-
PDF
- Split View
-
Views
-
Cite
Cite
Kristina Lossow, Irm Hermans-Borgmeyer, Maik Behrens, Wolfgang Meyerhof, Genetic Labeling of Car4-expressing Cells Reveals Subpopulations of Type III Taste Cells, Chemical Senses, Volume 42, Issue 9, November 2017, Pages 747–758, https://doi.org/10.1093/chemse/bjx048
Close - Share Icon Share
Abstract
Carbonic anhydrases form an enzyme family of 16 members, which reversibly catalyze the hydration of carbon dioxide to bicarbonate and protons. In lung, kidney, and brain, presence of carbonic anhydrases is associated with protons and bicarbonate transport in capillary endothelium of lung, reabsorption of bicarbonate in proximal renal tubules, and extracellular buffering. In contrast, their role in taste is less clear. Recently, carbonic anhydrase IV expression was detected in sour-sensing presynaptic taste cells and was associated with the taste of carbonation, yet the precise role and cell population remained uncertain. To examine the role of carbonic anhydrase 4-expressing cells in taste reception, we generated a mouse strain carrying a modified allele of the carbonic anhydrase 4 gene in which the coding region of the red fluorescent protein monomeric Cherry is attached to that of carbonic anhydrase 4 via an internal ribosome entry site. Monomeric Cherry fluorescence was detected in lingual papillae as well as taste buds of soft palate and naso-incisor duct. However, expression patterns on the tongue differ between posterior and fungiform papillae. Whereas monomeric Cherry auto-fluorescence was almost always co-localized with presynaptic cell markers aromatic L-amino-acid decarboxylase, synaptosomal-associated protein 25 or glutamic acid decarboxylase 67 in fungiform papillae and taste buds of palate and naso-incisor duct, monomeric Cherry-positive cells in posterior tongue papillae represent only a subpopulation of presynaptic cells. We conclude that this model is well suited for detailed investigation into the role of carbonic anhydrase in gustation and other processes.
Introduction
Carbonic anhydrases catalyze the conversion of carbon dioxide into bicarbonate and protons. The enzyme family consists of 16 members, varying in their enzymatic properties, subcellular localizations, and sites of expression (reviewed in Esbaugh and Tufts (2006); Supuran (2008)). They participate in various comparatively well understood biological processes, including carbon dioxide transport, pH homeostasis, production of body fluids, electrolyte secretion, ureagenesis, gluconeogenesis, and fertilization (reviewed in Sly and Hu (1995); Parkkila (2000)). Recently, the carbonic anhydrase isoform 4 (Car4), a glycosylphosphatidyl inositol-anchored enzyme on the cell surface, has been associated with the taste of carbonation (Chandrashekar et al. 2009).
Taste stimuli are sensed by assemblies of 50 to 100 cells referred to as taste buds that are composed of at least 4 morphologically distinct cell types (Murray 1973; Finger and Simon 2000). The Type I cells are considered as glia-like cells (Murray 1973; Pumplin et al. 1997; Lawton et al. 2000; Bartel et al. 2006; Dvoryanchikov et al. 2009; Sinclair et al. 2010), Type IV or basal cells constitute progenitor cells (Suzuki 2007; Yee et al. 2013; Miura et al. 2014). Type II cells have been identified to respond to sweet (Nelson et al. 2001; Li et al. 2002), umami (Nelson et al. 2002) or bitter substances (Chandrashekar et al. 2000; Mueller et al. 2005) by releasing the transmitter substance ATP to excite afferent fibers (Finger et al. 2005) and Type III cells (Huang et al. 2009). Their tuning properties depend on the expression of the type of taste receptors (Tas1rs, Tas2rs). Whereas heterodimers of Tas1r2 and Tas1r3 assemble to form a universal sweet taste receptor, the Tas1r1-Tas1r3 heteromer represents a umami receptor (Bachmanov et al. 2001; Kitagawa et al. 2001; Max et al. 2001; Montmayeur et al. 2001; Nelson et al. 2001; Nelson et al. 2002; Li et al. 2002). In contrast, the Tas2rs which differ in number in different species function as monomeric or homo-oligomeric bitter receptors (Adler et al. 2000; Chandrashekar et al. 2000; Matsunami et al. 2000; Kuhn et al. 2010; Meyerhof et al. 2010; Behrens et al. 2014; Lossow et al. 2016). Type III or presynaptic cells are thought to be sour responsive cells that form, unlike Type II cells, synaptic contacts with primary sensory afferent terminals (DeFazio et al. 2006; Huang et al. 2006; Huang et al. 2008). They are characterized by the expression of aromatic L-amino-acid decarboxylase (AADC), an essential enzyme for the synthesis of dopamine and serotonin (Seta et al. 2007), synaptosomal-associated protein 25 (SNAP25), a component of the vesicular release machinery (Yang et al. 2000; Clapp et al. 2006), glutamic acid decarboxylase 67 (GAD67) that converts glutamate to the neurotransmitter γ-aminobutyric acid (Tamamaki et al. 2003), as well as polycystic kidney disease 2-like 1 protein (PKD2L1) (Huang et al. 2006; Ishimaru et al. 2006; LopezJimenez et al. 2006; Kataoka et al. 2008), protein gene product 9.5 (PGP9.5) (Yee et al. 2001), neural cell adhesion molecule (NCAM) (Takeda et al. 1992; Nelson and Finger 1993; Smith et al. 1993; Yee et al. 2001), and serotonin (5-HT) (Kim and Roper 1995). Type III cells do not only respond directly to sour taste stimuli but also receive input from Type II cells (Tomchik et al. 2007). Type III cells release serotonin in response to taste stimulation and excite afferent fibers via 5-HT3 receptors (Larson et al. 2015). Thus, Type III cells produce secondary responses to sweet, umami, and bitter stimuli, but their contribution to taste coding remains unclear. Type III cells are also involved in sensing carbon dioxide in carbonated beverages (Chandrashekar et al. 2009). Carbonic anhydrase isoform 4, an ectoenzyme located on the cell surface of Type III cells, converts onsite carbon dioxide to bicarbonate and protons which are thought to stimulate sour transduction pathways locally in these cells. However, it remained unclear why the taste of carbonation differs from pure sourness (Chandrashekar et al. 2009). Recent studies further associate Type III cells with amiloride-insensitive sodium taste (Oka et al. 2013; Lewandowski et al. 2016). Silencing the synaptic machinery in PKD2L1 cells not only eliminated acid-evoked responses but also significantly reduced their high-salt responses (Oka et al. 2013). Based on calcium imaging recordings of isolated taste cells, it was further shown that amiloride-insensitive salt responses were observed in a subset of acid-responsive Type III taste cells (Lewandowski et al. 2016). Moreover, Type III cells also mediate taste responses to water by a yet unknown mechanism (Zocchi et al. 2017).
The aforementioned functional variety suggests that Type III cells do not constitute a homogeneous population but could be further divided into subpopulations. This assumption is supported by data from co-localization experiments. For example, Car4 is co-expressed with the sour cell marker PKD2L1; however, not all PKD2L1-positive cells showed Car4 expression (Chandrashekar et al. 2009) suggesting that the sensors of carbonation form only a subset of sour- dedicated cells. However, with regard to the overlap between PKD2L1 and other Type III cell markers the published data showed discrepancies. Kataoka et al. reported that about 10% and 7% of PGP9.5- and NCAM-immunoreactive taste bud cells were not co-localized with PKD2L1, respectively (Kataoka et al. 2008), whereas others report a nearly complete overlap between GAD67 and PKD2L1 in posterior tongue papillae (Horio et al. 2011). Thereby, some outline a total overlap of AADC with serotonin, PGP9.5, and NCAM (Seta et al. 2007), while others do not (DeFazio et al. 2006). Furthermore, Car4 has been shown to partially co-localize with the alpha subunit of the epithelial sodium channel (αENaC) (Chandrashekar et al. 2010). Whereas Car4-negative αENaC-expressing cells are postulated to be table salt-mediating taste cells, the function of Car4-expressing αENaC cells is unknown.
Taken together, Type III cells appear to represent different subsets of taste cells, yet a detailed classification and functional characterization of these subsets is still missing. In order to facilitate a mutual demarcation of the different Type III cells we engineered mice to express a fluorescent protein in all Car4 cells. This model represents a powerful tool to morphologically characterize Car4 cells in sections from gustatory tissues and to study their function in and ex vivo.
Materials and methods
Generation of gene-targeted animals
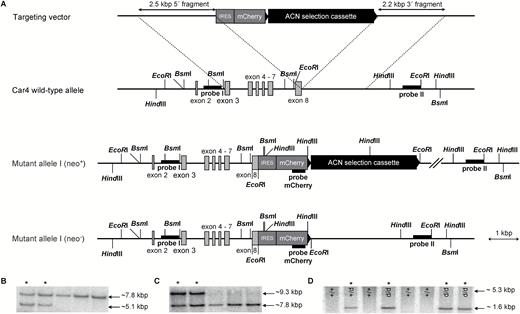
In Car4 knock-in mice the transcribed region of Car4 was extended by an internal ribosomal entry site (IRES) and monomeric cherry (mCherry) followed by a LoxP flanked selection cassette. The strategy for the generation of Car4 knock-in mice ensured that the Car4-coding sequence remained intact and is illustrated in Figure 1A. Fragments used for the 5′and 3′ flanking regions were amplified by polymerase chain reaction (PCR) using a bacterial artificial chromosome bMQ-262p07 (Source BioScience LifeScience, Nottingham, UK) derived from the 129/S7 mouse strain as template, to amplify a 2.5 kbp and 2.2 kbp fragment, respectively. Homologous fragments were cloned into pKO-V901-DTA plasmid (Lexicon Genetics, The Woodlands, USA), next to the IRES element, mCherry coding sequence and a Cre-recombinase- and neomycin resistance gene-containing selection cassette (ACN) flanked by LoxP sites (Voigt et al. 2012). The targeting vector was introduced into R1 embryonic stem cells (Nagy et al. 1993) by electroporation. The neomycin resistance gene in the ACN cassette and the diphtheria toxin A-chain (DTA) gene of the plasmid backbone were used for positive and negative selection, respectively. In total, 284 G418-resistant colonies were selected; genomic DNA was digested with HindIII (probe I) or BsmI (probe II) and analyzed with a random-primed alpha-32P-labeled probe by Southern blot analysis. To identify homologous recombinant clones, 2 external probes (probe I and II, upstream and downstream of the homologous fragments, respectively; see Figure 1A) were used, amplified by using oligonucleotides 5′-GAGATGGAAGTGGGAGATGGTTCTTGGTAG-3′, 5′-ATCTCAGAATCCTGCCCTGGCCTTC-3′ and 5′-CAACAAC CGCTACTACATCCTTCAGGCTAGAG-3, 5′-ACATTGAACCTGTAGCTCACTCTCTGGGCT-3′, respectively. Two correctly targeted clones were injected into C57BL/6J blastocysts, and chimeric mice were bred with C57BL/6J animals to obtain mutated offspring. Offspring was genotyped by PCR analysis with oligonucleotides 5′-ACATGAAGGACAATGTGAGGCCCCTG-3′ and 5′-CAGAGATCCACATGTCCCTGTCACCCT-3′ for wild-type and 5′-GTGAGGGTTAATTGCTAGAGCTCGCTGATC-3′ and 5′-CATGTCCTCTAAAGGCTGAGGTCAGAGGTC-3′ for the mutated allele, resulting in amplification of a 327 bp and 226 bp fragment, respectively. Furthermore, genomic modification was verified by Southern blot analysis after EcoRI digestion with an internal probe (probe mCherry; see Figure 1A), generated by using oligonucleotides 5′-CCCACCCCCCAGAATAGAATGACACCTACT-3′ and 5′-GAGATCAAGCAGAGGCTGAAGCTGAAGGAC-5′, validating the deletion of the ACN selection cassette by the Cre-LoxP-mediated excision. Based on the expression of Cre recombinase in the germ line of male chimeric mice ACN selection cassette was excised from the genome leaving only 1 LoxP site behind (neo−; see Figure 1A). Heterozygous animals were interbred to produce homozygous offspring B6;129SvJ-Car4tm-IRES-mCherry/Hhtg.
Generation of Car4 knock-in mice. A Simplified illustration showing the structure of Car4 gene and the strategy for generating knock-in mice. The targeting construct inserted the coding region for an internal ribosomal entry site (IRES), the fluorescent protein monomeric cherry (mCherry) and the selection cassette ACN (Angiotensin-Cre-Neomycin) flanked with LoxP sites (indicated by triangle) directly after the coding sequence of Car4. B-C Southern blot analysis of embryonic stem cell clones. Genomic DNA of neomycin-resistant stem cell clones were subjected to Southern blot analysis with 5′-(probe I; B) and 3′-flanking probes (probe II; C), allowing the identification of homologous recombined stem cell clones (indicated by asterisks). D Southern blot analysis of Car4 wild-type (+/+), heterozygous (+/d), and homozygous (d/d) mice. EcoRI-digested genomic DNAs extracted from tail biopsies were subjected to Southern blot analysis with an internal probe (mCherry probe) that distinguishes wild-type and knock-in alleles of Car4 locus. Furthermore, the size of the knock-in band allows conclusions about the presence (neo+) or absence (neo−) of the selection cassette ACN (band at 1.6 kbp indicate absence, whereas a band at 5.3 kbp demonstrate presence).
Animal experiments
Animal care and experiments were conducted following national and institutional guidelines, approved by the local animal experimentation committee and animal welfare committee of the University of Hamburg (Germany; permit number V1/591-00.33, 128/12) and the German Institute of Human Nutrition Potsdam-Rehbruecke (Germany). Mice were housed in polycarbonate cages and kept under 12:12 h light:dark cycle with food and water ad libitum. Car4-IRES-mCherry mice were interbred with Tas2r131-BL-IRES-hrGFP (Voigt et al. 2012) and TRPM5-IRES-Cre/eR26-τGFP mice (Kusumakshi et al. 2015) to gain Car4-IRES-mCherry/Tas2r131-BL-IRES-hrGFP and Car4-IRES-mCherry/TRPM5-IRES-Cre/eR26-τGFP animals, respectively. Car4-IRES-mCherry mice were hetero- and homozygous, whereas double and triple transgenic mice in this study were heterozygous for corresponding knock-in alleles. Studies were performed on tissue sections of adult mice (>8 weeks) of both sexes on mixed C57BL/6 and 129/Sv genetic background.
Tissue preparation
Tissues were obtained from adult mice, anesthetized by intraperitoneal injection of 150 mg/kg body weight Narcoren (Merial, Hallbergmoos, Germany) and perfused transcardially with phosphate buffered saline (PBS; 0.01 M Na2HPO4, 1.764 mM KH2PO4, 2.683 mM KCl, 0.1369 M NaCl, pH 7.4) followed by ice-cold 4% paraformaldehyde (PFA) in PBS. Corresponding tissues were removed, postfixed for 2 h in 4% PFA, cryoprotected in 30% sucrose overnight at 4 °C, and finally frozen in tissue-freezing OCT compound (Leica, Wetzlar, Germany). Serial 14 µm-thick sections were generated using a cryostate (Microm HM560; Leica) and thaw-mounted onto Superfrost Plus slides (Menzel, Roth, Germany).
In situ hybridization
Colorimetric in situ hybridization was performed as described previously (Behrens et al. 2007). Fluorescent in situ hybridization were mainly performed as described in (Voigt et al. 2012) with some adaptations. PKD2L1-specific riboprobes were hybridized at 55 °C with 40% formamide concentration, RNase buffer contained 0.83 µg/ml RNase A, washing steps were performed at 50 °C with 0.4X saline-sodium citrate buffer, and Fluorescein-tyramide (PerkinElmer; 1:50) in diluent was applied onto the slides for exactly 15 min.
The probe for Car4 was based on Allen Brain Atlas (Allen Institute for Brain Science), whereas the template for the mCherry probe was generated by using following oligonucleotides 5′-ATGGTGAGCAAGGGCGAGGAGGATAAC-3′ and 5′-TTACTTGTACAGCTCGTCCATGCCGCC-3′. Probe sequence of PKD2L1 was amplified by using oligonucleotides 5′-CCCA CTCCTGGACAGTTTGT-3′ and 5′-AGAATGTTCCAGACGCTG CT. Fragments were cloned into pBluescript II KS+ (Stratagene, Amsterdam, Netherlands; Car4 and PKD2L1) or TOPO TA vector (Thermo Fisher Scientific, Schwerte, Germany; mCherry), respectively. The cloned cDNAs were used for in vitro transcription reactions in the presence of Dig-dUTP as before (Voigt et al. 2012). Colorimetric in-situ hybridization was digitalized by using upright microscope (Nikon Eclipse Ni U), whereas fluorescent tissue sections were detected by using confocal laser scanning microscope (Leica TCS SP8). The brightness of micrographs of colorimetric in-situ hybridizations was adjusted using the CorelDraw Software to optimize signals; identical settings were applied equally to all micrographs.
Immunohistochemistry
Tissue sections were fixed in 4% PFA in PBS, rinsed with PBS and, if necessary, incubated in preheated target retrieval solution (Dako, Hamburg, Germany) at 80 °C for 10 min (AADC, GAD67, and SNAP25). Subsequently, sections were washed with PBS, incubated in PBS with 0.5% Triton X-100 for 10 min, rinsed with TNT buffer (0.1 M Tris, 0.15 M NaCl, 0.05% Tween 20), treated with blocking solution (TNB; 0.1 M Tris, 0.15 M NaCl, 0.5% blocking reagent (Roche, Mannheim, Germany)) for 1 h at room temperature, followed by overnight incubation of the primary antibody in TNB at 4 °C. Primary antibodies included Car4 (1:100; AF2414, R&D Systems, Wiesbaden-Nordenstadt, Germany), dsRed (anti-mCherry; 1:200; 632496, Clontech Laboratories, Mountain View, USA), nucleoside triphosphate diphosphohydrolase 2 (NTPDase, 1:500; Bartel et al. 2006), phospholipase Cβ2 (PLCβ2, 1:500; sc-206, Santa Cruz Biotechnology, Heidelberg, Germany), transient receptor potential cation channel subfamily M member 5 (TrpM5, 1:5000; Kusumakshi et al. 2015), AADC (1:500; BZL02908, Biozol, Eching, Germany), GAD67 (1:100; sc-7512, Santa Cruz Biotechnology), and SNAP25 (1:100; GTX89577, GeneTex, Irvine, USA). After washing with TNT buffer, tissues were incubated for 1 h at room temperature with appropriate secondary antibodies. Immunofluorescence of labeled cells and mCherry auto-fluorescence were observed by using confocal laser scanning microscope. Collected data reflect results from tissue sections of a minimum of 5 mice.
Results
Generation of Car4-IRES-mCherry mice
For the generation of Car4-IRES-mCherry knock-in mice, the sequences for IRES-mCherry were integrated directly behind exon 8 of the Car4 coding sequence by homologous recombination. To this end, we electroporated the linearized targeting construct into R1 embryonic stem cells and picked 284 neomycin-resistant embryonic stem cell clones (neo+; see Figure 1A). Genomic Southern blot analysis identified correctly and incorrectly modified stem cell clones (Figure 1B, C). In total, 54 correctly targeted clones were detected, corresponding to a targeting frequency of 19%.
We expanded 2 of these correctly targeted clones and injected them into C57BL/6J blastocysts from which Car4-IRES-mCherryneo+ chimeric mice were derived. These animals were backcrossed with C57BL/6J mice. Offspring animals revealed the targeted modification of the Car4 locus, confirming germ-line transmission of the mutated allele (Figure 1D). Furthermore, Southern blot analyses demonstrated the successful self-excision of the neomycin-resistance selection cassette (Car4-IRES-mCherryneo-, Figure 1A, D). No differences were observed concerning the number and co-localization of mCherry-positive cells in different lingual papillae between the 2 lines of mice that were derived from independent embryonic stem cell clones. Therefore, the subsequent experiments were performed with only one of these lines. Animals of both sexes appeared healthy and showed normal viability and fertility.
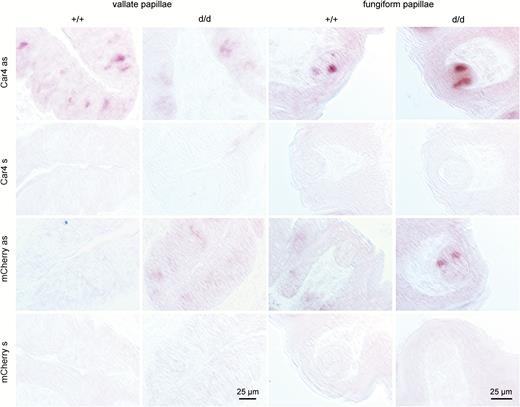
The modification at the Car4 locus resulted in the transcription of a bicistronic messenger RNA from which Car4 and mCherry are independently translated. The presence of Car4/mCherry transcripts in vallate and fungiform papillae of Car4-IRES-mCherry mice was confirmed by in situ hybridization (Figure 2). Car4 mRNA was observed in tissue sections of wild-type and homozygous Car4-IRES-mCherry mice. Expression of mCherry transcripts was detected in both vallate and fungiform papillae of Car4-IRES-mCherry mice, but not in sections of wild-type mice. Control experiments with the sense riboprobes did not label any cells confirming the specificity of the observed signals.
Messenger RNA expression of Car4 and mCherry in taste tissue of Car4 mice. Chromogenic in situ hybridization in vallate and fungiform papillae of wild-type (+/+) and homozygous (d/d) Car4 knock-in mice. In situ hybridization experiments demonstrate that Car4 is expressed in a subset of vallate and fungiform taste bud cells of wild-type and homozygous Car4 mice, whereas mCherry was only detectable expressed in tissue sections of knock-in mice when sections were treated with corresponding antisense (as) riboprobes. Tissue sections treated with sense (s) riboprobes did not reveal any signals, independent of genotype or probe.
Expression of Car4 in gustatory tissue
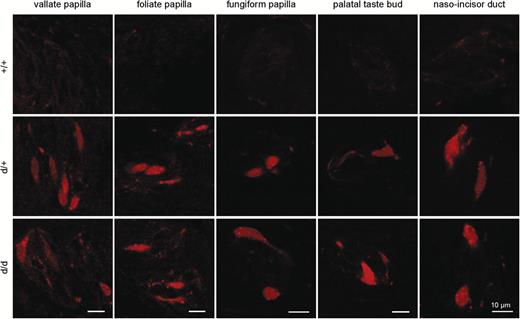
Monomeric Cherry expression occurred in taste buds of all gustatory papillae (Figure 3). In total, we analyzed 2103 taste buds containing 5802 mCherry-positive cells. On average, we detected 1.7, 3.1, 2.9, 2.7, and 2.0 mCherry-positive cells per taste bud section of fungiform, foliate, vallate papillae, palate and NID, respectively.
Presence of mCherry auto-fluorescence in taste papillae. Auto-fluorescence of mCherry in tissue sections of various taste papillae of Car4 wild-type (+/+), heterozygous (+/d), and homozygous (d/d) animals.
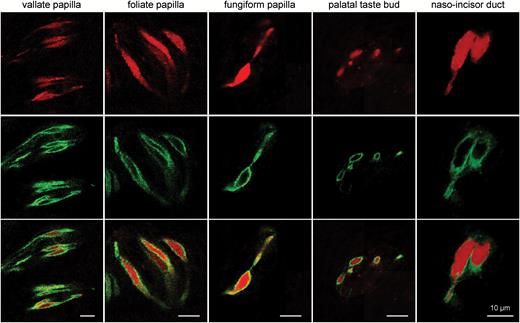
In order to demonstrate correct mCherry expression we co-localized mCherry auto-fluorescence with indirect Car4 immunofluorescence using a specific Car4 antiserum. Overall, in fungiform, foliate, vallate, and palatal taste cells as well as in taste cells of the naso-incisor duct 99.9% of the mCherry-positive cells showed Car4 immunofluorescence, vice versa 96.1% of the Car4 immunofluorescent taste cells demonstrated mCherry fluorescence. This congruence indicates correct expression of mCherry (Figure 4).
Expression of mCherry auto-fluorescence in Car4-positive cells. Immunohistochemistry for Car4 directly illustrates cellular co-expression of Car4 immunofluorescence (green; middle panel) and mCherry auto-fluorescence (red; upper panel) in all taste papillae, indicating acute expression of mCherry in Car4-expressing cells.
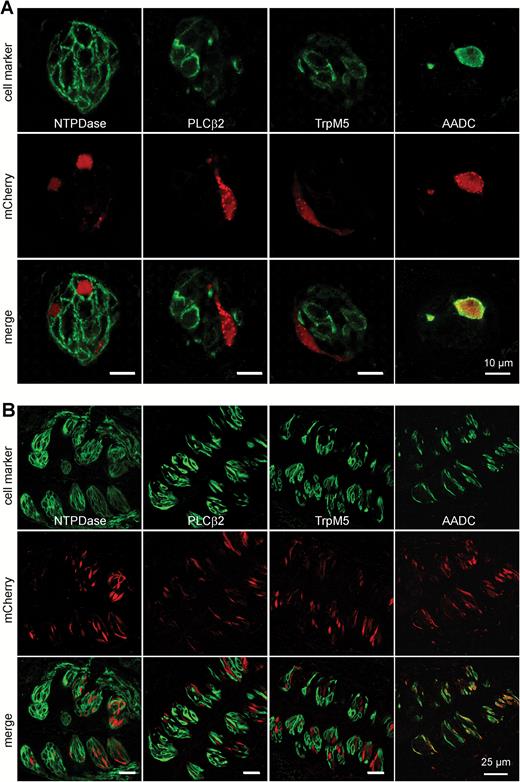
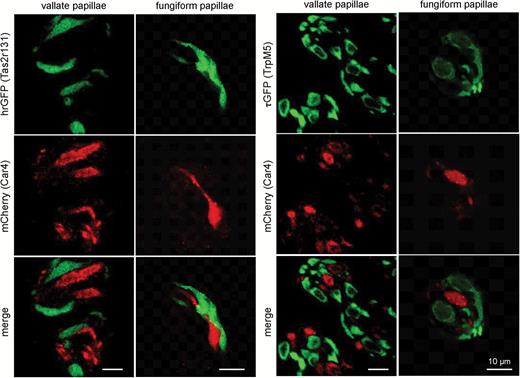
To characterize mCherry-positive cells in Car4-IRES-mCherry mice in more detail, we performed co-localization studies using indirect immunofluorescence and antisera for various cell type markers. These experiments revealed that mCherry is co-expressed with the Type III cell marker AADC but not with NTPDase2 which marks Type I cells. Complete lack of co-expression was also evident for PLCβ2 and TrpM5, which are indispensable components in sweet, umami, and bitter taste transduction and label Type II cells (Figure 5). To further exclude mCherry expression in Type II cells, we crossed Car4-IRES-mCherry mice with TRPM5-IRES-Cre/eR26-τGFP and Tas2r131-BL-IRES-hrGFP mice which express τGFP in TRPM5 cells and hrGFP in bitter-sensing cells. No overlap was observed between cells showing mCherry and τGFP or hrGFP auto-fluorescence in lingual tissue sections of these animals (Figure 6). Thus, the results confirm that mCherry fluorescence selectively labels Car4-positive presynaptic Type III cells.
Car4 is selectively expressed in presynaptic taste bud cells. Double-labeled images of fungiform (A) and vallate papillae (B) sections of homozygous Car4-IRES-mCherry mice stained for markers of Type I (NTPDase 2), Type II (PLCβ2, TrpM5) and Type III (AADC) taste cells. Monomeric Cherry auto-fluorescence shown in red, immunofluorescence of cell markers in green. Overlay images show that taste cells positive for mCherry do not express Type I and II cell markers, but co-localized with the AADC cell marker.
Expression of mCherry and GFP auto-fluorescence in tissue sections of vallate and fungiform papillae of Car4-IRES-mCherry/Tas2r131-BL-IRES-hrGFP and Car4-IRES-mCherry/TRPM5-IRES-Cre/eR26-τGFP mice. Co-localization of auto-fluorescences reveal no overlap between hrGFP fluorescence, indicating Tas2r131 expression (green, right panel), or τGFP fluorescence, indicating TrpM5 expression (green, left panel), and mCherry fluorescence, indicating Car4 expression (red) independent of taste papillae.
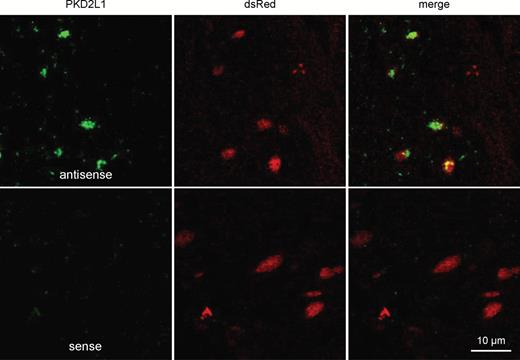
To examine if Type III cells form a homogeneous population or are composed of different subsets of cells we investigated the co-localization of mCherry with marker proteins by in situ hybridization and immunohistochemistry experiments. We performed in situ hybridization experiments with riboprobes for the sour cell marker PKD2L1 in vallate papillae of Car4-IRES-mCherry mice. We found that 97% of the PKD2L1-positive cells were also positive for the dsRed indicative of Car4 expression (Figure 7). However, in the reverse case, only 80% of the dsRed-positive taste cells were also labeled by the PKD2L1 antisense riboprobe suggesting that 20% of the Car4 cells represent a subpopulation devoid of PKD2L1.
Car4 co-localizes with PKD2L1 in vallate papillae. Confocal images of vallate taste bud sections of homozygous Car4-IRES-mCherry mice, treated with antisense and sense riboprobes for PKD2L1 (green) and labeled with anti-mCherry immunofluorescence (dsRed; red). Expression of PKD2L1 mRNA could only be co-localized with immunostaining for mCherry.
Based on immunohistochemical approaches we analyzed various taste papillae. In fungiform papillae we analyzed 123 taste buds and found that 98% of mCherry-positive cells co-expressed AADC and vice versa 99% of AADC-positive cells showed mCherry auto-fluorescence suggesting that Type III cells represent a homogeneous population which expresses both Car4 and AADC (Figure 5, 8, Table 1). In contrast, in 389 analyzed vallate taste buds only 81% of the AADC-positive cells were also positive for mCherry (Figure 5, Table 1A), whereas all mCherry-positive taste cells showed AADC immunofluorescence (Table 1B). Similar results were obtained for foliate papillae. In 300 investigated foliate taste buds, 86% of AADC-positive cells also showed mCherry expression (Table 1A), yet all mCherry-positive cells showed AADC immunoreactivity (Table 1B). We obtained similar results by using antisera against both Car4 and AADC. In this case, 99%, 86%, and 90% of the AADC-positive cells revealed Car4-immunoreactivity in fungiform, vallate, and foliate papillae, respectively. Thus, though fungiform Type III cells appear to be homogeneous regarding Car4 and AADC expression, the posterior Type III cells can be divided into 2 subpopulations, one that comprises 80–90% of the cells and expresses both Car4 and AADC and a population of 10–20% that does express AADC but not Car4.
Percent of immunohistochemical markers co-localized with mCherry auto-fluorescence (A) and vice versa (B)
| A . | |||||
|---|---|---|---|---|---|
| . | fungiform papillae . | foliate papillae . | vallate papillae . | palatal taste buds . | taste buds of naso-incisor duct . |
| Car4 | 99.5% (181/182) | 96.2% (655/681) | 96.2% (1127/ 1171) | 93.2% (300/322) | 96.7% (88/91) |
| AADC | 98.5% (200/203) | 85.6% (924/ 1079) | 81.1% (1802/ 2221) | 95.9% (579/604) | 97.6% (124/127) |
| SNAP25 | 95.2% (40/42) | 82.5% (146/177) | 78.8% (167/212) | 99.4% (165/166) | 100% (18/18) |
| GAD67 | 100% (14/14) | 83.1% (157/189) | 87.8% (172/196) | 97.0% (162/167) | 97.8% (44/45) |
| B | |||||
| fungiform papillae | foliate papillae | vallate papillae | palatal taste buds | taste buds of naso-incisor duct | |
| Car4 | 100% 181/181) | 100% (655/655) | 99.8% (1127/ 1129) | 99.7% (300/301) | 100% (88/88) |
| AADC | 97.6% (200/205) | 100% (924/924) | 100% (1802/ 1802) | 98.6% (579/587) | 98.4% (124/126) |
| SNAP25 | 100% (40/40) | 100% (146/146) | 100% (167/167) | 100% (165/165) | 100% (18/18) |
| GAD67 | 93.3% (14/15) | 100% (157/157) | 100% (172/172) | 97.6% (162/166) | 91.7% (44/48) |
| A . | |||||
|---|---|---|---|---|---|
| . | fungiform papillae . | foliate papillae . | vallate papillae . | palatal taste buds . | taste buds of naso-incisor duct . |
| Car4 | 99.5% (181/182) | 96.2% (655/681) | 96.2% (1127/ 1171) | 93.2% (300/322) | 96.7% (88/91) |
| AADC | 98.5% (200/203) | 85.6% (924/ 1079) | 81.1% (1802/ 2221) | 95.9% (579/604) | 97.6% (124/127) |
| SNAP25 | 95.2% (40/42) | 82.5% (146/177) | 78.8% (167/212) | 99.4% (165/166) | 100% (18/18) |
| GAD67 | 100% (14/14) | 83.1% (157/189) | 87.8% (172/196) | 97.0% (162/167) | 97.8% (44/45) |
| B | |||||
| fungiform papillae | foliate papillae | vallate papillae | palatal taste buds | taste buds of naso-incisor duct | |
| Car4 | 100% 181/181) | 100% (655/655) | 99.8% (1127/ 1129) | 99.7% (300/301) | 100% (88/88) |
| AADC | 97.6% (200/205) | 100% (924/924) | 100% (1802/ 1802) | 98.6% (579/587) | 98.4% (124/126) |
| SNAP25 | 100% (40/40) | 100% (146/146) | 100% (167/167) | 100% (165/165) | 100% (18/18) |
| GAD67 | 93.3% (14/15) | 100% (157/157) | 100% (172/172) | 97.6% (162/166) | 91.7% (44/48) |
Taste cells immunoreactive for one of the analyzed markers were checked for co-localization. Cells were scored as positive, as they exhibited fluorescent above background levels of reactivity. All entries reflect data from a minimum of 5 mice.
Percent of immunohistochemical markers co-localized with mCherry auto-fluorescence (A) and vice versa (B)
| A . | |||||
|---|---|---|---|---|---|
| . | fungiform papillae . | foliate papillae . | vallate papillae . | palatal taste buds . | taste buds of naso-incisor duct . |
| Car4 | 99.5% (181/182) | 96.2% (655/681) | 96.2% (1127/ 1171) | 93.2% (300/322) | 96.7% (88/91) |
| AADC | 98.5% (200/203) | 85.6% (924/ 1079) | 81.1% (1802/ 2221) | 95.9% (579/604) | 97.6% (124/127) |
| SNAP25 | 95.2% (40/42) | 82.5% (146/177) | 78.8% (167/212) | 99.4% (165/166) | 100% (18/18) |
| GAD67 | 100% (14/14) | 83.1% (157/189) | 87.8% (172/196) | 97.0% (162/167) | 97.8% (44/45) |
| B | |||||
| fungiform papillae | foliate papillae | vallate papillae | palatal taste buds | taste buds of naso-incisor duct | |
| Car4 | 100% 181/181) | 100% (655/655) | 99.8% (1127/ 1129) | 99.7% (300/301) | 100% (88/88) |
| AADC | 97.6% (200/205) | 100% (924/924) | 100% (1802/ 1802) | 98.6% (579/587) | 98.4% (124/126) |
| SNAP25 | 100% (40/40) | 100% (146/146) | 100% (167/167) | 100% (165/165) | 100% (18/18) |
| GAD67 | 93.3% (14/15) | 100% (157/157) | 100% (172/172) | 97.6% (162/166) | 91.7% (44/48) |
| A . | |||||
|---|---|---|---|---|---|
| . | fungiform papillae . | foliate papillae . | vallate papillae . | palatal taste buds . | taste buds of naso-incisor duct . |
| Car4 | 99.5% (181/182) | 96.2% (655/681) | 96.2% (1127/ 1171) | 93.2% (300/322) | 96.7% (88/91) |
| AADC | 98.5% (200/203) | 85.6% (924/ 1079) | 81.1% (1802/ 2221) | 95.9% (579/604) | 97.6% (124/127) |
| SNAP25 | 95.2% (40/42) | 82.5% (146/177) | 78.8% (167/212) | 99.4% (165/166) | 100% (18/18) |
| GAD67 | 100% (14/14) | 83.1% (157/189) | 87.8% (172/196) | 97.0% (162/167) | 97.8% (44/45) |
| B | |||||
| fungiform papillae | foliate papillae | vallate papillae | palatal taste buds | taste buds of naso-incisor duct | |
| Car4 | 100% 181/181) | 100% (655/655) | 99.8% (1127/ 1129) | 99.7% (300/301) | 100% (88/88) |
| AADC | 97.6% (200/205) | 100% (924/924) | 100% (1802/ 1802) | 98.6% (579/587) | 98.4% (124/126) |
| SNAP25 | 100% (40/40) | 100% (146/146) | 100% (167/167) | 100% (165/165) | 100% (18/18) |
| GAD67 | 93.3% (14/15) | 100% (157/157) | 100% (172/172) | 97.6% (162/166) | 91.7% (44/48) |
Taste cells immunoreactive for one of the analyzed markers were checked for co-localization. Cells were scored as positive, as they exhibited fluorescent above background levels of reactivity. All entries reflect data from a minimum of 5 mice.
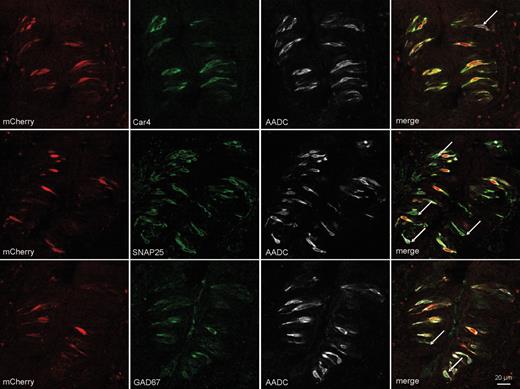
Differential expression of Car4 in presynaptic taste cells of vallate papillae. Confocal images of triple-labeled sections of vallate taste buds labeled with markers for Type III cells Car4, SNAP25, GAD67 (green), AADC (white), and mCherry auto-fluorescence (red). Monomeric Cherry and Car4 are present in AADC-, SNAP25-, or GAD67-immunoresponsive taste bud cells. However, not all AADC-, SNAP25-, or GAD67-positive cells showed mCherry or Car4 auto- and immunofluorescence, respectively (indicated by arrows), indicating the presence of Type III-positive cells in vallate papillae, which do not express Car4.
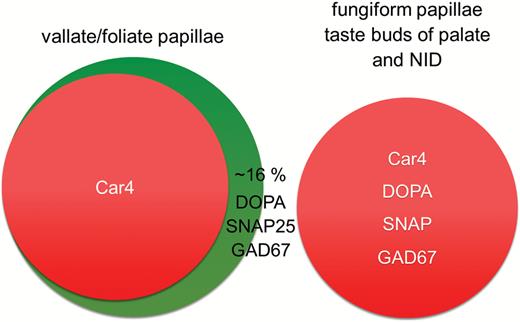
In sections of Car4-IRES-mCherry mice mCherry fluorescence was co-localized with immunoreactivity for the marker proteins SNAP25, a component of the vesicular release machinery, and GAD67 which converts glutamate to the neurotransmitter γ-aminobutyric acid. In vallate and foliate papillae, only 81% and 86% of the SNAP25 and GAD67 immunofluorescent taste cells also showed mCherry fluorescence, respectively, whereas in fungiform papillae 95% of the cells with SNAP25 immunoreactivity and all cells positive for GAD67 immunoreactivity also demonstrated mCherry fluorescence (Table 1). Vice versa, mCherry-positive cells of fungiform papillae were 100% SNAP25 and 93% GAD67 immunoreactive (Table 1B). We also determined the overlap of AADC-, SNAP25-, and GAD67-immunoreactivity to be >99% (Figure 8) in all types of taste papillae. AADC-, SNAP25-, and GAD67-immunoreactive cells co-expressed mCherry fluorescence to similar extent (Table 1A). These results suggest that all presynaptic taste cells in fungiform papillae express Car4, AADC, SNAP25, and GAD67, whereas in the lingual posterior papillae only a subpopulation of about 84% of the AADC-, SNAP25-, and GAD67-positive cells additionally expresses Car4 (Figure 9). Taste cells in the soft palate, Geschmacksstreifen, and NID resemble fungiform taste cells in that they co-express all 4 marker proteins (Figure 9). Thus, it appears that in taste buds that are innervated by the 7th cranial nerve sour/carbon dioxide-sensing cells that are characterized by Car4 equal the presynaptic cells characterized by the presynaptic proteins AADC, GAD67, and SNAP25. In taste buds innervated by the 9th cranial nerve, only 84% of the presynaptic cells appear to be capable of sensing sourness/carbonation as evidenced by Car4 expression whereas 16% do not.
Composition of Type III cell population depends on topography. Predicted Type III cell population based on immunofluorescent analysis. Co-localization studies revealed differences in Type III cell populations in posterior tongue papillae and fungiform papillae. Type III cell population of taste buds in palate and naso-incisor duct (NID) is equivalent to fungiform papillae.
Discussion
In the present study, we used a genetic approach to visualize Car4-expressing cells in vivo based on a knock-in strategy. The modification at the Car4 locus resulted in the transcription of a bicistronic messenger RNA from which both Car4 and the fluorescent protein mCherry are translated.
As a control for the appropriate expression of the marker protein, the recombinant mCherry was visualized by its fluorescence properties in the lingual papillae. We detected mCherry-positive cells in vallate, foliate, and fungiform papillae as well as in taste buds of the palate or NID (Figure 3). These data are consistent with previous studies showing Car4 expression in all 3 tongue taste papillae and in the palate (Chandrashekar et al. 2009; Chandrashekar et al. 2010). Using a specific Car4-antiserum we found that almost 100% of the cells with mCherry-fluorescence also showed immunoreactivity for Car4 in all types of oral taste buds analyzed which confirms faithful expression of the mCherry reporter in our Car4-IRES-mCherry mice (Figure 4).
Gustatory expression of Car4
Within the gustatory system, we did not observe mCherry fluorescence in cells positive for NTPDase-, PLC-, or TrpM5-immunoreactivity (Figure 5) demonstrating that Car4 is not expressed in Type I and Type II cells, which was further verified by the absence of co-labeling after intercrossing Car4-IRES-mCherry animals with Tas2r131-BL-IRES-hrGFP and TrpM5-IRES-Cre/eR26-τGFP mice, representing a bitter taste receptor cell subpopulation and the entire Type II taste cell population, respectively (Figure 6). In contrast, mCherry fluorescence co-localized with immunoreactivity for the Type III cell markers, PKD2L1, AADC, SNAP25, and GAD67 (Figures 7 and 8) confirming and extending previous reports (Chandrashekar et al. 2009; Chandrashekar et al. 2010).
In fungiform papillae and taste buds of the palate and NID, mCherry signals completely overlapped with immunoreactivities for the presynaptic proteins AADC, SNAP25, and GAD67 (Figure 8; Table 1B) suggesting that in taste tissues supplied by the 7th cranial nerve the Type III cells form a homogenous population combining presynaptic properties and the ability to sense acidic stimuli as well as carbonation or external water. In contrast, in the foliate and vallate papillae only about 84% of the cells positive for AADC-, SNAP25-, or GAD67 immunoreactivity express mCherry or Car4 and therefore combine presynaptic and sour/carbon dioxide/water-sensing properties whereas a substantial population of cells (16%) do express the AADC-, SNAP25-, or GAD67 presynaptic markers in the absence of mCherry/Car4 (Figure 8). Intriguingly, previous experiments characterizing Type III taste cells in GAD67-GFP mice showed that about 80% of the cells in vallate taste buds co-express GFP and a neurotransmitter of Type III cells 5-HT, whereas about 20% expressed only 5-HT (Tomchik et al. 2007). In the past, the fact that the genetic ablation of PKD2L1-expressing cells affected only the sour sensing abilities of mice, whereas the other taste qualities remained unimpaired was put forward as an argument against the role of presynaptic Type III cells as broadly tuned integrators of multiple taste qualities (Huang et al. 2006; Chandrashekar et al. 2010). From the heterogeneous appearance of Type III cells reported here it is tempting to speculate that the Car4-negative Type III cells indeed facilitate transmission of multiple taste qualities (Tomchik et al. 2007), while the Car4-positive taste cells could represent the PDK2L1-expressing sour-sensitive subpopulation of Type III cells. However, our data are not fully consistent with the hypothesis that Car4 is always co-localized with PKD2L1 (Figure 7). Even though cells labeled by PKD2L1 antisense riboprobe almost always showed dsRed immunofluorescence, not all dsRed-positive cells were co-localized with PKD2L1 mRNA. This discrepancy could be due to an insufficient sensitivity to detect low-level PKD2L1-expressing cell by in situ hybridization. Immunohistochemical analyses of others indeed indicate an opposite situation that not all PKD2L1-positive taste cells were also labeled by Car4 antisera (Chandrashekar et al. 2009), again indicating the existence of subpopulations of presynaptic taste cells. Accordingly, Yee and coworkers reported that approximately 10% of PGP-9.5-immunoresponsive taste bud cells did not express PKD2L1 (Yee et al. 2001). However, others reported a nearly complete overlap between SNAP25, AADC, GAD67, serotonin, and PKD2L1 (DeFazio et al. 2006; Tomchik et al. 2007; Kataoka et al. 2008; Horio et al. 2011). Whereas Car4-positive cells as well as PKD2L1-positive cells might represent subpopulations of Type III cells, our data also indicate that the often used Type III cell markers AADC, SNAP25, and GAD67 show a nearly complete overlap indicating that all of these markers label the entire Type III cell population. Consistently, Tomchik et al. reported that nearly 100 % of GAD67-positive taste cells express SNAP25 and 5-HT based on immunohistochemistry (Tomchik et al. 2007), and DeFazio et al. showed that a high degree of overlap for SNAP25, NCAM, and AADC based on single cell RT-PCR (DeFazio et al. 2006). Furthermore, a substantial co-expression of AADC, PGP9.5, and NCAM was reported by Seta et al. (2007), whereas Yee et al. (2001) observed that most NCAM-positive cells also contained 5-HT and PGP9.5, however some NCAM-positive taste cells did not display 5-HT or PGP9.5. Hence, the Type III cell population seems to be dominantly characterized by the co-expression of SNAP25, 5-HT, GAD67, AADC, and PGP9.5, with subpopulations expressing additionally NCAM, PKD2L1, and Car4. Future studies will be necessary to elucidate whether the subpopulations characterized by the latter markers represent a single cell type characterized by the expression of all 3 molecules or subpopulations somewhat independent of each other.
In conclusion, our Car4-IRES-mCherry mice represent a reliable tool to visualize Car4-expressing cells in oral taste tissue. They also allowed us to assign Car4 expression selectively to Type III cells and furthermore were helpful to describe the subpopulation structure of these cells and their topographical differences. Finally, because we used a knock-in rather than a knock-out strategy Car4 remains intact in this strain of mice which ensures that the function of Car4 in these cells can be investigated in vivo and in vitro. Additional experiments will be necessary to assess the functional differences between presynaptic taste cells expressing Car4 or not.
Funding
This work was supported by the federal ministry for education and research (BMBF) and the ministry for science, research, and culture (MWFK) of the State of Brandenburg, Germany.
Acknowledgements
The authors thank Josefine Würfel and Stefanie Demgensky (Nuthetal) for technical assistance and Veit Flockerzi (Homburg) for generously providing the anti-TrpM5 antiserum. Furthermore, the authors thank Anja Voigt and Ulrich Boehm for providing Tas2r131-BL-IRES-hrGFP and TRPM5-IRES-Cre/eR26-τGFP mice, respectively.
Reference