth, C.M., Conrad, L.J., Zhang, J., Huang, H.C., Lee, V.M. and Klein, P.S. (
Stambolic, V., Ruel, L. and Woodgett, J.R. (
Dokucu, M.E., Yu, L. and Taghert, P.H. (
Mudher, A., Shepherd, D., Newman, T.A., Mildren, P., Jukes, J.P., Squire, A., Mears, A., Drummond, J.A., Berg, S., MacKay, D. et al. (
Padiath, Q.S., Paranjpe, D., Jain, S. and Sharma, V.K. (
Bhat, R.V., Budd Haeberlein, S.L. and Avila, J. (
Cohen, P. and Goedert, M. (
Bourouis, M. (
Papadopoulou, D., Bianchi, M.W. and Bourouis, M. (
Pai, L.M., Orsulic, S., Bejsovec, A. and Peifer, M. (
van de Wetering, M., Cavallo, R., Dooijes, D., van Beest, M., van Es, J., Loureiro, J., Ypma, A., Hursh, D., Jones, T., Bejsovec, A. et al. (
Peifer, M., Sweeton, D., Casey, M. and Wieschaus, E. (
Tomlinson, A., Strapps, W.R. and Heemskerk, J. (
Ruel, L., Stambolic, V., Ali, A., Manoukian, A.S. and Woodgett, J.R. (
Jackson, G.R., Wiedau-Pazos, M., Sang, T.K., Wagle, N., Brown, C.A., Massachi, S. and Geschwind, D.H. (
Kazemi-Esfarjani, P. and Benzer, S. (
Warrick, J.M., Chan, H.Y., Gray-Board, G.L., Chai, Y., Paulson, H.L. and Bonini, N.M. (
Kim, W.Y., Fayazi, Z., Bao, X., Higgins, D. and Kazemi-Esfarjani, P. (
Hay, D.G., Sathasivam, K., Tobaben, S., Stahl, B., Marber, M., Mestril, R., Mahal, A., Smith, D.L., Woodman, B. and Bates, G.P. (
Sittler, A., Lurz, R., Lueder, G., Priller, J., Lehrach, H., Hayer-Hartl, M.K., Hartl, F.U. and Wanker, E.E. (
Smith, D.F. (
Nonaka, S. and Chuang, D.M. (
Wei, H., Qin, Z.H., Senatorov, V.V., Wei, W., Wang, Y., Qian, Y. and Chuang, D.M. (
McQuade, R., Leitch, M.M., Gartside, S.E. and Young, A.H. (
Hamamura, T., Lee, Y., Ohashi, K., Fujiwara, Y., Miki, M., Suzuki, H. and Kuroda, S. (
Camus, M., Hennere, G., Baron, G., Peytavin, G., Massias, L., Mentre, F. and Farinotti, R. (
Manji, H.K., Moore, G.J. and Chen, G. (
Bao, Y.P., Cook, L.J., O'Donovan, D., Uyama, E. and Rubinsztein, D.C. (
Brand, A.H. and Perrimon, N. (
Lin, M.H., Bour, B.A., Abmayr, S.M. and Storti, R.V. (
Freeman, M. (
Bonini, N.M., Bui, Q.T., Gray-Board, G.L. and Warrick, J.M. (
Therrien, M., Wong, A.M., Kwan, E. and Rubin, G.M. (
Perrimon, N. and Mahowald, A.P. (
Wieschaus, E. and Riggleman, R. (

![Figure 1. Lithium rescues polyglutamine-mediated toxicity. (A) Photographs of visible rhabdomeres in Drosophila eyes 2 days after eclosion in gmr-httQ120 flies. Expression of the N-terminal fragment of huntingtin with 120Q driven by the gmr promoter leads to progressive rhabdomere loss, which is partially rescued by rearing on lithium (0.18 mg/ml in fly food, ∼4.2 mm—also used for other panels). (B) Quantification of visible rhabdomeres in gmr-httQ120 flies reared in the absence or presence of lithium, 2 days after eclosion. About 360 ommatidia (24 flies) were analysed in each experiment (P<0.0001). A representative experiment is shown (four experiments with similar results were performed at the same time point). (C) Pseudopupil analysis of gmr-httQ120 flies reared in the presence or absence of lithium, 10 days after eclosion. Flies were treated both during larval and adult stages. The experiment was done as in (B). Frequencies of ommatidia with different numbers of rhabdomeres are shown (P<0.001). (D) Lethality of flies expressing a Q108 transgene is partially rescued by lithium. Females carrying the neuronal driver elav-GAL4C155 were crossed to Q108/TM3 and the percentage of adult flies carrying the Q108 transgene was determined (P=0.003). Error bars: SEM. We evaluated 24 bottles (∼900 flies). (E) Lithium does not affect the relative survival of flies carrying a TM3 balancer. Females of genotype elav-GAL4c155 were crossed to Q22/TM3 and the percentage of adult flies containing Q22 transgene was determined. The ratio of flies with Q22 and TM3 was compared in control and lithium-containing fly food (P=0.781). We evaluated eight bottles (∼1000 flies). (F) Flies expressing NLS-Q97 under the control of ey-GAL4 exhibit abnormal eyes characterized by abnormal shape (arrow in middle panel points to the irregular shape of the eye) and occasionally the eyes are absent (left panel). Expression of NLS-Q25 under control of ey-GAL4 (right panel) does not lead to any abnormalities. (G) Lithium reduces the percentage of NLS-Q97 flies with rough/small eyes. Female ey-GAL4 flies were crossed to males of genotype UAS-NLS-Q97/CyO. To facilitate comparison across multiple experiments, untreated flies were taken as 100%. [Odds ratios—see Materials and Methods, OR=0.232; 95% CI (0.118–0.457); P<0.001]. Error bars represent 95% confidence intervals. We evaluated 10 bottles (∼350 flies, fewer than in most other experiments as there is some lethality caused by the expression of long polyglutamines in other parts of the fly than eyes, data not shown).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/hmg/14/20/10.1093_hmg_ddi331/1/m_ddi33101.jpeg?Expires=1716441194&Signature=dORzGBnHMDr3sUyOr2759qk3zoEQprPmGoAZWacEE-LAl9i4J13qPb6XT92UV9KQT9Ih6OdsEa-5BJLF78BkNPSm1jH7W2cDFPVElp3JadHjitXp7X4IzJuoMuWIi9kcrARi7YnInv3bzAIfNGjc2jvxQq3o31o18a6MxR2vw4cntlhATDuI2S-YlC1Ty~3kJU0OOVHoZ8LhdhyPmy4lDleQOptqIXk38P30X2ZJooBsY7VJVi3lJYz88~WmTDS8OO1WTPHoypCas~zWGPbjYIefftuYNVQ0lIF6oRZFhxMGMRl9TIWijM2998YhOSur3pQyJIj4iCgo8RlVYwFrhA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. GSK3β-specific inhibitor AR-A014418 rescues polyglutamine-mediated toxicity. (A) Quantification of visible rhabdomeres in gmr-httQ120 flies treated with DMSO (vehicle) or with 20 µm AR-A014418 (GSK3β inhibitor). Rhabdomeres were scored 2 days after eclosion. Graph shows summary data from seven experiments (∼1600 ommatidia from 100 flies per group; P<0.001). (B) AR-A014418 reduces the percentage of Q127 flies with abnormal eyes. Females carrying ey-GAL4 were crossed to flies carrying UAS-127Q. To facilitate comparison across multiple experiments, untreated flies were taken as 100%. [OR=0.291; 95% CI (0.145–0.586); P=0.001]. Error bars: 95% confidence intervals. We evaluated 16 bottles (∼150 flies, as there is some lethality caused by the expression of long polyglutamines in other parts of the fly than eyes, data not shown). (C) AR-A014418 reduces the percentage of NLS-Q97 flies with abnormal eyes. Females carrying ey-GAL4 were crossed to flies of genotype UAS-NLS-Q97/CyO. To facilitate comparison across multiple experiments, untreated flies were taken as 100%. (OR=0.555; 95% CI (0.379–0.812); P=0.002). Error bars: 95% confidence intervals. We evaluated 12 bottles (∼500 flies).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/hmg/14/20/10.1093_hmg_ddi331/1/m_ddi33102.jpeg?Expires=1716441194&Signature=CTON0Qde-zFQjMnvnLgAJDxmd0XsSpmAD1C7d2xZEzEWK4qRL-mOAz-HCv2fVcW8Ayoelbg8WWKJ0I-yJZE~hqQsMrZzImzDMTnv-jRntOmUQnqgpbTIPuwZDifVkf1DJUXeONmkCj4he3MTOCRBJ3Z-yIdXAVYs4uQ4wX60Amht76b0qOesY6hBcBdFHinRFBu7ExGKZY2j~DjtHo7TyAGrCzFCgBA1K5D4~pyR~IKNtsJPBRB1J6gzDhVRRckm42J4UiDp4dSeM3qk5Svyj6eHnSS1YllvHPtJykdk3CUV3oYFGYIBhDVr1ZErctUKwQKw8M1Nn-J77yuo9agHVA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Lithium and GSK3β-specific inhibitor rescue polyalanine-induced toxicity. (A) Lithium decreases long polyalanine toxicity. Flies of genotype w; +; UAS-NLS-A37/TM6B were crossed to ey-GAL4 flies and the percentage of flies with abnormal eyes was evaluated in their progeny that carried both UAS-NLS-A37 and ey-GAL4. Two different lithium concentrations were used 0.18 mg/ml (lithium low, 4.2 mm) and 0.25 mg/ml (lithium high, 5.9 mm). Flies were raised at 28°C. [0.18 mg/ml: OR (used for Odds ratio)=0.091; 95% CI (0.059–0.140); P<0.0001 and 0.25 mg/ml: OR=0.030; 95% CI (0.001–0.061); P<0.001]. We evaluated 16 bottles, ∼1100 flies. Error bars: 95% confidence intervals. (B) AR-A014418 decreases toxicity of long polyalanines. Experiment was done as in (A) but flies were treated either with DMSO (vehicle) or with 100 µm AR-A014418. Flies were raised at 25°C (OR=0.059; 95% CI (0.008–0.444); P=0.006). We evaluated 18 bottles (∼1100 flies). Error bars: 95% confidence intervals. (C) AR-A014418 increases survival to adult stage of flies with long polyalanines. Flies were crossed as in (A) and the percentage of flies with the A37 transgene was calculated. Flies were treated either with DMSO (vehicle) or with 100 µm AR-A014418. Flies were raised at 29°C [OR=1.838; 95% CI (1.274–2.652); P=0.001]. We evaluated 12 bottles (∼800 flies). (D) AR-A014418 does not decrease survival of TM6B flies. Flies of genotype w; +; UAS-EGFP-NLS-A7/TM6B were crossed to ey-GAL4 flies and the percentage of progeny with the UAS-EGFP-NLS-A7 transgene was calculated. Flies were treated either with DMSO (vehicle) or with 100 µm AR-A014418. Flies were raised at 29°C. We evaluated 12 bottles (∼600 flies). (C and D) Error bars: SEM. (E) Overexpression of dTCF decreases the percentage of flies with rough eyes. Females of genotype ey-GAL4/CyO; UAS-NLS-A37/TM6B were crossed to UAS-EGFP (control) or UAS-dTCF (TCF). Progeny was raised at 28°C, and the percentages of ey-GAL4 UAS-NLS-A37 flies with rough eyes were scored. We evaluated 14 bottles (∼300 flies) [OR=0.047; 95% CI (0.020–0.107); P<0.001]. Error bars: 95% confidence intervals.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/hmg/14/20/10.1093_hmg_ddi331/1/m_ddi33104.jpeg?Expires=1716441194&Signature=w4WYaWolMz3YUdZ1vyS60DsVguEqUxcqRO9mu~XFUX7AMkm467jVPgjxG4Nao4n8roMQt17wmc0~JJlMrOksiNgEjSnCElByspsy4iQUd4GxPp~S9HGPTOkk05NVzvAtD6tde0B-lU21GA~dVW3oNL79-DFfxriUE-Nn0ci9SbAkKIFsRuxUjvr2BJ0ZPA7Hhf~aR5vx-tw~KzEGCuX9KkTDvWK9Bi6ZKm39Oa38jJoH6~uHTE2tRg7b6wmLb~quY9YG1wlRVNTrlRZPaGAnH54Pu5ANsAjvMoIDmyL7esl9o9YmEcLLkVPyMZuuflqPdOARyv5zZa~M6YYwUyWYEA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
