-
PDF
- Split View
-
Views
-
Cite
Cite
Thomas A. Schoenfeld, Thomas A. Cleland, Anatomical Contributions to Odorant Sampling and Representation in Rodents: Zoning in on Sniffing Behavior, Chemical Senses, Volume 31, Issue 2, February 2006, Pages 131–144, https://doi.org/10.1093/chemse/bjj015
Close - Share Icon Share
Abstract
Odorant sampling behaviors such as sniffing bring odorant molecules into contact with olfactory receptor neurons (ORNs) to initiate the sensory mechanisms of olfaction. In rodents, inspiratory airflow through the nose is structured and laminar; consequently, the spatial distribution of adsorbed odorant molecules during inspiration is predictable. Physicochemical properties such as water solubility and volatility, collectively called sorptiveness, interact with behaviorally regulable variables such as inspiratory flow rate to determine the pattern of odorant deposition along the inspiratory path. Populations of ORNs expressing the same odorant receptor are distributed in strictly delimited regions along this inspiratory path, enabling different deposition patterns of the same odorant to evoke different patterns of neuronal activation across the olfactory epithelium and in the olfactory bulb. We propose that both odorant sorptive properties and the regulation of sniffing behavior may contribute to rodents' olfactory capacities by this mechanism. In particular, we suggest that the motor regulation of sniffing behavior is substantially utilized for purposes of “zonation” or the direction of odorant molecules to defined intranasal regions and hence toward distinct populations of receptor neurons, pursuant to animals' sensory goals.
Introduction
Odorant sampling is a spatially and temporally dynamic behavior displayed by many animal species that draws odorant molecules into contact with the olfactory organ (arthropod antenna or antennule or vertebrate nose) in necessary conformity with the principles of fluid dynamics (Koehl, 2005; Zhao et al., 2005). Consequently, odorant-induced activity in the brain reflects the spatiotemporal patterns associated with odorant sampling (Buonviso et al., 2005; Kepecs et al., 2005; Scott, 2005; Vickers, 2005). A major question, and one addressed by many of the authors in this collection, is to what extent such sampling and sampling-dependent activities contribute measurably to odorant discrimination.
It is clear that the structure of odorant molecules normally suffices to enable their discrimination via the binding of their component odotopes to specific membrane receptors on olfactory receptor neurons (ORNs), thus potentially rendering variations in sampling behavior superfluous to the process of stimulus perception. However, there is increasing evidence that the distribution of odorant molecules in fluid streams during sampling could be a source of additional information useful for determining the identity of an odorant and/or its source in the external environment (Koehl, 2005; Mainland and Sobel, 2005; Scott, 2005; Vickers, 2005; Zhao et al., 2005). Moreover, in keen-smelling terrestrial vertebrates such as rodents, there is corresponding evidence that the spatial organization of the olfactory epithelium (OE), including the restricted distribution patterns of odorant receptor (OR) genes and hence of ORN molecular receptive ranges, is aligned with the patterning of inspiratory airflow (Scott, 2005; Zhao et al., 2005) and could serve to extract information about odorant physicochemical properties and convey this to the main olfactory bulb (MOB).
We examine in this article how the spatial organization of ORNs and MOB circuitry in rodents is aligned with the spatial dimensions of inspiratory airflow, how this provides an anatomical basis for differentiating among odorants on the basis of their deposition patterns during inspiration, and how sniffing behavior, an active sampling process, could use this differentiation for investigating and discriminating odorants. A more focused version of this discussion appears elsewhere (Schoenfeld and Cleland, 2005).
The rodent olfactory system maps olfactory airspace in the nose onto the MOB
The specialized anatomy of rodent nasal passages induces structured inspiratory and expiratory airflow through the nose (Zhao et al., 2005). Inspiratory airflow through the olfactory recesses is guided by the orientation of the ethmoid turbinates (Figure 1). Each nasal cavity is divided into separable, longitudinally oriented medial and lateral recesses that comprise separate but parallel medial and lateral air channels, much like the two cavities themselves. As demonstrated initially using physical models (Morgan et al., 1991) and subsequently confirmed and extended with computational modeling (Kimbell et al., 1997; Zhao et al., 2005), inspiratory airflow courses first through a central domain associated with the dorsal meatus that is common to both the medial and lateral channels and then, more caudally, diverges into the medial and lateral recesses (Figure 2). While diverging, the airflow enters more peripheral domains within the cavity (more ventral, lateral, and even dorsal than the central domain) before exiting through the internal naris at the septal window into the nasopharynx (see also Schoenfeld and Knott, 2002; Schoenfeld and Knott, 2004). The epithelial surface can thereby be coarsely segregated into central and peripheral domains and into lateral and medial channels (Figure 1). While the lateral and medial channels are in many ways comparable, computational modeling indicates that air flows more slowly through the lateral than the medial channel, potentially affecting odorant deposition (Kimbell et al., 1997). Given the relatively small diameter of these passageways, airflow through the rodent nose is reliably laminar over a wide range of flow rates, in contrast to the turbulence that characterizes flow through the larger human nose during sniffing (Kimbell et al., 1997; Zhao et al., 2005). Although the significance of this difference is not clear, laminar flow could permit minute differences in odorant concentration within the carrier airstream to have a more predictable effect on the number of molecules that diffuse laterally to the mucosal wall.
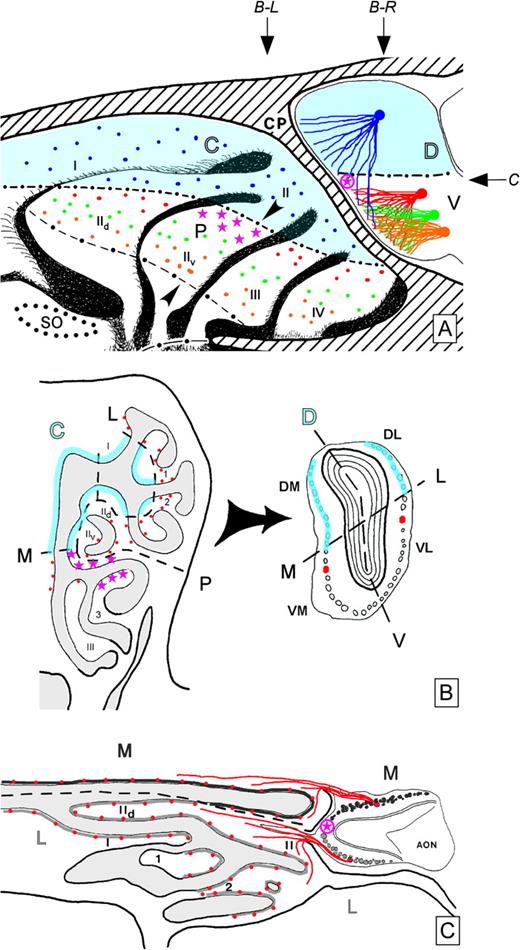
Relationship between rhinotopic and odotopic organization in the rodent olfactory system, illustrated with drawings of the hamster nose and MOB. (A) Sagittal view of exposed medial aspects of the nose (left) and MOB (right), separated by the cribriform plate (CP). Zonally distributed ORNs (colored dots) associated with central (C) and peripheral (P) domains of the olfactory recesses project convergently onto glomeruli (colored lines leading to filled circles) in the dorsal (D) and ventral (V) MOB, respectively (e.g., Wang et al., 1998; Alenius and Bohm, 2003). Focally restricted ORNs (magenta stars) lie in a patch near the C-P and M-L boundaries in the olfactory recesses and project convergently onto glomeruli (encircled magenta star) near comparable boundaries in the MOB (e.g., Strotmann et al., 2000). Blue shading denotes staining of the central-dorsal projections with reduced nicotinamide adenine dinucleotide phosphate (NADPH) diaphorase histochemistry (Schoenfeld and Knott, 2002; Alenius and Bohm, 2003). Arrowheads on endoturbinate IIv denote the approximate position of a known M-L boundary (see Schoenfeld et al., 1994; Bozza and Kauer, 1998; Levai et al., 2003). Arrows denote the approximate levels of section shown in the other figure panels (B-L: panel B, left; B-R: panel B, right; C: panel C). Black dots at the lower left mark the encircled position of the SO, where it would be found on the septum opposite to the lateral wall shown. (B) Coronal sections through the nose (left) and MOB (right). Blue shading is as in panel A. Red dots represent peripheral domain ORN populations near the C-P border in the medial (M) and lateral (L) channels, expressing the same OR gene, for example, P2, but projecting separately to glomeruli on the M and L hemispheres of the MOB, respectively, near the D-V border, as in panel A (e.g., Ressler et al., 1994; Vassar et al., 1994; Alenius and Bohm, 2003). Magenta stars denote focally restricted ORNs as in panel A. The MOB section represents a mid-level coronal plane caudal to that containing the glomerular target (encircled magenta star) of the focally restricted ORNs. Note that medial and lateral pairs of homologous glomeruli are not typically found in the same coronal plane, as illustrated, but are oriented with the lateral glomerulus positioned more rostrally (as depicted in panel C). (C) Horizontal section through the nose and MOB (rostral to the left). Projections to M and L glomeruli (dark gray and light gray circles, respectively) arise from spatially segregated populations of ORNs associated with the M and L recesses (dark gray and light gray lines, respectively) (Schoenfeld et al., 1994; Levai et al., 2003). Red dots and convergent projections to glomeruli are as described in panels A and B and illustrate the mutually exclusive nature of M-L divergence. The encircled magenta star denotes the approximate position of the glomerular target of focally restricted ORNs, as depicted in panel A. AON, anterior olfactory nucleus; DL, dorsal–lateral; DM, dorsal–medial; VL, ventral–lateral; VM, ventral–medial; I, IId, IIv, III, IV, endoturbinates; 1, 2, 3, ectoturbinates.
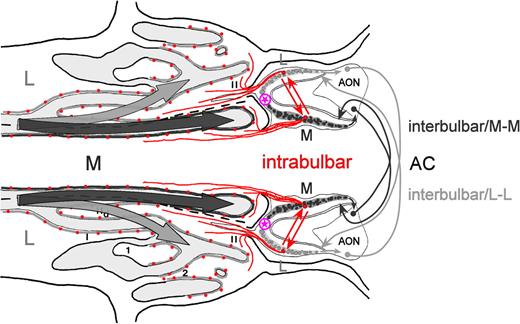
Spatial relationships between the diverging medial and lateral paths of inspiratory airflow in the nose and the neural connections of two MOB circuits. The drawing represents a horizontal section through the nose and cranium (cf., Figure 1C) that includes both nasal cavities (left) and both MOBs (right). Arrows from left denote medial (dark gray shading; M) and lateral (light gray shading; L) paths of inspiratory airflow, stimulating the OE of the medial channel (dark gray line) and lateral channel (light gray line), respectively. Medial OE projects exclusively to glomeruli of the medial MOB (dark gray shading and circles), whereas lateral OE projects exclusively to lateral MOB glomeruli (light gray shading and circles). Red dots and convergent projections to glomeruli are as shown in Figure 1C, as is the encircled magenta star. Red arrows denote the reciprocal projections of the intrabulbar associational system that interconnects neurons associated with homologous glomeruli within each MOB that are innervated by ORNs expressing the same OR gene, in this case, the medial and lateral red-dot populations and their respective projections to homologous medial and lateral red glomeruli (Schoenfeld et al., 1985; Liu and Shipley, 1994; Lodovichi et al., 2003). The dark gray curved arrows at right denote the part of the interbulbar commissural system (Schoenfeld and Macrides, 1984; Scott et al., 1985) that interconnects the medial hemispheres of the left and right MOBs (M-M), via neurons in the pars externa of the anterior olfactory nucleus (AON) whose axons cross in the anterior commissure, whereas the light gray curved arrows at right denote the part of this system that interconnects the lateral hemispheres (L-L), also via the AON, pars externa.
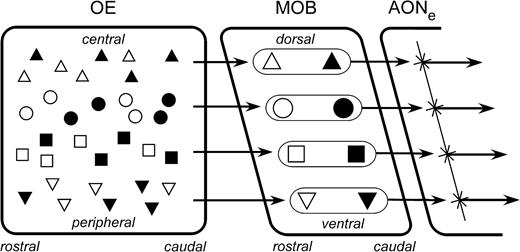
We use the term “olfactory airspace” to refer to the space within these channels and domains of the nose that are lined by OE and through which air flows during inspiration and/or expiration (Clancy et al., 1994; Schoenfeld and Knott, 2004; Schoenfeld and Cleland, 2005). The distribution of ORN populations within particular domains and channels of olfactory airspace determines to a large extent the locations in the MOB to which these ORN populations project their axons (Figure 1). ORNs distributed within local “neighborhoods” of the OE project to glomeruli located within corresponding neighborhoods positioned in longitudinally oriented sectors of the MOB, establishing a coordinate system of axonal projections based upon rhinotopy (Astic and Saucier, 1986; Saucier and Astic, 1986; Stewart and Pedersen, 1987; Clancy et al., 1994; Schoenfeld et al., 1994; Schoenfeld and Knott, 2002, 2004; Miyamichi et al., 2005). Specifically, central domain ORNs in the nose project to the dorsal MOB, whereas those expressed in peripheral domains project to the ventral MOB. ORNs lining the medial air channel project to the medial MOB, while those lining the lateral channel project to the lateral MOB (Figure 1).
It is well established that discrete populations of ORNs that express specific OR genes project convergently to discrete glomeruli in the MOB (Mombaerts et al., 1996; Miyamichi et al., 2005). These ORN populations are intermingled, but each is distributed within a delimited region of the OE that conforms to the rhinotopic coordinate system; that is, most ORN populations are centered at distinct positions along the central–peripheral (C-P) path of inspiratory airflow. Early reports described the distribution of most ORN populations as forming three or four discrete, invariant zones within the OE. ORNs associated with the central domain are found in what was designated as the dorsal-most zone in the earliest descriptions (zone 1, Ressler et al., 1993; zone 4, Vassar et al., 1993; dorsal zone, Strotmann et al., 1994), whereas those associated with the peripheral domain were originally assigned to one of 2–3 additional discrete zones. However, more recent work (Iwema et al., 2004; Miyamichi et al., 2005) indicates that the distribution of ORN populations cannot be easily reconciled to three or four discrete zones; that is, while each population has restrictive zonal boundaries along the C-P axis, the boundaries of different populations do not necessarily coincide. These zonal ORN populations can be further divided into two distinct subpopulations associated with the medial and lateral channels that project, respectively, to medial and lateral glomeruli within the MOB. These matched pairs of glomeruli lie at homologous dorsal–ventral (D-V) and rostral–caudal (R-C) positions in the medial and lateral hemispheres of the MOB (Levai et al., 2003; Miyamichi et al., 2005).
In contrast to the similarity of the medial and lateral channels, the central and peripheral domains of OE in rodents display substantially different patterns of OR gene expression and axonal convergence. Central domain ORNs in mice express in aggregate about 50% of all OR genes (Zhang et al., 2004) but are confined to only about 25% of the OE by surface area (Ressler et al., 1993). This disproportion is also reflected in the overall pattern of convergence of ORNs onto the MOB in hamsters (Figure 1) and so appears to be a general feature of rhinotopic and odotopic organization in rodents (Schoenfeld and Knott, 2004; see later discussion). Moreover, ORNs in the central domain OE express both class I and class II OR genes, whereas peripheral domain ORNs express only class II genes (Conzelmann et al., 2000; Zhang and Firestein, 2002; Zhang et al., 2004). Class I genes are virtually the only type found in teleost fish, whereas both classes are found in amphibians and in purely terrestrial vertebrates (Ngai et al., 1993; Freitag et al., 1998; Zhang and Firestein, 2002; Niimura and Nei, 2005). This pattern has led to suggestions that class I ORs are specialized toward detecting hydrophilic odorants (Freitag et al., 1998), a hypothesis that has received some empirical support (Malnic et al., 1999; Mezler et al., 2001; see later discussion).
Most ORN populations follow these zonal patterns of expression. However, some populations are distributed within more focally restricted patches of OE and exhibit different projection properties. These populations fall into two categories. The first forms the septal organ (SO), an island of OE that lies rostral to the main OE on the septal wall (Figure 1). The ORNs of the SO project broadly to the ventral–medial MOB, consistent with their association with the peripheral domain of the medial channel. Likewise, most of these ORNs express OR genes common to peripheral domain ORNs in the main OE and so are likely to share glomerular targets in common with main OE ORNs of the medial channel (Kaluza et al., 2004; Tian and Ma, 2004). Some SO ORNs, however, express unique OR genes that are not also expressed by ORNs in the main OE, such that there is no homologous population of ORNs in the lateral channel OE; consequently, these SO ORNs target glomeruli in the ventral–medial MOB have no homologues in the ventral–lateral MOB. Owing to its apparent association with part of the inspiratory airstream (see Zhao et al., 2005) and the broad tuning of its ORNs (Marshall and Maruniak, 1986), the SO was originally proposed to serve an alerting function (Rodolfo-Masera, 1943; Negus, 1958). This hypothesis has been discounted by subsequent behavioral lesion studies (Giannetti et al., 1995), but the issue may bear further investigation.
The second category of focally restricted ORNs is confined to a patch of OE on the ethmoid turbinates, centered approximately at the nexus of the C-P and medial–lateral (M-L) axes (Figure 1). These ORNs, comprising several families of OR genes (Strotmann et al., 1994, 2000; Pyrski et al., 2001; Hoppe et al., 2003), tend to project to single glomeruli that together lie at or near the true rostral pole of the MOB, that is, at the nexus of its D-V and M-L meridians (Figure 1). The special function of these ORNs, if any, is unknown, although their unique spatial position is suggestive.
Finally, two extrinsic circuits within and between the rodent MOBs are keyed to inputs from parallel air channels in the nose and so are positioned to operate on sensory information conveyed to the MOB in accordance with its rhinotopic and odotopic maps (Figure 2). First, an intrabulbar associational system interconnects homologous glomeruli and associated neurons of the medial and lateral hemispheres of each MOB; that is, it interconnects glomeruli that receive input from the medial and lateral subsections of the same C-P zones (Schoenfeld et al., 1985; Liu and Shipley, 1994; Belluscio et al., 2002; Lodovichi et al., 2003). Second, an interbulbar commissural system interconnects homologous groups of glomeruli and associated neurons in the two MOBs that receive input from homologous C-P positions in the two nasal cavities (Schoenfeld and Macrides, 1984; Scott et al., 1985). The function of these circuits is unknown.
The map of olfactory airspace onto the rodent MOB organizes the representation of odorant sorptiveness
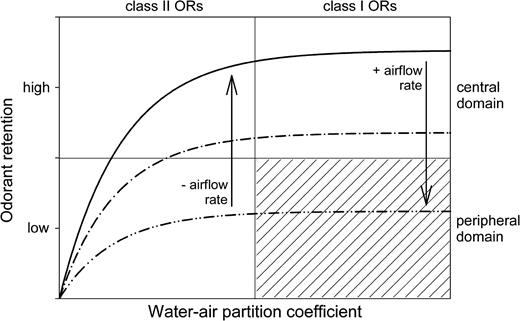
The terrestrial vertebrate nose acts like a gas chromatograph, differentially retaining or adsorbing odorant molecules according to nonodotopic properties such as volatility, hydrophobicity, and water solubility (Mainland and Sobel, 2005; Scott, 2005; Zhao et al., 2005). As originally demonstrated by Mozell and colleagues in frogs (Mozell, 1964, 1970; Mozell and Jagodowicz, 1973), and subsequently confirmed in rats (Morris et al., 1986; Medinsky et al., 1993; Kent et al., 1996) and humans (Kurtz et al., 2004), it is now clear that differential sorption of odorants across the nasal cavity during inspiration is a general characteristic of the vertebrate nose. This has been experimentally demonstrated by measuring the net retention of odor molecules as they are drawn through the nose and across the OE and by comparing the retention of different odorants that vary in some measure of sorptiveness. For example, as represented in Figure 3, odorant retention is an exponential function of the water–air partition coefficient, an index of sorptiveness, and also varies with carrier airflow rate (reviewed in Schoenfeld and Cleland, 2005).
Impact of odorant sorptiveness and airflow rate on odorant retention in the nose. As modeled here, based on empirical data (Morris et al., 1986; Medinsky et al., 1993; Kent et al., 1996; Kurtz et al., 2004; cf., Schoenfeld and Cleland, 2005), retention is an exponential function of the water–air partition coefficient, an index of sorptiveness, rising to an asymptotic maximum at moderate to high values of this coefficient. Increases in airflow rate reduce retention, whereas reductions in airflow rate enhance retention. Odorant retention is likely to be greatest in the central domain along early portions of the inspiratory path; hence, highly sorptive odorants should primarily activate neurons in the central domain projecting to the dorsal MOB. On the other hand, poorly retained odorants of low sorptiveness should instead be more likely to activate neurons in the peripheral domain projecting to ventral MOB as they are carried quickly through the central domain and then trapped by the more highly convoluted peripheral domain OE. Increased airflow rates should deposit even highly sorptive odorants into increasingly more peripheral regions. Reduced airflow rates, in contrast, should favor the retention of poorly sorptive odorants that might otherwise migrate through the nose without significant activation of the OE. Class I ORs may be particularly attuned to odorant ligands of high sorptiveness, whereas class II ORs are also well stimulated by odorants of low sorptiveness (Freitag et al., 1998). The precise relationship between OR class and ligand sorptiveness, including as indexed by the water–air partition coefficient, is unknown. Class I ORs are restricted to the central domain (Zhang et al., 2004), so the activation of ORNs expressing these ORs is not predicted to involve the peripheral domain (hatched area).
Differential retention, in turn, leads to the differential migration of odorant molecules along the intranasal flow path. This is a fundamental principle of chromatography (Jennings et al., 1997) and has been demonstrated empirically for odorants drawn through a frog's nose, in which it is possible to correlate physiological responses from the anterior and posterior aspects of the OE with measurements of net odorant retention across the same space (Mozell and Jagodowicz, 1973; Mozell et al., 1991). Thus, odorants with different sorptive properties are expected to migrate differently along the inspiratory flow path in all terrestrial vertebrate noses. As applied to the rodent nose and considering that odorants are carried in inspired air flowing from the central to the peripheral domain (Kimbell et al., 1997; Zhao et al., 2005), odorants of high sorptiveness (e.g., low volatility, water-soluble, hydrophilic) should exhibit high retention and slow net migration along this path, resulting in a sorption gradient in which higher odorant concentrations adsorb to the central domain epithelium than to the peripheral domain OE (Figure 3). In contrast, odorants of low sorptiveness (e.g., high volatility, water-insoluble, hydrophobic) should exhibit lower retention and faster net migration along this path, resulting in negligible variations in concentration across the C-P axis or even adsorption of greater concentrations in the peripheral domain. Recent fluid dynamic modeling of mass transport between a carrier airstream and the rat nasal mucosa using odorants that differ in sorptiveness (Zhao et al., 2005) has yielded results consistent with this prediction. Note that inspiratory airflow courses in parallel along separable medial and lateral channels (Kimbell et al., 1997; Figure 2), although the slower rate of flow in the lateral channel should enhance retention and slow migration of odorant molecules coursing laterally as compared to medially.
As reviewed in the preceding section of this paper, populations of OR gene–specific ORNs are spatially restricted (zonally or focally) along the C-P axis, in alignment with the inspiratory path. In particular, class I ORs are expressed exclusively in the central domain, whereas class II ORs are found throughout the OE (Zhang et al., 2004). Thus, class I ORs, which are the exclusive class in the OE of fish and in some regions of the amphibian OE and appear to be most responsive to water-soluble odorants, reside in a region of the rodent OE where high retention of highly sorptive odorants is expected (Figure 3). Class II ORs, which are believed to be more heterogeneous in their origin and function, are the exclusive general class in the peripheral domain and predominate centrally as well (Niimura and Nei, 2005). The responsiveness of ORN populations along the C-P axis reflects a similar rhinotopic alignment. Electroolfactogram (EOG) recordings from the exposed rat OE demonstrate that populations of ORNs in the central domain are more responsive to odorants with substantial polarity and water solubility, such as benzaldehyde, than are ORNs in the peripheral domain, whereas peripheral domain ORNs are more responsive to odorants that are nonpolar and less water soluble, such as hexane (Scott et al., 2000; Scott-Johnson et al., 2000; Scott, 2005) (Figure 3). Calcium imaging of isolated ORNs (Bozza and Kauer, 1998; Malnic et al., 1999; Bozza et al., 2002; Feinstein et al., 2004) and c-fos imaging of OE sections (Norlin et al., 2005) are in general agreement with this result, although the sampling is more limited than that achieved in the EOG studies by Scott.
Physiological studies of odorant-induced activity in the MOB demonstrate similar patterns of rhinotopy; that is, more sorptive odorants preferentially induce activity in the dorsal MOB and less sorptive odorants in the ventral MOB. Slotnick et al. (1987) were the first to notice that propionic acid preferentially stimulates 2-deoxyglucose (2DG) uptake in the dorsal–medial MOB of rats, a result later confirmed using Fos immunocytochemistry (Onoda, 1992). Imamura et al. (1992) and Katoh et al. (1993) extended this work with extensive single-unit recordings from the rabbit MOB, showing that the dorsal MOB was particularly responsive to aliphatic acids and other odorants with polar functional groups, whereas the ventral MOB was particularly responsive to aromatic hydrocarbons lacking these polar groups. Their recent efforts using intrinsic imaging techniques in mice (Uchida et al., 2000; Nagao et al., 2002; Takahashi et al., 2004; Igarashi and Mori, 2005; Mori et al., 2005) have generally confirmed this basic pattern, although the intrinsic imaging could only be attempted from the dorsal and lateral aspects of the bulb that are directly accessible and amenable to study with this technique. Moreover, in both approaches, the dorsal and ventral aspects of the bulb were studied with largely different odorant sets having only selective overlap, so it is difficult to conclude from this work that odorant sorptiveness per se should be considered to be an important source of response variability. Comprehensive maps of odor-specific 2DG uptake patterns across the entire MOB of rats have been developed by Johnson, Leon and colleagues using a wide range of odorants (Johnson et al., 1998, 1999, 2002, 2004, 2005a,b; Johnson and Leon, 2000a,b; Linster et al., 2001; Leon and Johnson, 2003), while Inaki et al. (2002) mapped Zif268 expression patterns using a similar approach in mice but with a more limited odorant test battery. In general, these studies have indicated that the most sorptive odorants, including methyl benzoate, the enantiomers of carvone, benzaldehyde, and propionic acid, tend to produce prominent focal activity in the dorsal MOB, whereas the least sorptive odorants, such as nonane and limonene, tend to evoke activity predominantly in the ventral MOB. However, this pattern has apparent exceptions. Ethylbenzene, for example, is as poorly sorptive as limonene and yet elicits substantial dorsal activity in the vicinity of the foci elicited by the carvones (Johnson et al., 2005a). On the other hand, there appears to be little evidence for substantial and exclusive ventral activity elicited by highly sorptive odorants.
In general, we see from these studies that both the extent of odorant retention in the nose and the relative location of maximal odorant-induced activity along the C-P axis in the OE and the D-V axis in the MOB are related to odorant sorptiveness. This is modeled in Figure 3, using the water–air partition coefficient as an index of sorptiveness, summarizing an analysis presented elsewhere (Schoenfeld and Cleland, 2005). Assuming that moderate to high retention largely confines an odorant to the central domain, whereas moderate to low retention allows it to migrate through to the peripheral domain, this model predicts that odorants of low to moderate sorptiveness should stimulate neurons in both domains. Interestingly, odorants of lower sorptiveness are likely to bind to class II ORs, which are distributed in both domains. Correspondingly, the model predicts that odorants of higher sorptiveness would be adsorbed primarily within the central domain, where they are likely to bind to class I ORs, only found in the central domain. The model also illustrates that increased flow rate acts to reduce the retention of highly sorbed odorants, promoting increased peripheral migration and the increased stimulation of peripheral ORNs. On the other hand, a reduced flow rate is predicted to increase the retention of poorly sorbed odorants, thereby increasing the stimulation of central ORNs. Such a result has been demonstrated directly by Scott and colleagues in the rat (Scott-Johnson et al., 2000; Scott, 2005) and is consistent with earlier demonstrations that variations in airflow have physiological consequences in the terrestrial vertebrate nose (Mozell et al., 1984, 1991, 1992; Kurtz and Mozell, 1985; Kent et al., 1996).
Disproportionate mapping of the C-P dimension could lead to rhinotopic variation in stimulus sensitivity and resolving power
The number of ORNs converging onto a single glomerulus is not uniform across the olfactory bulb in rodents. Specifically, the ORNs lining the central domain of the OE express about half of the OR genes (in mice: Zhang et al., 2004) and project to half of the MOB (in hamsters: Schoenfeld and Knott, 2004) but constitute only 25% of all ORNs and occupy only 25% of the total surface area of the OE (Schoenfeld and Knott, 2004). Thus, the average convergence ratio for ORNs associated with the peripheral domain, which project to the ventral MOB, is threefold higher than that for ORNs associated with the central domain. In contrast, the ratios of convergence onto the medial and lateral MOB are comparable (Schoenfeld and Knott, 2004). While the convergence ratios for individual populations of OR gene–specific ORNs have yet to be determined, and rough estimates using incomplete samples indicate that the variability of these ratios may be substantial (Iwema et al., 2004), the overall pattern suggests that variation in ORN convergence ratios onto MOB glomeruli may be linked to rhinotopic properties in the nose.
The greater convergence ratios are found among ORNs lining the peripheral domains of the medial and lateral channels, where adsorbed odorant concentrations are likely to be lower. It has been proposed in theoretical (Van Drongelen et al., 1978; Cleland and Linster, 1999) and experimental studies (Duchamp-Viret et al., 1989; Meisami, 1989) that greater ORN convergence ratios lead to improved stimulus sensitivity, observable in the odor responses of postconvergence secondary neurons (e.g., mitral cells). The principle is that a mitral cell can be successfully activated even when only a small fraction of its convergent ORNs are responding (to a very weak stimulus), though increasing mitral cell sensitivity in this manner also increases the probability of false-positive responses. Indeed, when ORN and mitral cell stimulus–evoked spike patterns were compared in frogs, response thresholds within mitral cells were significantly lower than those in ORNs when the entire epithelium was stimulated with odorant (Duchamp-Viret et al., 1989).
However, maximizing sensitivity per se may not be a critical problem for olfactory processing at this stage. Theoretical studies have suggested that individual ORNs can be made arbitrarily sensitive to low odorant concentrations irrespective of their receptor affinities for the odorant by regulating the gain of coupling between the metabotropic OR itself and its effector channels, such that activation of 1% of the membrane receptor population might activate 75% of the maximum effector current (Cleland and Linster, 1999). The simplest illustration of this concept is receptor overexpression with respect to its coupled effectors (Zhao et al., 1998), known as spare receptor capacity or receptor reserve, but it is functionally equivalent to other observed mechanisms of gain in the intracellular transduction cascades of ORNs (e.g., Balasubramanian et al., 1996; Müller et al., 1998). However, while such intrinsic ORN mechanisms may increase absolute sensitivity for odorants, they do not improve the signal to noise ratio upon which the measure of “least detectable difference” depends. This ratio is improved by increasing the number of independent redundant samples, that is, the number of ORNs that converge onto a single glomerulus. Thus, the increased ORN convergence ratios in the periphery could indeed help compensate for the reduced concentrations of adsorbed odorants expected to reach the olfactory receptors in the peripheral channels of the rodent nose.
ORN convergence has also been implicated in broadening the intensity tuning range (ITR) of glomeruli. The ITR represents the range of odorant concentrations over which a cell or a convergent population of cells (projecting to a given glomerulus) is able to respond to a small change in concentration with an observable change in response and has been quantified as the range of concentrations evoking activation levels between 10% and 90% of maximum. Convergent populations incorporating ORNs with identical chemoreceptive fields but different absolute sensitivities (owing to different degrees of intracellular gain) will broaden the population ITR of the glomerulus over those of the individual ORNs without altering its chemoselectivity (Cleland and Linster, 1999). Indeed, such a broadening of glomerular dynamic range over that of individual ORNs has been observed in mice (Wachowiak and Cohen, 2001). In the current context, broader ITRs will improve the degree to which sorptiveness can be used to differentiate among odor stimuli. The extent to which the benefits of increased convergence ratios may serve to broaden glomerular ITRs or to reduce the least detectable difference among stimuli depends upon the distribution of intracellular gains mediated by ORN intracellular cascades within convergent ORN populations. It is interesting to note that these gains appear to be under neuromodulatory control (Balasubramanian et al., 1996; Müller et al., 1998), suggesting the possibility that this may be another point at which the sampling behavior and internal state of the animal may dynamically influence olfactory stimulus representations.
Some of the quantitative anatomical and physiological differences observed between the central and peripheral domains are also reflected in the differences between the olfactory systems of different species. Olfactory sensitivity can be increased either by increasing ORN sensitivity to odorants or by increasing convergence ratios. All else being equal, the latter can provide a better signal to noise ratio at low stimulus intensities and can support broader ITRs but requires the maintenance of larger numbers of ORNs. Narrower selectivity of ORNs for odorants can improve discrimination capacity but will result in reduced sensitivity for many odorants unless the number of different OR-specific ORN populations is increased. Rats exhibit somewhat higher average ORN response thresholds in individual ORNs than do frogs, perhaps due to narrower ORN molecular receptive ranges. However, the reduced average olfactory system sensitivity that consequently would be expected is counteracted by higher ORN convergence ratios (Duchamp-Viret et al., 2000). This tradeoff theoretically enables greater reliability in rats' responsiveness to weak stimuli, as well as a potentially greater discriminative capacity for odorants, but requires the maintenance both of larger numbers of ORNs in convergent populations as well as a larger number of different OR-specific populations.
The higher convergence ratios of peripheral ORN populations upon ventral MOB glomeruli may confer heightened sensitivity to low concentrations of poorly sorbed odorants. In contrast, ORNs located in the central domain of the OE exhibit convergence ratios upon dorsal MOB glomeruli that are three times lower. What tradeoff may have been made for the theoretical loss of sensitivity and/or reliability that these lower convergence ratios predict? Each of the zonally positioned OR gene–specific populations appears to be restricted to an equivalent area of OE, comprising roughly 25% of its total area, or 12.5% when divided into separate medial and lateral channel components (Ressler et al., 1993; Levai et al., 2003; Miyamichi et al., 2005). In hamsters, this 12.5% corresponds to ∼20 mm2 of surface area (Schoenfeld and Knott, 2004); in mice, it is ∼10 mm2 (Pomeroy et al., 1990). Within these zones, individual ORNs are dispersed randomly and intermingle with ORNs of different populations (Ressler et al., 1993; Vassar et al., 1993; Iwema et al., 2004; Miyamichi et al., 2005). Hence, reductions in convergence ratios cannot be attributed to the sampling of smaller regions of OE. The dendritic knobs of mature ORNs also show a relatively constant luminal surface density across the OE, which for rodents and many other species is in the range of 5 × 104 to 10 × 104 per mm2, with hamsters at the lower end and mice at the higher end of this range (Menco, 1983; Farbman et al., 1988; Mackay-Sim et al., 1988; Mackay-Sim and Kittel, 1991; Ma et al., 1999; Schoenfeld and Knott, 2004). This constancy is maintained despite substantial fluctuation in the population of immature ORNs without knobs that determines the thickness of the OE from region to region.
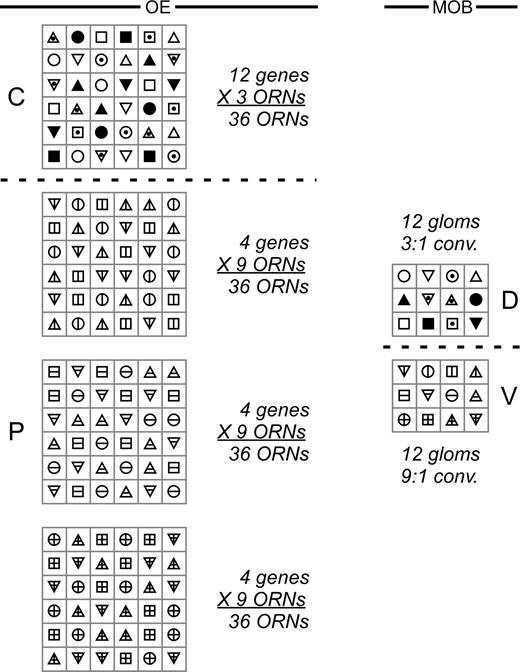
The tradeoff for lower convergence ratios is an elevated “genetic diversity” among central domain ORNs. Genetic diversity refers to the number of OR gene-specific populations represented in a given region of the OE. Given the relative constancy of the surface areas of OR gene expression zones and of the total knob density across the OE, a reduction in convergence ratios implies a reduction in ORN population sizes and hence in the fractional density (proportion of the total knob density) attributable to each population represented. This in turn implies a corresponding increase in the genetic diversity of the central domain (Figure 4). That is, where convergence ratios to the MOB are relatively low, fractional density will also be low but genetic diversity will be high. This characterizes the central domain, in which the average convergence ratio in hamsters is about 1333 knobbed ORNs per glomerulus, the average fractional density of one population is about 100 knobs per mm2 (0.2%), and the estimated number of gene populations represented in 20 mm2 is 500–7501. Where convergence is relatively high, fractional density will also be high but genetic diversity will be low. This characterizes the peripheral domain, in which the average convergence ratio in hamsters is about 4000 knobbed ORNs per glomerulus, the average fractional density is about 300 knobs per mm2 (0.6%), and the estimated number of gene populations represented in 20 mm2 is only 167–2502. In principle, having a larger number of ORN populations expressed within the central domain increases its capacity to discriminate among similar odorants and/or increases the diversity of odorants that can be detected. As a region incorporating both class I and class II ORs, through which all odorants must travel during inspiration and in which odorants are likely to be present at relatively high concentrations, the central channel OE may be better adapted to process and identify highly diverse and/or complex odor stimuli or perform other sensory analyses that benefit from a greater breadth and/or resolution of odor sampling.
Inverse relationship between the fractional density of any one ORN population in the OE and the total number of populations present (genetic diversity). Boxes at left represent discrete, same-sized patches of OE in the C and P domains that contain the same number of ORNs with knobs at the luminal surface (36 ORNs per patch in this example). Symbols in these boxes represent different OR gene–specific populations of ORNs. Boxes at right represent glomeruli in the D and V MOB and are marked by the same symbols as at left, indicating the sites of convergence of each OR gene–specific ORN population. Note that at left there are threefold more boxes and ORNs in the P domain as in the C domain, whereas there are equivalent numbers of glomeruli in the D and V MOB. This yields a threefold higher convergence ratio in the P-V projection than in the C-D projection (in this example, a 9:1 convergence ratio, P:V, compared with a 3:1 ratio, C:D). Given the constraints of constant surface density of all populations in the OE and the restriction to roughly the same surface area of OE for any one population, the fractional density and genetic diversity of intermingled populations will vary inversely across similar patches in different regions of the OE.
Interbulbar and intrabulbar circuits mediate interactions between homologous regions of the MOB
Extrinsic associative circuitry at the level of the MOB also reflects the rhinotopic and odotopic coordinate systems established by the zonal distribution and axonal convergence of ORN populations. First, an intrabulbar associational system reciprocally connects the homologous medial and lateral glomeruli within each olfactory bulb that receive convergent input from the distinct lateral and medial ORN subpopulations expressing the same OR gene, for example, the lateral and medial P2 glomeruli of each bulb (Figures 1 and 2) (Schoenfeld et al., 1985; Lodovichi et al., 2003). Second, an interbulbar commissural system reciprocally connects the homologous regions of the two olfactory bulbs (e.g., their extended dorsal–medial sectors) via the anterior olfactory nucleus, pars externa (Schoenfeld and Macrides, 1984; Scott et al., 1985). Both systems interconnect portions of the bulb that sample from parallel rhinotopic regions: the intrabulbar projections connect inputs from the parallel medial and lateral passages of each nasal cavity, while the interbulbar projections connect MOB regions sampling from corresponding locations in the two nasal cavities (Figure 2). Specifically, these connections are confined to particular D-V sectors of the MOB, representing corresponding C-P regions of origin in the nose. While certain local interneuronal circuits appear to mediate communication between different sectors along the dorsoventral axis (Macrides et al., 1985; Aungst et al., 2003), neither extrinsic circuit does so directly. Along the rostrocaudal axis of the MOB, the intrabulbar extrinsic projections connect specific glomerular modules located at specific rostrocaudal coordinates, while the interbulbar connections are distributed across the entire rostrocaudal range of a dorsoventrally restricted sector of the MOB (Figure 2). Interestingly, recent observations of the rostrocaudal arrangement of glomeruli with respect to the C-P position of their afferent ORNs (Miyamichi et al., 2005) demonstrate that overlapping ORN populations, which are interspersed within the same C-P zone, diverge in their bulbar projections to glomeruli arrayed along the rostrocaudal extent of the MOB at the same D-V level, in apparent alignment with the organization of the interbulbar commissural system (Figures 2 and 5). Based on these projection patterns, it is clear that the intrabulbar associational and interbulbar commissural systems are aligned so as to differentiate among ORN populations based upon their C-P locations corresponding to the D-V locations of their associated MOB glomeruli.
Relationship of the interbulbar commissural system to the zonal organization of ORN projections to the MOB. Different populations of OR gene–specific ORNs are arrayed zonally in the OE, confined to a particular zonal distribution along the C-P axis and represented here by symbols of different shapes. Within each zone, intermingled populations (filled or open symbols) further differentiate themselves anatomically by divergent projections to different glomeruli arrayed within the same D-V sector of the MOB but at different positions along the R-C axis (Miyamichi et al., 2005). The dual projections of zonal ORNs to homologous medial and lateral glomeruli are not depicted. MOB neurons arising from all the glomeruli arrayed within a specific D-V sector then project en masse to target neurons within a particular sector of the pars externa of the anterior olfactory nucleus (AONe) (Schoenfeld and Macrides, 1984). These target neurons then project to a homologous longitudinal array of glomeruli located at an identical D-V position in the contralateral MOB.
Both of these bulbo-bulbar systems interconnect regions corresponding in dorsal–ventral position within the paired MOBs, implying that they are keyed to odorant-evoked activity representing comparable levels of odorant sorptiveness. Their specificity is somewhat surprising because these sets of parallel passageways (two cavities, medial and lateral channels within each cavity) are likely to induce substantial variation in the rates of airflow and odorant migration that would tend to differentiate odorants by their sorptiveness. For example, variation in patency between the two cavities is common in humans and leads to variations in odorant perception that can be directly attributed to an interaction of odorant sorptiveness with odorant migration on the two sides (Mainland and Sobel, 2005). Computer modeling of the rodent nose indicates that there is likely to be a considerable difference in airflow between the medial and lateral channels: lateral airflow is slower, at least in the peripheral domain (Kimbell et al., 1997). The central domains of the two channels, in contrast, are contiguous at rostral levels and so probably do not induce different flow rates or rates of migration (Kimbell et al., 1997; see Figure 2).
Given these sources of intrinsic variability, the utility of these apparently redundant sampling circuits is not clear. The simplest hypothesis is that both the intrabulbar and interbulbar circuits are mediating a signal-averaging function, as opposed to, for example, contrast enhancement. That is, they may mitigate rather than accentuate variability among the four channels owing to their different retention properties, perhaps thereby obtaining a more reliable representation of sorptiveness. However, empirical evidence for this hypothesis is mixed and based on limited study. Both signal averaging and contrast enhancement in interbulbar interactions were observed by Leveteau and Macleod (1969) in a study of the effects of birhinal stimulation of the rabbit nose on activity recorded from the two MOBs. In this study, ipsilateral MOB activity evoked by presentation of a weak odorant stimulus to one naris was enhanced by a stronger contralateral stimulus but inhibited by a weaker contralateral stimulus; that is, the level of ipsilateral activity was drawn to the birhinal mean. However, when the ipsilateral stimulus was relatively strong, weaker contralateral stimulation evoked strong ipsilateral facilitation, generating a form of internaris contrast enhancement. This issue deserves more attention.
The zonation hypothesis
As reviewed above, odorant molecules with different sorptive properties are differentially adsorbed during inspiration and migrate differentially through the nasal passages. Different OR gene–specific populations of ORNs are distributed within zones that are restricted with respect to the C-P axis of the nasal cavity, that is, the inspiratory path. This structured distribution of ORs across olfactory airspace enables the sorptive properties of odorants to alter the neural representation of odors on the basis of these sorptive properties; this capacity, in turn, can be behaviorally exploited to enable animals to gather additional information about odors by regulating their sampling strategy (sniffing). Sorptiveness, a property of whole odorant molecules, constitutes a source of stimulus variance that is partially independent of odotopy, that is, the pattern of ligand–receptor affinities between the multiple molecular epitopes of the odorant molecule and the available complement of ORs. This is because odorant-binding sites (odotopes) are often common to multiple molecules with different overall sorptive properties; that is, these molecules may have identical affinities for a given set of receptors owing to these shared odotopes but will bind to them in differing ratios based upon their patterns of migration through the nasal passages, owing to the net sorptive properties of the different odorant molecules and the different locations of the zones of sensitive ORN populations along the inspiratory path. That is, an aldehyde group attached to a hydrophobic molecule will be differently distributed along the C-P axis than will the same aldehyde group attached to a more sorptive, hydrophilic molecule, and the ratios of activation among sensitive ORN populations will reflect this differential distribution (in addition to whatever differences in binding affinities may derive from subtle differences in partial charge distributions or receptor access to the binding site between the different molecules). Thus, the sorptive properties of molecules are expected to affect the pattern of activation of ORs and hence presumably influence the perception of odor quality.
The potency of sorptiveness as a relevant source of stimulus variance is realized primarily by what it implies about sniffing as an active sampling behavior. Sniffing behavior determines the flow rate of air through the nasal passages, and odorant retention and migration vary with flow rate. Moreover, flow rate interacts with sorptiveness in affecting retention (Figure 3). Specifically, an increased flow rate reduces the maximum retention of odorants of moderate-to-high sorptiveness, which should also promote more peripheral migration and possible activation of peripherally situated ORNs. In contrast, a reduced flow rate increases the retention of odorants of low sorptiveness, thereby promoting less peripheral migration and possibly an increased activation of central ORNs. This relationship has been demonstrated in rats (Scott-Johnson et al., 2000; Scott, 2005) and is consistent with earlier demonstrations that variation in airflow has physiological consequences in the terrestrial vertebrate nose (Mozell et al., 1984, 1991, 1992; Kurtz and Mozell, 1985; Kent et al., 1996). Furthermore, animals, including humans, are known to adjust the depth of sniffing (i.e., flow rate) in response to changes or variation in odorant concentration or sorptiveness (Youngentob et al., 1987; Sobel et al., 1999, 2000; Mainland and Sobel, 2005).
The “zonation hypothesis” proposes that these behavioral variations in sniffing will deposit odorants in predictable distributions along the inspiratory path, as a function of odorant sorptiveness, enabling animals to achieve particular sensory goals by actively regulating the motor parameters of sniffing. Zonation roughly resembles visual foveation in that it is based on actively directing sensory stimulus elements to a particular region of the sensory sheet, a process dependent both on motor control of sensory sampling and on the physical properties of the stimulus. Olfactory zonation, however, may contribute to a number of different sensory functions and goals. Zonation could in principle be employed to optimally identify known odors by directing odorants so as to maximize the similarity of the evoked glomerular activation pattern with a corresponding pattern that was previously learned. It could facilitate the detection of weak stimuli by directing odorant molecules toward their highest affinity ORs. It could enable animals to compensate for focal sensory damage by directing stimuli to other parts of the sensory sheet that remain functional and responsive (Slotnick and Bodyak, 2002). Finally, it could serve to improve difficult discriminations, in that multiple sniffs of differing parameters will separate multiple odorant components differentially in olfactory airspace, potentially enabling the disambiguation of odorant species that interfere with one another via activation (or blockade) of the same OR populations.
The hypothetical role of zonation as the core component of active, investigative sniffing by rodents underscores how odorant sampling resembles investigative behaviors in other sensory modalities, such as visual saccades or tactile manipulation (Macrides, 1977; Youngentob et al., 1987; Kepecs et al., 2005; Mainland and Sobel, 2005). While simple discriminations can be performed quickly and speed may be favored over accuracy in some circumstances (Uchida and Mainen, 2003), more difficult problems of odor identification in complex natural scenes may require repeated or extended sniffs with varying sampling parameters (Mainland and Sobel, 2005). In such contexts, odor representations may be constructed by integrating the information gained from multiple sequential samples, as in saccades, utilizing subtleties in the odor signature that could not be appreciated with only a single sniff. This is supported by the observation that more difficult discriminations require a longer temporal integration of olfactory sampling than do simple discriminations to achieve similar levels of performance (Abraham et al., 2004). Furthermore, neural activity in some higher-order olfactory centers depends on active sniffing in addition to the presence of odorants (Sobel et al., 1998; Bensafi et al., 2003; Kareken et al., 2004), suggesting an integral role for active sampling in the construction of odor representations.
To assess the role of zonation in olfactory sampling and perception, both sniffing behavior and the sorptive properties of odorants will need to be manipulated and/or measured in a combination of physiological and behavioral test paradigms. For example, artificial sniffing protocols in anesthetized animals (Macrides and Chorover, 1972; Macrides, 1977; Morris et al., 1986; Scott-Johnson et al., 2000) would permit manipulation of inspiratory parameters such as airflow rate and sniff volume, frequency, and duration while recording the spatial patterning and dynamic range of odorant-induced activity in the OE and MOB in relation to the predicted patterns of migration through the olfactory recesses of odorants varying in sorptiveness. Observations of freely behaving animals (Youngentob et al., 1987; Sobel et al., 2000; Uchida and Mainen, 2003; Johnson et al., 2005b), on the other hand, would permit evaluation of the dynamic adjustments in sniffing behavior displayed by animals when presented with odorants or odorant mixtures differing in concentration and sorptiveness or in the context of different operant tasks and could also be used to test the extent to which higher-order odorant representations and behavioral performance measures depend on sniffing parameters, particularly in the context of complex natural scenes and differing behavioral motivations.
Concluding remarks
The role of active, motivated sampling of odor stimuli is a critical issue in the study of olfaction. The zonation hypothesis offers a mechanism by which behaviorally mediated changes in the delivery of odorants to ORNs during sniffing can be translated into different patterns of glomerular activation in the MOB. Rodent nasal anatomy facilitates structured inspiratory airflow, leading to predictable chromatographic separation of odorants along discrete air passages. Spatially segregated zones of OR expression along these air passages enable this separation to be translated into differential patterns of neuronal activation in the OE and MOB, which in turn can be regulated and modified by the motor dynamics of sniffing behavior. The ability to regulate sampling in this manner has many potential benefits; for example, it could improve the discriminability of odorants or, in contrast, facilitate recognition of the similarities between odotopes on molecules of very different sorptive properties. The utility of controlled variability in stimulus representations suggested by directed sampling, in general, and zonation, in particular, undoubtedly will become clearer as we learn more about the contributions of odor memory, behavioral context, and higher-order sensorimotor networks on the construction and processing of odor representations (Wilson and Stevenson, 2003; Mainland and Sobel, 2005).
The upper limit (750) corresponds to the number of glomeruli in each hemisphere of the dorsal MOB of hamsters innervated by 20 mm2 (50%) of central OE (Schoenfeld and Knott, 2004), assuming homotypical innervation (Treloar et al., 2002). The lower limit (500) corresponds approximately to the number of OR genes expressed in the central OE, assuming that hamsters have roughly the same number of functional OR genes as do mice and rats (Young et al., 2002; Zhang and Firestein, 2002; Gibbs et al., 2004; Godfrey et al., 2004; Zhang et al., 2004).
The upper limit (250) corresponds to the number of glomeruli in each hemisphere of the ventral MOB of hamsters innervated by 20 mm2 (16.7%) of peripheral OE (Schoenfeld and Knott, 2004), again assuming homotypical innervation (Treloar et al., 2002). The lower limit (167) corresponds approximately to the number of OR genes expressed in a comparable portion of the peripheral OE, again assuming that the number of functional OR genes in hamsters is comparable to that in mice and rats (Young et al., 2002; Zhang and Firestein, 2002; Gibbs et al., 2004; Godfrey et al., 2004; Zhang et al., 2004).
Our own work, as cited here, has been generously supported by the National Institutes of Health. The views expressed in this paper are entirely our own.
References
Abraham, N.M., Spors, H., Carleton, A., Margrie, T.W., Kuner, T. and Schaefer, A.T. (
Alenius, M. and Bohm, S. (
Astic, L. and Saucier, D. (
Aungst, J.L., Heyward, P.M., Puche, A.C., Karnup, S.V., Hayar, A., Szabo, G. and Shipley, M.T. (
Balasubramanian, S., Lynch, J.W. and Barry, P.H. (
Belluscio, L., Lodovichi, C., Feinstein, P., Mombaerts, P. and Katz, L.C. (
Bensafi, M., Porter, J., Pouliot, S., Mainland, J., Johnson, B., Zelano, C., Young, N., Bremner, E., Aframian, D., Khan, R. and Sobel, N. (
Bozza, T., Feinstein, P., Zheng, C. and Mombaerts, P. (
Bozza, T.C. and Kauer, J.S. (
Buonviso, N., Amat, C. and Litaudon, P. (
Clancy, A.N., Schoenfeld, T.A., Forbes, W.B. and Macrides, F. (
Cleland, T.A. and Linster, C. (
Conzelmann, S., Levai, O., Bode, B., Eisel, U., Raming, K., Breer, H. and Strotmann, J. (
Duchamp-Viret, P., Duchamp, A. and Chaput, M.A. (
Duchamp-Viret, P., Duchamp, A. and Vigouroux, M. (
Farbman, A.I., Brunjes, P.C., Rentfro, L., Michas, J. and Ritz, S. (
Feinstein, P., Bozza, T., Rodriguez, I., Vassalli, A. and Mombaerts, P. (
Freitag, J., Ludwig, G., Andreini, I., Rossler, P. and Breer, H. (
Giannetti, N., Saucier, D. and Astic, L. (
Gibbs, R.A., Weinstock, G.M., Metzker, M.L., Muzny, D.M., Sodergren, E.J., Scherer, S., Scott, G., Steffen, D., Worley, K.C., Burch, P.E., Okwuonu, G., Hines, S., Lewis, L., DeRamo, C., Delgado, O., Dugan-Rocha, S., Miner, G., Morgan, M., Hawes, A., Gill, R., Celera, Holt, R.A., Adams, M.D., Amanatides, P.G., Baden-Tillson, H., Barnstead, M., Chin, S., Evans, C.A., Ferriera, S., Fosler, C., Glodek, A., Gu, Z., Jennings, D., Kraft, C.L., Nguyen, T., Pfannkoch, C.M., Sitter, C., Sutton, G.G., Venter, J.C., Woodage, T., Smith, D., Lee, H.M., Gustafson, E., Cahill, P., Kana, A., Doucette-Stamm, L., Weinstock, K., Fechtel, K., Weiss, R.B., Dunn, D.M., Green, E.D., Blakesley, R.W., Bouffard, G.G., De Jong, P.J., Osoegawa, K., Zhu, B., Marra, M., Schein, J., Bosdet, I., Fjell, C., Jones, S., Krzywinski, M., Mathewson, C., Siddiqui, A., Wye, N., McPherson, J., Zhao, S., Fraser, C.M., Shetty, J., Shatsman, S., Geer, K., Chen, Y., Abramzon, S., Nierman, W.C., Havlak, P.H., Chen, R., Durbin, K.J., Egan, A., Ren, Y., Song, X.Z., Li, B., Liu, Y., Qin, X., Cawley, S., Worley, K.C., Cooney, A.J., D'Souza, L.M., Martin, K., Wu, J.Q., Gonzalez-Garay, M.L., Jackson, A.R., Kalafus, K.J., McLeod, M.P., Milosavljevic, A., Virk, D., Volkov, A., Wheeler, D.A., Zhang, Z., Bailey, J.A., Eichler, E.E., Tuzun, E., Birney, E., Mongin, E., Ureta-Vidal, A., Woodwark, C., Zdobnov, E., Bork, P., Suyama, M., Torrents, D., Alexandersson, M., Trask, B.J., Young, J.M., Huang, H., Wang, H., Xing, H., Daniels, S., Gietzen, D., Schmidt, J., Stevens, K., Vitt, U., Wingrove, J., Camara, F., Mar, A.M., Abril, J.F., Guigo, R., Smit, A., Dubchak, I., Rubin, E.M., Couronne, O., Poliakov, A., Hubner, N., Ganten, D., Goesele, C., Hummel, O., Kreitler, T., Lee, Y.A., Monti, J., Schulz, H., Zimdahl, H., Himmelbauer, H., Lehrach, H., Jacob, H.J., Bromberg, S., Gullings-Handley, J., Jensen-Seaman, M.I., Kwitek, A.E., Lazar, J., Pasko, D., Tonellato, P.J., Twigger, S., Ponting, C.P., Duarte, J.M., Rice, S., Goodstadt, L., Beatson, S.A., Emes, R.D., Winter, E.E., Webber, C., Brandt, P., Nyakatura, G., Adetobi, M., Chiaromonte, F., Elnitski, L., Eswara, P., Hardison, R.C., Hou, M., Kolbe, D., Makova, K., Miller, W., Nekrutenko, A., Riemer, C., Schwartz, S., Taylor, J., Yang, S., Zhang, Y., Lindpaintner, K., Andrews, T.D., Caccamo, M., Clamp, M., Clarke, L., Curwen, V., Durbin, R., Eyras, E., Searle, S.M., Cooper, G.M., Batzoglou, S., Brudno, M., Sidow, A., Stone, E.A., Venter, J.C., Payseur, B.A., Bourque, G., Lopez-Otin, C., Puente, X.S., Chakrabarti, K., Chatterji, S., Dewey, C., Pachter, L., Bray, N., Yap, V.B., Caspi, A., Tesler, G., Pevzner, P.A., Haussler, D., Roskin, K.M., Baertsch, R., Clawson, H., Furey, T. S., Hinrichs, A.S., Karolchik, D., Kent, W.J., Rosenbloom, K.R., Trumbower, H., Weirauch, M., Cooper, D.N., Stenson, P.D., Ma, B., Brent, M., Arumugam, M., Shteynberg, D., Copley, R.R., Taylor, M.S., Riethman, H., Mudunuri, U., Peterson, J., Guyer, M., Felsenfeld, A., Old, S., Mockrin, S. and Collins, F. (
Godfrey, P.A., Malnic, B. and Buck, L.B. (
Hoppe, R., Breer, H. and Strotmann, J. (
Igarashi, K.M. and Mori, K. (
Imamura, K., Mataga, N. and Mori, K. (
Inaki, K., Takahashi, Y.K., Nagayama, S. and Mori, K. (
Iwema, C.L., Fang, H., Kurtz, D.B., Youngentob, S.L. and Schwob, J.E. (
Jennings, W., Mittlefehldt, E. and Stremple, P. (
Johnson, B.A., Farahbod, H. and Leon, M. (
Johnson, B.A., Farahbod, H., Saber, S. and Leon, M. (
Johnson, B.A., Farahbod, H., Xu, Z., Saber, S. and Leon, M. (
Johnson, B.A., Ho, S.L., Xu, Z., Yihan, J.S., Yip, S., Hingco, E.E. and Leon, M. (
Johnson, B.A. and Leon, M. (
Johnson, B.A. and Leon, M. (
Johnson, B.A., Woo, C.C., Hingco, E.E., Pham, K.L. and Leon, M. (
Johnson, B.A., Woo, C.C. and Leon, M. (
Kaluza, J.F., Gussing, F., Bohm, S., Breer, H. and Strotmann, J. (
Kareken, D.A., Sabri, M., Radnovich, A.J., Claus, E., Foresman, B., Hector, D. and Hutchins, G.D. (
Katoh, K., Koshimoto, H., Tani, A. and Mori, K. (
Kent, P.F., Mozell, M.M., Murphy, S.J. and Hornung, D.E. (
Kepecs, A., Uchida, N. and Mainen, Z.F. (
Kimbell, J.S., Godo, M.N., Gross, E.A., Joyner, D.R., Richardson, R.B. and Morgan, K.T. (
Koehl, M.A.R. (
Kurtz, D.B. and Mozell, M.M. (
Kurtz, D.B., Zhao, K., Hornung, D.E. and Scherer, P. (
Leon, M. and Johnson, B.A. (
Levai, O., Breer, H. and Strotmann, J. (
Leveteau, J. and MacLeod, P. (
Linster, C., Johnson, B.A., Yue, E., Morse, A., Xu, Z., Hingco, E.E., Choi, Y., Choi, M., Messiha, A. and Leon, M. (
Liu, W.-L. and Shipley, M.T. (
Lodovichi, C., Belluscio, L. and Katz, L.C. (
Ma, M., Chen, W.R. and Shepherd, G.M. (
Mackay-Sim, A., Breipohl, W. and Kremer, M. (
Mackay-Sim, A. and Kittel, P.W. (
Macrides, F. (
Macrides, F. and Chorover, S.L. (
Macrides, F., Schoenfeld, T.A., Marchand, J.E. and Clancy, A.N. (
Mainland, J. and Sobel, N. (
Malnic, B., Hirono, J., Sato, T. and Buck, L.B. (
Marshall, D.A. and Maruniak, J.A. (
Medinsky, M.A., Kimbell, J.S., Morris, J.B., Gerde, P. and Overton, J.H. (
Meisami, E. (
Menco, B.Ph.M. (
Mezler, M., Fleischer, J. and Breer, H. (
Miyamichi, K., Serizawa, S., Kimura, H.M. and Sakano, H. (
Mombaerts, P., Wang, F., Dulac, C., Chao, S.K., Nemes, A., Mendelsohn, M., Edmondson, J. and Axel, R. (
Morgan, K.T., Kimbell, J.S., Monticello, T.M., Patra, A.L. and Fleishman, A. (
Mori, K., Takahashi, Y.K., Igarashi, K. and Nagayama, S. (
Morris, J.B., Clay, R.J. and Cavanagh, D.G. (
Mozell, M.M. (
Mozell, M.M. and Jagodowicz, M. (
Mozell, M.M., Kent, P.F. and Murphy, S.J. (
Mozell, M.M., Kent, P.F. and Murphy, S.J. (
Mozell, M.M., Sheehe, P.R., Swieck, S.W. Jr, Kurtz, D.B. and Hornung, D.E. (
Müller, F., Bonigk, W., Sesti, F. and Frings, S. (
Nagao, H., Yamaguchi, M., Takahash, Y. and Mori, K. (
Negus, V. (
Ngai, J., Dowling, M.M., Buck, L., Axel, R. and Chess, A. (
Niimura, Y. and Nei, M. (
Norlin, E.M., Vedin, V., Bohm, S. and Berghard, A. (
Onoda, N. (
Pomeroy, S.L., LaMantia, A.-S. and Purves, D. (
Pyrski, M., Xu, Z., Walters, E., Gilbert, D.J., Jenkins, N.A., Copeland, N.G. and Margolis, F.L. (
Ressler, K.J., Sullivan, S.L. and Buck, L.B. (
Ressler, K.J., Sullivan, S.L. and Buck, L.B. (
Rodolfo-Masera, T. (
Saucier, D. and Astic, L. (
Schoenfeld, T.A., Clancy, A.N., Forbes, W.B. and Macrides, F. (
Schoenfeld, T.A. and Cleland, T.A. (
Schoenfeld, T.A. and Knott, T.K. (
Schoenfeld, T.A. and Knott, T.K. (
Schoenfeld, T.A. and Macrides, F. (
Schoenfeld, T.A., Marchand, J.E. and Macrides, F. (
Scott, J.W. (
Scott, J.W., Brierley, T. and Schmidt, F.H. (
Scott, J.W., Ranier, E.C., Pemberton, J.L., Orona, E. and Mouradian, L.E. (
Scott-Johnson, P.E., Blakley, D. and Scott, J.W. (
Slotnick, B. and Bodyak, N. (
Slotnick, B., Graham, S., Laing, D. and Bell, G. (
Sobel, N., Khan, R.M., Hartley, C.A., Sullivan, E.V. and Gabrieli, J.D.E. (
Sobel, N., Khan, R.M., Saltman, A., Sullivan, E.V. and Gabrieli, J.D.E. (
Sobel, N., Prabhakaran, V., Desmond, J.E., Glover, G.H., Goode, R.L., Sullivan, E.V. and Gabrieli, J.D.E. (
Stewart, W.B. and Pedersen, P.E. (
Strotmann, J., Conzelmann, S., Beck, A., Feinstein, P., Breer, H. and Mombaerts, P. (
Strotmann, J., Wanner, I., Helfrich, T., Beck, A., Meinken, C., Kubick, S. and Breer, H. (
Takahashi, Y.K., Kurosaki, M., Hirono, S. and Mori, K. (
Tian, H. and Ma, M. (
Treloar, H.B., Feinstein, P., Mombaerts, P. and Greer, C.A. (
Uchida, N. and Mainen, Z.F. (
Uchida, N., Takahashi, Y.K., Tanifuji, M. and Mori, K. (
Van Drongelen, W., Holley, A. and Døving, K.B. (
Vassar, R., Chao, S.K., Sitcheran, R., Nuñez, J.M., Vosshall, L.B. and Axel, R. (
Vassar, R., Ngai, J. and Axel, R. (
Vickers, N.J. (
Wachowiak, M. and Cohen, L.B. (
Wang, F., Nemes, A., Mendelsohn, M. and Axel, R. (
Wilson, D.A. and Stevenson, R.J. (
Young, J.M., Friedman, C., Williams, E.M., Ross, J.A., Tonnes-Priddy, L. and Trask, B.J. (
Youngentob, S.L., Mozell, M.M., Sheehe, P.R. and Hornung, D.E. (
Zhang, X. and Firestein, S. (
Zhang, X., Rogers, M., Tian, H., Zhang, X., Zou, D.J., Liu, J., Ma, M., Shepherd, G.M. and Firestein, S.J. (
Zhao, H., Ivic, L., Otaki, J.M., Hashimoto, M., Mikoshiba, K. and Firestein, S. (
Author notes
1Department of Physiology and Program in Neuroscience, University of Massachusetts Medical School, Worcester, MA 01655, USA and 2Department of Neurobiology and Behavior, Cornell University, Ithaca, NY 14853, USA