-
PDF
- Split View
-
Views
-
Cite
Cite
Joel Mainland, Noam Sobel, The Sniff Is Part of the Olfactory Percept, Chemical Senses, Volume 31, Issue 2, February 2006, Pages 181–196, https://doi.org/10.1093/chemse/bjj012
Close - Share Icon Share
Abstract
In this review, we use data obtained primarily from humans to argue that sniffs are not merely a stimulus carrier but are rather a central component of the olfactory percept. We argue that sniffs 1) are necessary for the olfactory percept, 2) affect odorant intensity perception and identity perception, 3) drive activity in olfactory cortex, 4) are rapidly modulated in an odorant-dependent fashion by a dedicated olfactomotor system, and 5) are sufficient to generate an olfactory percept of some sort even in the absence of odor.
Introduction
Odorant transduction, the process of odorant binding at olfactory receptors culminating in an action potential, is traditionally referred to as the first stage in the olfactory process. However, in the behaving animal, there is a process that precedes transduction, and that is olfactory sampling or sniffing. The sniff is often viewed as a simple delivery method designed to transport odorous molecules from the entrance of the nares to the epithelium several centimeters inside the nasal passage. Such a simplified view of sniffing, however, fails to capture the significance of this earliest stage of olfaction. The sniff is as integral to olfactory perception as the eye movement is to visual perception. Just as oculomotor adjustments during the smooth pursuit of a moving object are an active process intimately tied to visual perception (Lisberger, 1988), so do the muscles regulating the sniff make constant adjustments to sniff volume and duration in response to the stimulus (Sobel et al., 2000a; Johnson et al., 2003). Just as deviations in eye position can distort visual perception (Murphy, 1978), so do deviations in nasal airflow distort olfactory perception (R. Teghtsoonian and M. Teghtsoonian, 1984). Just as when the eye is completely motionless relative to the visual scene, no image is relayed to the brain (Pritchard, 1961), so does the olfactory scene disappear when no air flows in the nasal cavity (Bocca et al., 1965). Indeed, far from being a simple stimulus delivery method, the sniff is necessary and sufficient for generating neural activity in olfactory brain areas as well as necessary and sufficient for olfactory perception. Here we will argue these claims using data collected primarily from humans.
What is a sniff?
The dictionary defines the word sniff as 1) perceive by inhaling through the nose and 2) inhale audibly through the nose (WordNet, 2003). The first definition focuses on the sniff as integral to perception, while the second refers only to the respiratory act. Indeed, this duality is borne out in textbooks as well, with texts on respiration defining the sniff as a respiratory act: “Sniffing is one or more small, quick inhalations through the nose. It is a mechanism for bringing ambient air into contact with the olfactory receptors in the nose without carrying the air (which may contain irritant, toxic materials) deep into the lung” (Comroe, 1974). Olfactory texts more often focus on the sniff either as a preparatory action, “Air must be drawn in through the external nares, presented to the olfactory membranes after cleaning and humidifying, and then expelled” (Stoddart, 1980), or as a periodic form of presentation, such as “[A] periodic movement associated with acquisition of the stimulus [to] intermittently expose receptor cells to their chemosensory environment” (Ache, 1991).
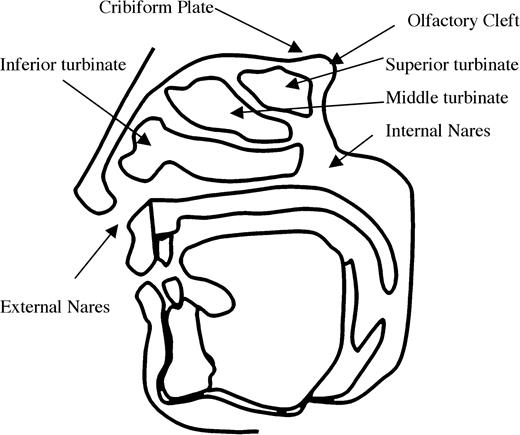
A typical human sniff has a duration of 1.6 s, an average inhalation velocity of 27 l/min, and a volume of 500 cm3 (Laing, 1983). During a sniff, air enters through the opening of the nostrils (anterior nares), passes through the nasal cavity, and continues out the posterior nares to the top of the throat (pharynx). The nasal cavity contains the anterior, middle, and superior turbinate (Figure 1). Olfactory epithelium, the sensory portion of the epithelium containing olfactory receptors, lines the roof of the nasal cavity below the cribiform plate and the superior turbinate. During resting breathing, only a fraction of the inspired air encounters the olfactory epithelium. In an early investigation of airflow, researchers lined a cadaver's nasal passages with litmus paper and found that the bulk of ammonia injected into the nasal passages ascended and reached a peak in the middle of the nasal cavity, falling considerably short of the olfactory epithelium (Paulsen, 1882). More recent computational models constructed from computed tomography or magnetic resonance imaging images verify that while airflow in the nose depends strongly on individual anatomy, only approximately 5–10% of airflow entering the external nares reaches the epithelium (Hahn et al., 1994; Keyhani et al., 1995; Zhao et al., 2004, 2005).
Saggital cross section of the human head outlining the nasal passages.
The sniff is necessary for an olfactory percept
Olfaction typically consists of both sniffing (airflow in the nostril regardless of odor presence) and smelling (the percept of odor regardless of airflow in the nostril). Although olfactory perception is usually assumed to reflect the latter, it is largely dependent on the former. Proetz (1941) demonstrated this dependency of coherent olfactory perception on sniffing elegantly and simply in two experiments proposed in a section of his book devoted to experiments suggested to accompany lectures. The first experiment attempted to address the issue by delivering odorants to the human epithelium in aqueous solution.
Although Proetz asserted no odor detection under these conditions of no sniffing, others reported conflicting results with this method (discussion in Moncrieff, 1946). For example, consistent with Proetz, Weber (1847) reported the absence of an olfactory percept after pouring a solution of eau de cologne into his nostrils. By contrast, Veress (1903) argued that under similar conditions an olfactory precept did ensue but that it differed from the percept of odorants diluted in air. The conflicting results may reflect the technical complications of conducting these experiments. It is quite difficult to fill the olfactory cleft with water, and warm water with physiological osmolarity must be used to ensure the solution does not damage the epithelium (indeed, we would cringe at the prospect of getting these ca. 1940s “do it yourself” experiments past a current Human Subjects Panel). Furthermore, it is unclear what measures, if any, these experiments took to prevent mechanical stimulation of the epithelium that may have mimicked the somatosensory stimulation of a sniff.To a few ounces of a physiological solution of sodium chloride solution add spirit of eau de cologne drop by drop until the mixture is definitely scented. Place the student upon a table and allow his head to hang over the edge, with his chin in a vertical plane above his external auditory meatuses. Fill his nose with the solution. Although it obviously reaches his olfactory area he will not detect the odor. There may be some tingling through stimulation of the nerve endings (p. 366).
Proetz (1941) also argued that “While the evaporation of certain substances in a quiet atmosphere undoubtedly reaches the nose by diffusion, still the actual smell impression is accomplished invariably by a slight sniffing and hence by convection.” His second experiment used a second technique to deliver odor without concomitant airflow, namely, diffusion during velopharyngeal closure.
Although these “try this at home” experiments provided support for the notion that sniffs are essential for olfactory perception, the strongest evidence was obtained in the laboratory, not in the classroom. Bocca et al. (1965) injected odorants intravenously during apnea. This method brings odorants to the epithelium through the blood stream and thus enables odorant delivery in the complete absence of sniffing. Bocca et al. found that when the subjects did not sniff, no odor was perceived. Only when subjects breathed normally through the nose or when odorless nitrogen was injected into the nostril so as to mimic a sniff, did they perceive the intravenous odor. This led them to conclude that mechanical stimulation of the epithelium concomitant with odorant delivery was necessary for perception to take place. In other words, the sniff is necessary for olfactory perception. Consistent with this view, the olfactory impairment in neurodegenerative diseases may in fact be in part a sniffing impairment. For example, Parkinson's patients sniffed less vigorously than healthy controls, and critically, when taught to sniff with more vigor, their olfactory performance improved (Sobel et al., 2001).Hold the breath, and insure against any air currents passing through the nose by pressing the lips together and forcing the column of air from the chest against the tightly closed velum palati, as though preparing to blow a trumpet. Strongly odorous substances may now be brought close to the nose without being detected by the subject, although odor may permeate the room if they are left uncorked. A slight sniffing is required to record smell impressions. (p. 365).
Aspects of the olfactory percept most influenced by sniffing
Considering that sniffs are necessary for proper olfactory perception, one may ask what aspects of the olfactory percept are most influenced by sniffing and in what manner.
Sniffing influences perception of olfactory intensity
The manner in which environmental stimuli are presented to the receptor plays an important part in sensory perception. When sniffing, one can alter various parameters such as sniff duration, sniff airflow, sniff volume, and overall number of sniffs. A change in one of these parameters might be expected to change the perception of an odorant. How much of an effect do sniff parameters have on performance? Le Magnen (1945) conducted a study looking at the influence of sniff airflow and sniff volume on detection thresholds. He found that while thresholds varied with nasal airflow, they were insensitive to overall volume. Threshold was found to vary with airflow in two further studies (Laing, 1983; Sobel et al., 2000a), with Laing stipulating that the total inspired volume must exceed 200 cm3, presumably due to a lack of appreciable olfactory stimulation at very low volumes.
The effect of sniff airflow on intensity estimates at suprathreshold concentrations is less clear. In a series of studies, Teghtsoonian and colleagues (Teghtsoonian et al., 1978; R. Teghtsoonian and M. Teghtsoonian, 1982, 1984) suggested that the information about sniff content is combined with the information about perceived effort to produce an invariant precept of odorant strength. In other words, airflow does not change suprathreshold intensity estimates despite having an effect on the number of molecules reaching the epithelium. They suggested a concentration constancy model to explain their results. A vigorous sniff of a low-concentration odorant or a weak sniff of a high-concentration odorant may transport a similar quantity of odorant molecules to the olfactory receptors. The quantity of odorant present at the epithelium, therefore, is not sufficient information to determine the concentration of odorant at the source. In order to accurately estimate concentration, the olfactory system must gather and integrate information regarding the sniff.
The number of molecules that reach the epithelium is determined both by the concentration of the odor source and by the airflow in the nostril. How does the olfactory system disentangle the two? The olfactory system can compute airflow using nonolfactory cues—either motor action that produces the sniff or somatosensory stimulation resulting from airflow in the nostril. A separate possibility is that the olfactory system uses a relational mechanism that compares a target odor to background odor; in other words, whereas the absolute number of molecules arriving at the epithelium would change with airflow, the relative number of target odor molecules compared to background odor molecules would not change with airflow.
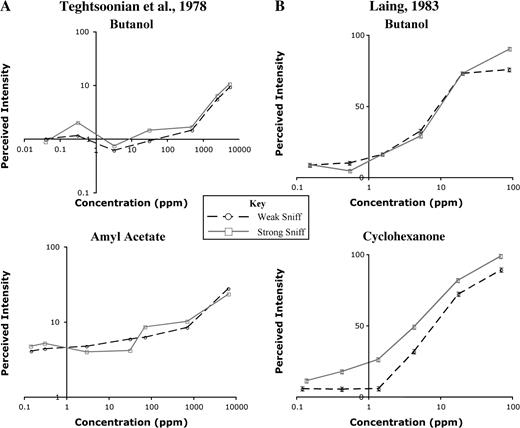
The Teghtsoonian model proposed that the olfactory system maintained concentration constancy by computing perceived effort during the sniff. Perceived effort is proportional to sniff pressure, so the model predicts that when subjects vary sniff pressure, concentration estimates will not vary with airflow. This was confirmed for both butanol and amyl acetate, as shown in (Figure 2A). The model also predicts that when airflow changes without a concomitant change in perceived effort, concentration constancy will no longer hold, and an increase in airflow will cause an increase in intensity perception. This situation occurs when sniff resistance is varied—the change in resistance alters airflow, but perceived effort remains constant. Under these conditions, the Teghtsoonian model predicts that an increase in airflow will cause an increase in intensity estimates. This has been shown in cases where external resistance was varied using different diameter delivery tubes (Rehn, 1978; R. Teghtsoonian and M. Teghtsoonian, 1984), mesh screens of varying resistance (Youngentob et al., 1986), and nasal dilators (Hornung et al., 1997).
Influence of sniffing technique on perceived intensity. (A) Mean perceived intensity ratings as a function of concentration for weak sniffs (circles) and strong sniffs (squares) (adapted from Teghtsoonian et al., 1978). No significant difference in intensity ratings at the two different airflow rates was seen for either butanol or amyl acetate across a wide range of concentrations. This implies that the olfactory system shows concentration constancy—dissociating the concentration of the source odor from the concentration of molecules at the epithelium. (B) Mean perceived intensity ratings of butanol as a function of concentration for natural single-sniff (circles) and strong single-sniff (squares) sampling techniques (adapted from Laing, 1983). As in (A), intensity ratings for butanol were not significantly different when using the two different sampling techniques. When subjects rated the intensity of cyclohexanone, however, higher airflow led to significantly higher intensity ratings, violating the Teghtsoonian model of concentration constancy.
Laing performed a study similar to the original Teghtsoonian study and replicated their result with butanol. The same study using cyclohexanone, however, did not generate the expected outcome (Figure 2B). In other words, he found that when subjects varied sniff pressure while sampling cyclohexanone, their intensity estimates were consistently higher when taking a large sniff. This suggests that the Teghtsoonian model might not apply to all odorants or under all conditions.
Sniffing influences perception of olfactory identity
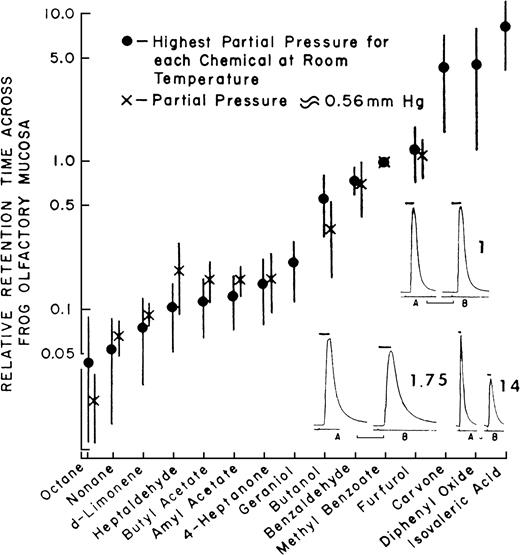
It is clear from the studies described earlier that the sniff is part of the intensity percept, but could the sniff contribute to additional aspects of the odor percept beyond intensity? The framework for such a possibility was set by the pioneering work of Mozell and colleagues, who demonstrated that odorants sorb to, and cross, the olfactory mucosa at different rates (Mozell and Jagodowicz, 1973; Mozell et al., 1991). They established this phenomenon by measuring the relative sorption rates of 15 odorants across the mucosa of the bullfrog (Figure 3).
Sorption rates of 15 different odorants across the mucosa of the frog (data from Mozell and Jagodowicz, 1973).
Mozell and colleagues later found that the effect of an odorant on the magnitude of response in the olfactory nerve of the frog results from an interaction between the particular sorption rate of that odorant and the velocity at which it flows across the olfactory mucosa: A high–sorption rate odorant will induce a large response when delivered at a high airflow and a smaller response when delivered at a lower airflow. In contrast, a low–sorption rate odorant will induce a small response when delivered at a high airflow and a larger response when delivered at a lower airflow. A theoretical explanation to this is as follows (Mozell et al., 1991). When a bolus of low-sorption odorant enters the nostril at a low velocity, there is a weak vector along the epithelium (low velocity) and a weak vector across the mucus (low sorption). This results in an even distribution of odorant molecules along the epithelium whereby a large epithelial surface area is affected, and the resulting response is large. In turn, when the same bolus of low-sorption odorant is flown rapidly across the mucosa, there is a strong vector along the epithelium (high velocity) and a weak vector across the mucus (low sorption). This results in a proportion of the molecules never sorbing before they are cleared into the respiratory system, an uneven distribution of odorant molecules along the epithelium whereby only the posterior end of the epithelial surface area is affected, and the resulting response is small. In contrast, when a bolus of high-sorption odorant enters the nostril at a low velocity, there is a weak vector along the epithelium (low velocity) and a strong vector across the mucus (high sorption). This results in an uneven distribution of odorant molecules along the epithelium whereby the full bolus saturates the anterior portion of the epithelium, a small epithelial surface area is affected, and the resulting response is small. In turn, when the same bolus of high-sorption odorant is flown rapidly across the mucosa, there is a strong vector along the epithelium (high velocity) and a strong vector across the mucus (high sorption). This results in an even distribution of odorant molecules along the epithelium whereby a large epithelial surface area is affected, and the resulting response is large (Mozell et al., 1991). This interaction may be the driving force behind the odorant-specific spatial patterns of activity across the olfactory epithelium (Mustaparta, 1971; Moulton, 1976; Thommesen and Doving, 1977; Mackay-Sim et al., 1982; Edwards et al., 1988; Kent and Mozell, 1992; Scott and Brierley, 1999; Kent et al., 2003). More recently, converging evidence from receptor distribution patterns and preservation of epithelial chromatographic mapping at the level of the olfactory bulb suggest that the nose is optimized to take advantage of chromatographic separation of odorants across the epithelium (Schoenfeld and Cleland, 2005; Scott, 2005). Together, these results add an additional aspect beyond intensity coding where the nature of the sniff strongly modulates, and is part of, the olfactory percept.
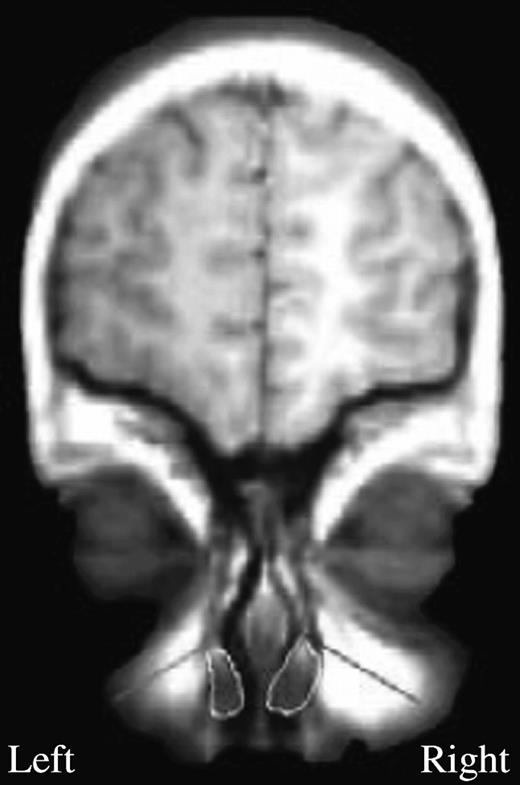
The above findings suggest that particular airflow velocities will optimize perception for particular odorants. High velocities will optimize perception of higher sorption rate odorants and low velocities will optimize perception of lower sorption rate odorants. In mammals, the velocity of airflow is usually higher in one nostril than in the other. This occurs because a bilateral highly vascularized structure, the nasal turbinate, swells with blood flow in either one nostril or the other, increasing the resistance to airflow in one nostril in comparison to the other (Principato and Ozenberger, 1970; Bojsen-Moller and Fahrenkrug, 1971; Hasegawa and Kern, 1977) (Figure 4). The nostril with higher airflow velocity, left or right, alternates on an ultradian rhythm of uncertain periodicity (Gilbert and Rosenwasser, 1987; Mirza et al., 1997). Considering the previously described findings in the frog, one may predict that the difference in airflow velocity between the nostrils in humans will result in a different olfactory percept in each nostril as a function of the interaction between airflow velocity and odorant sorption rates. Accordingly, Sobel et al. (1999) hypothesized that the high-velocity nostril is better tuned to high–sorption rate odorants and the low-velocity nostril is better tuned to low–sorption rate odorants.
A coronal image of the nasal passage. The turbinates are traced in white. In this subject, the right turbinate is expanded, largely blocking the right nostril. Thus, this subject has an open left nostril with high airflow rate (green) and an occluded right nostril with low airflow rate (red).
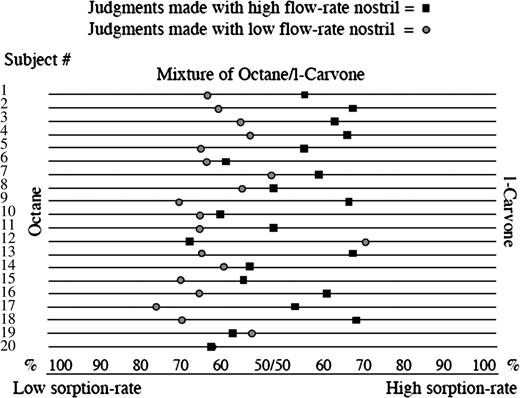
To test this, 20 subjects performed a task in which on each trial an olfactometer produced an equally proportioned mixture of the low–sorption rate odorant octane and the high–sorption rate odorant l-carvone (Figure 5). Although subjects were deceived and told that the mixtures would be slightly different on every trial, they were actually identical mixtures. The subject then 1) took a monorhinal fixed-duration sniff of the mixture with either the high-velocity nostril or low-velocity nostril, 2) smelled each component odorant individually, and then 3) judged the composition of the mixture on a proportion scale (ranging from 100% octane to 50/50 octane/l-carvone to 100% l-carvone, as illustrated at the bottom of Figure 5). Each subject performed 20 trials with each nostril. All experimental components were counterbalanced to prevent confounds of cross-adaptation and learning.
Nostril-specific odor tuning. Mean judgment of 10 trials made by each of the 20 subjects using the high–flow rate nostril (square) and the low–flow rate nostril (circle) separately to estimate the contents of the same mixture. The mixture was composed of 50% octane and 50% l-carvone. Using the high–flow rate nostril, the average judgment was that the mixture was composed of 55% l-carvone and 45% octane. Using the low–flow rate nostril, the average judgment was that the same mixture was composed of 61% octane and 39% l-carvone (t(19) = 3.74, P = 0.001) (Sobel et al., 1999).
Although the mixture was always the same, we predicted that when smelling the mixture with the high-velocity nostril, the high–sorption rate odorant would seem more prominent in the mixture, and when smelling the same mixture with the low-velocity nostril, the low–sorption rate odorant would seem more prominent. As predicted, 17 of the 20 subjects judged the same mixture to have a higher l-carvone content (high–sorption rate odorant) when using the high-velocity nostril and a higher octane content (low–sorption rate odorant) when using the low-velocity nostril (binomial, P < 0.002) (Figure 7). This finding demonstrated that the olfactory content obtained from each nostril in a given sniff was different and related to sniff airflow velocity. Each nostril was slightly better tuned to odorants that optimally sorb to the mucosa at the current airflow velocity in that nostril (Sobel et al., 1999). Critically, this effect consists of a clear demonstration that sniff velocity alters the percept of odorant identity when smelling mixtures. We have since replicated this finding with additional odorants (Bensafi et al., 2004) and found various experimental parameters that will either heighten or eliminate this effect (Mainland et al., 2005b). For example, it is critical that sniff duration is held equal across nostrils by matching sniff duration to a tone (as in the natural sniff). If subjects are permitted to sniff at will, they tend to sniff for a longer duration when using the low-velocity nostril, and this compensatory behavior eliminates the effect of nostril-specific, airflow-dependent odor tuning.
One important test of this theory is retronasal olfaction. During a sniff, odors are drawn across the epithelium from anterior to posterior. In retronasal olfaction, food odors reach the olfactory epithelium from the mouth via the posterior nares. If chromatographic separation is important to perception, then flowing odors in the opposite direction would lead to an inverted separation across the epithelium. For example, a high-sorption odorant sampled at a low velocity would saturate the anterior epithelium and be relatively absent in the posterior epithelium. In contrast, the same high-sorption odorant sampled at the same low velocity in the retronasal direction would saturate the posterior epithelium and be relatively absent in the anterior epithelium. Indeed, retronasal olfaction results in an altered olfactory percept (Rozin, 1982) as well as distinct odor-evoked potentials (Heilmann and Hummel, 2004) and functional magnetic resonance imaging (fMRI) activity (Cerf-Ducastel and Murphy, 2001; Small et al., 2005). It remains to be seen if perception is altered in a manner consistent with chromatographic separation of odorants and epithelial receptor distribution patterns.
Sniff-induced neural activity in the olfactory system
Given that odorant intensity and identity perception are influenced by patterns of sniffing, one may claim that the sniff itself is part of the olfactory percept. As such, one might expect the sniff to be represented in olfactory bulb and cortex. Since the pioneering work of Adrian (1942) and Freeman (1960), it has been known that two particular frequency domains dominate neural activity throughout the olfactory system (reviewed in Buonviso et al., 2005). The first is the slow θ rhythm (typically 3–12 Hz) related to sniffing, and the second is the γ rhythm (typically 30–100 Hz), an oscillation that rides on the sniffing wave. Both these activity types can be driven by sniffs without odorant. When Adrian first described these oscillations he, noted that “in spite of their olfactory origin the waves seem to depend more on the mechanical effect of the air current than on its smell.” Although Adrian (1950a, 1951) later suggested that filtered air did not stimulate olfactory areas, Ueki and Domino (1961) were able to elicit responses using purified air in both dogs and nonhuman primates.
Sniffs of odorless air drive activity patterns in the bulb (Hughes et al., 1969) and cortex (Sobel et al., 1998a) of humans as well. Activity in the human olfactory bulb was examined during therapeutic neurosurgical operations. When subjects were at rest with no odor stimulation, background activity in the human olfactory bulb was similar to intrinsic waves reported by Adrian (1950b). When an odorant was presented, rhythmical bursts were observed, again similar to the induced waves observed by Adrian. Signal amplitude also reflected behavior—successful odor detection was accompanied by an increase in the amplitudes of the responses recorded in the olfactory bulb (Hughes et al., 1969). Similarly, for suprathreshold stimuli, signal amplitude increased with increases in odorant intensity.
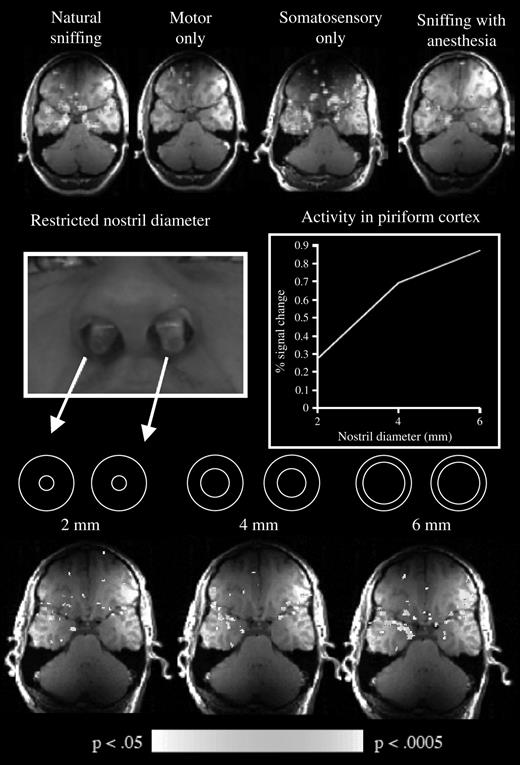
Human sniff-induced activity in primary olfactory cortex was identified with fMRI. Considering the temporal resolution of fMRI, it is unclear whether this global signal reflected the summation of the θ rhythm, the γ rhythm, or both. What functional aspect of the sniff drives this activity in primary olfactory cortex? Does it reflect the motor action of sniffing, the somatosensory stimulation of sniff airflow in the nostrils, attentional mechanisms, or the process of analyzing the odor content of a sniff even if no odorant was present? Using fMRI to address these possibilities, we found that 1) sniffing clean air induced activity in primary olfactory cortex, 2) the motor effort alone of trying to sniff with the nostrils occluded did not induce this activity, 3) artificially blowing air at the nostrils of otherwise passive subjects did induce this activity, 4) topical anesthesia of the nostril reduced this activity while not hampering olfaction, and 5) when systematically varying the airflow and resistance of sniffs, activity in primary olfactory cortex increases with increased airflow (Figure 6) (Sobel et al., 1998a). Thus, fMRI activity in piriform cortex was primarily driven by the somatosensory component of the sniff, namely, airflow in the nostrils [it is noteworthy that this sniff-induced activity may not be evident in positron emission tomography data (Kareken et al., 2004)]. It is tempting to link this activity to the slow θ rhythm. This is not to say that piriform cortex is simply somatosensory cortex or that the slow θ rhythm reflects solely airflow encoding. The ultimate role of olfactory cortex is to encode odor, not airflow. Indeed, when the identical odorless sniff is generated in and out of an olfactory context, the olfactory odorless sniff induces significant fMRI activity in piriform cortex and the respiratory odorless sniff induces significantly less activity (Zelano et al., 2005). Thus, it is our view that this global fMRI signal reflects the encoding of airflow as it factors into the computation of odor intensity and identity within olfactory cortex. When one factors out the sniff-induced activity, odorant-induced activity is revealed, reflecting the neural process of odorant encoding (Sobel et al., 2000b). Again, it is tempting to link this odor-induced component of the fMRI signal to the γ rhythm.
Influence of sniffing on fMRI signal in piriform cortex. Top row from left to right: magnetic resonance activity induced by natural sniffing, by trying to sniff with the nostrils occluded, by artificial airflows directed at the nostrils, and by sniffing with topical anesthesia in the nostrils. Middle row from left to right: the implants used to restrict nasal flow and the effect of this on fMRI signal in piriform cortex. Bottom row: the corresponding activation patterns. Taken together, these results demonstrated that activity in primary olfactory cortex is strongly affected by the somatosensory stimulation of sniffing (Sobel et al., 1998a).
These θ and γ oscillations occur in both the olfactory bulb and the primary olfactory cortex in a correlated manner (Eeckman and Freeman, 1990) and are present in rodents (Adrian, 1942; Ueki and Domino, 1961; Macrides and Chorover, 1972; Bressler and Freeman, 1980; Ketchum and Haberly, 1993) and perhaps in humans (Hughes et al., 1969). Although these oscillations are key to the process of olfaction, the exact functional significance of these patterns remains unclear (reviewed in Buonviso et al., 2005). Several researchers have suggested that the γ frequencies, coupled with the exceedingly large number of back-projecting pyramidal axons from anterior piriform cortex to the bulb, categorize olfactory stimuli in an increasingly specific fashion over successive sniff cycles. (Bressler, 1990; Freeman and Barrie, 1994; Bhalla and Bower, 1997). Although temporal ordering of neural activity is a well-described encoding strategy in the olfactory system of insects (reviewed in Laurent, 2002), the suggestion of temporal coding in the mammalian system was met with hesitation by a field that has been dominated by the notion of spatial encoding (Stewart et al., 1979; Jourdan et al., 1980; Lancet et al., 1982; Shepherd, 1985; Kauer, 1988; Guthrie and Gall, 1995; Mori and Yoshihara, 1995; Johnson et al., 1998, 2002; Rubin and Katz, 1999; Uchida et al., 2000; Meister and Bonhoeffer, 2001; Inaki et al., 2002). That said, a recent study by Spors and Grinvald (2002) begins to bridge the gap between these two schools (Friedrich, 2002). Spors and Grinvald combined optical imaging with voltage-sensitive dyes to obtain high spatial (10–20 μm) and temporal (50–200 Hz) resolution measurements from the olfactory bulbs of rodents. Using these methods, the authors found odorant-specific glomerular modules of activity similar to those previously described with optical imaging. However, the added temporal resolution revealed a highly dynamic spatial representation across the glomeruli that was constantly modified both within a sniff and across consecutive sniffs. In other words, odor was represented by a combined spatiotemporal pattern of activity. Although it is not yet clear how this spatiotemporal information translates into perception (reviewed in Kepecs et al., 2005), the brain may read this spatiotemporal pattern as a sequence of discreet spatiotemporal events like frames in a movie (Hopfield and Brody, 2001; Friedrich, 2002). On the other hand, these successive representations may represent stages of an ongoing computation in the bulb, in which case “time” is a computational variable in the construction of the odor representation at the bulbar level (Bhalla and Bower, 1997; Friedrich and Laurent, 2001). In both cases, the temporal information about odor may be carried by the intrinsic oscillations whereby it would alter the phase of activity in a specific manner (Hopfield, 1995).
In addition to directly participating in encoding of odorant content, the rapid oscillations may reflect the organization of network activity (Wilson and Bower, 1992; Protopapas and Bower, 2001). Specifically, current source-density analysis suggests that each γ oscillation decomposes each inspiratory cycle into temporal bins of about 20-ms duration (Ketchum and Haberly, 1993). One possibility is that the olfactory system uses these temporal bins to pair afferent input from the olfactory bulb with intrinsically generated associational activity and inhibition in piriform cortex in order to subserve this region's primary function as odor association cortex (Haberly, 1985, 1998, 2001; Wilson and Bower, 1992).
Neural control of the olfactomotor response
Given that sniffs are necessary for an olfactory percept and are represented in olfactory cortex, one may predict a dedicated neural subsystem for sniff generation, namely, an olfactomotor system. However, surprisingly little is known about the neural mechanisms devoted to the control of nasal respiration in the context of olfaction.
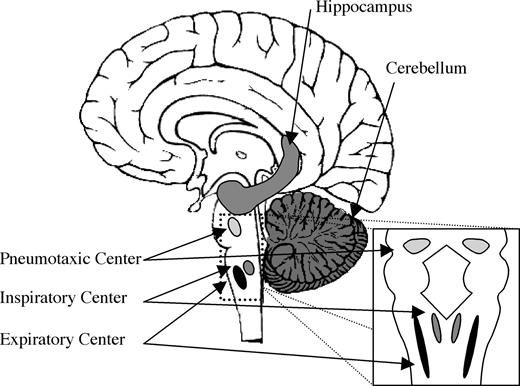
We speculate that the olfactory sniff both shares neural mechanisms with nonolfactory respiration and is likely subject to olfactory-related control through a dedicated olfactomotor system. Automatic nonolfactory control of respiration is regulated by three respiratory “centers”: the inspiratory center in the medial medulla, the expiratory center in the lateral medulla, and the pneumotaxic center at the pontomedullary junction (Figure 7). A typical nonolfactory respiratory cycle is initiated by stimulation of neurons in the inspiratory center by carbon dioxide levels in the blood. These chemosensory neurons then stimulate motor neurons supplying the diaphragm and intercostal muscles via reticulospinal connections. Expansion of the lung then drives expiratory neurons, which in turn inhibit inspiratory centers—this is known as the Hering–Breuer reflex. Expiration then occurs as a passive process, resulting from the relaxation of respiratory muscles.
Brain areas involved in the olfactomotor system. Sniff adjustments are thought to be mediated by either the cerebellum or hippocampus, leading to a final common pathway in the brain stem respiratory centers that control the diaphragm and intercostal muscles.
In addition to conscious control, sniffing may be reflexively elicited by chemicals, functioning as either irritants or odors in the nose (Tomori et al., 1994; Benacka and Tomori, 1995). When an odorant is encountered during passive breathing, it may be this reflex which leads to an exploratory sniff. The sniff reflex may also be induced by nonchemosensory stimuli. Puffs of chemically pure air, dispersion of dust particles into the nose, and mechanical stimulation of the pharynx all evoke the sniff reflex (Tomori, 1965; Berger and Mitchell, 1976; Widdicombe, 1986; Wallois et al., 1994). In studies conducted mostly in cats, mechanical and electrical stimulation of the nasal cavity have been used to determine the latency between activity in the inspiratory centers and muscle contraction in the diaphragm. Inspiratory neurons near the fasiculus solitarius have a low threshold to mechanical stimulation and respond to ipsilateral stimulation of the nasal cavity. Firing in these neurons leads the diaphragm by 40 ms. Inspiratory neurons near nucleus ambiguus, on the other hand, have a high threshold to mechanical stimulation and lead the diaphragm by 25 ms or less (Batsel and Lines, 1973). Stimulation of the glossopharyngeal nerve leads the diaphragm by 20–30 ms (Nail et al., 1969). These latencies refer to stimulation initiating a sniff. In contrast, the discharge frequency of neurons during a sniff can reach 400 Hz, allowing for modification of an ongoing sniff in approximately 4 ms (Batsel and Lines, 1973). In other words, the sniff mechanism can be initiated and modulated very rapidly.
Given that sniffs are generated and modulated as part of the olfactory percept, one would predict anatomical pathways mediating communication between olfactory areas and inspiratory centers. However, the identity of such olfactomotor pathways remains unknown. In searching for such substrates, one should consider neural loci that are privy to first-order olfactory information and also have direct input to respiratory control downstream of the inspiratory center in the medial medulla. Preliminary evidence implicates two structures, the hippocampus (Vanderwolf, 2001) and the cerebellum (Sobel et al., 1998b; Mainland et al., 2005b), as possibly involved in an olfactomotor circuit.
Hippocampus
In 1917, Ramon y Cajal considered, based on anatomical connectivity, that the hippocampal formation was part of the olfactory system (DeFelipe and Jones, 1988). The hippocampus is suited for an olfactomotor role in that it receives significant olfactory input via the entorhinal cortex and the perforant pathway (Switzer et al., 1985; McLean and Shipley, 1992) and has several descending projections that may synapse in respiratory centers (reviewed in Holstege, 1991). Similar to piriform cortex, the hippocampus exhibits slow wave activity that phase locks to sniffing (Macrides, 1975). However, unlike activity in piriform cortex, it is not specific to olfaction. Namely, this slow wave activity neither is affected by olfactory stimuli that do not elicit a motor response nor is it specific to motor responses triggered by olfactory stimuli (Vanderwolf, 1992). The hippocampus also exhibits fast wave (15–30 Hz) activity in response to olfactory stimuli. This activity does not diminish with repeated presentation of the odor and develops even in the absence of motor activity.
Cerebellum
It is particularly tempting to implicate the cerebellum in olfactomotor control because of its role in other senses. The cerebellum has been implicated in sensorimotor control, specifically to optimize sensory processing in both vision and somatosensation (Bower, 1997a,b). For example, cerebellar involvement in the optokinetic reflex helps stabilize visual signals during head and body movement (Robinson, 1976; Lisberger and Sejnowski, 1992). In light of the anatomical uniformity of cerebellar circuits (Palay and Chan-Palay, 1974), the cerebellum may play a similar role in olfaction, namely, optimize sniffing for olfactory processing (Sobel et al., 1998b; Mainland et al., 2005a).
A cerebellar role in olfactomotor control is plausible considering three recent lines of evidence suggesting that the cerebellum receives olfactory input. First, cerebellar activity has been consistently observed in functional imaging studies of olfaction (Small et al., 1997; Yousem et al., 1997; Sobel et al., 1998b; Qureshy et al., 2000; Savic et al., 2000; Zatorre et al., 2000; Cerf-Ducastel and Murphy, 2001; Ferdon and Murphy, 2003). Second, genetically modified mice with cerebellar abnormalities have impaired olfaction (Feron and Baudoin, 1992, 1993; Baudoin et al., 1994; Deiss and Baudoin, 1997). Third, human patients with cerebellar degeneration or lesions exhibit olfactory impairments (Abele et al., 2003; Connelly et al., 2003) contralateral to the side of the lesion (Mainland et al., 2005a). The path of olfactory input to the cerebellum remains unknown, although it may occur via neurons in the ventral tegmental area that send collaterals to both piriform cortex and both hemispheres of the cerebellum (Ikai et al., 1992, 1994).
Finally, the cerebellum is functionally well situated to generate and/or modulate olfactomotor responses. The cerebellum may interact with either spinal inspiratory facilitatory reflexes or supraspinal inspiratory inhibitory reflexes to modify the diaphragm and intercostal muscles that control the sniff (Decima and von Euler, 1969). Depression of cerebellar function in the cat by ischemia, topical administration of procaine, or ablation results in an augmentation of inspiratory activity (Glasser et al., 1966). In addition, electrical stimulation of the anterior lobe of the cerebellum inhibits the inspiratory discharge driven by the medullary respiratory mechanism (Moruzzi, 1940; Decima and von Euler, 1969). Taken together, these results suggest that the cerebellum provides a tonic, primarily inhibitory influence on inspiratory mechanisms of the lower brain stem. Furthermore, patients with cerebellar lesions often have weakness in the muscles of the diaphragm and show respiratory ataxia (Mavlov and Chavdarov, 1968; Mier-Jedrzejowicz and Green, 1988).
Olfaction as an active process
Given a dedicated olfactomotor system, one may ask what physical aspects of the sniff are modulated by this system. The neural response to odorants is highly dependent on airflow velocity, and as previously noted, sniff velocity combines with odorant solubility to produce different patterns of neural response (Mozell et al., 1991). Ideally, the olfactory system would optimize sniff velocity, duration, and number of sniffs in a bout for each odorant it set out to detect and each task it set out to perform. This, however, is only possible when setting out for olfactory probing of a previously learned stimulus. For example, when placing one's nose within the neck of a bottle of milk that has been sitting in the refrigerator for too long, one may optimize the sniff for that particular olfactory note denoting spoiled milk. Likewise, specified sniffs may be generated for detection of stereotyped odors such as predator, prey, or mate. It is noteworthy, however, that this is a prediction we are making as we are unaware of current evidence for target-specific sniffing of this kind. In contrast to pretailoring sniffs to an anticipated odor, a second role for the olfactomotor system is to adjust ongoing sniffing in real time to account for the odor being perceived. There are several lines of evidence for this type of olfactomotor processing, revealing real-time modulation of sniff airflow, sniff duration, and number of sniffs in bout.
Airflow modulation
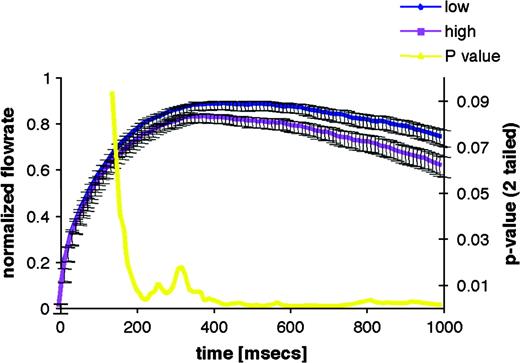
When the olfactory system encounters a concentrated odorant, sniff vigor is reduced in real time; when it encounters a diluted odorant, sniff vigor is increased in real time. This inverse relationship between sniff vigor (reflected in the airflow velocity and resultant sniff volume) and odor concentration holds for a variety of odors and across a broad range of intensities (Laing, 1983; Warren et al., 1994; Sobel et al., 2001; Walker et al., 2001). A similar interaction reflects odorant pleasantness: real-time sniff vigor increases when smelling a pleasant odor and decreases when smelling an unpleasant odor (Bensafi et al., 2003). The predicted reduction in sniff vigor in response to an intense odorant is sufficiently robust such that its absence is sufficient for clinical diagnosis of olfactory impairments (Frank et al., 2003). To probe the latency of this olfactomotor response, Johnson et al. (2003) used careful recordings of sniff airflow combined with tight temporal control over odorant delivery. They found that sniffs were initially uniform but were then modulated in accordance with odorant concentration by as early as 160 ms following sniff onset (Figure 8). This psychophysical finding may offer some insight as to the neural organization of the olfactomotor system. Specifically, the duration of the olfactory transduction cascade is estimated at ∼150 ms (Firestein et al., 1990; Duchamp-Viret et al., 2000). In turn, the typical latency for olfactory cortical-evoked potentials is between 171 and 400 ms (Hummel and Kobal, 1992; Livermore et al., 1992; Murphy et al., 2000). In other words, the olfactomotor system modulates sniffs at a latency that barely trails transduction and precedes cortical responses. This points to subcortical substrates of olfactomotor function and is consistent with a predicted role for the cerebellum in this mechanism.
Time course of concentration-dependent sniff modulation. The first second of the mean sniff to low and high concentrations of propionic acid is shown. The blue line is the mean sniff to the low concentration, and the purple line is the mean sniff to the high concentration. Bars are standard error. The P value from the associated paired t-test is shown in yellow. Sniffs of propionic acid are significantly concentration dependent by 160 ms.
Duration modulation
The above-described concentration-dependent sniff modulation is typically reflected in sniff duration as well. In other words, sniffs of higher as compared to lower concentration odorants are typified by a reduction in sniff velocity but also tend to terminate earlier. In turn, we have found one instance where the olfactomotor system modulates sniff duration independent of sniff velocity. This was revealed in tests of unilateral olfactory detection. As previously noted, the flow of air is usually greater into one nostril than into the other because there is a slight swelling of the turbinate on one side (Kayser, 1895; Principato and Ozenberger, 1970; Hasegawa and Kern, 1977). Considering that airflow influences olfactory performance, one would therefore expect different olfactory performance across nostrils as a function of airflow. Paradoxically, however, previous tests of olfactory detection had revealed equal performance across nostrils despite pronounced differences in airflow (Eccles et al., 1989; Frye, 1995). We hypothesized that this paradoxical equal performance across nostrils differing in airflow velocity may have reflected olfactomotor compensation. Specifically, when forced to sniff through the low-airflow nostril, subjects may have either sniffed longer or sniffed stronger with that nostril in order to optimize performance. To address this, rather than measure airflow in each nostril before and/or after the task as previous studies had done, we measured airflow during the task (Sobel et al., 1999). We found that when forced to sniff through the low-airflow nostril, subjects automatically sniffed longer with that nostril and thus equated performance across nostrils. Furthermore, when we eliminated this compensatory mechanism by preventing the low-velocity nostril from increasing sniff duration, detection performance dropped in that nostril as compared to the higher velocity nostril. It is noteworthy that this compensatory olfactomotor mechanism that obscured differences in detection threshold across the high- and low-velocity nostrils can also obscure the previously described velocity-dependent, nostril-specific odorant tuning. To prevent this, it is essential to equate sniff duration across nostrils during testing by instructing subjects to match their sniff duration to an auditory tone that is set at the duration of a typical sniff for that task (∼1.6 s) (Mainland et al., 2005b). Although this line of studies negated the paradox, it remains unclear why, when forced to sniff under conditions of restricted flow, the olfactomotor system opted to sniff longer rather than stronger.
Number of sniffs in a bout
Upon encountering an odorant, many humans take multiple sniffs despite the fact that both odor presence and strength can normally be determined in one sniff (Laing, 1986; Uchida and Mainen, 2003). Multiple sniffs may be necessary in natural conditions where complex mixtures of molecules are present in the environment. As previously noted, the olfactory system may use successive sniffs to categorize olfactory stimuli in an increasingly specific fashion over successive sniff cycles. Haberly (2001) has theorized that the network properties of piriform cortex resemble computational models of associative networks. Each sniff drives activity in the piriform cortex at a frequency of 40 Hz, which, in simulations, corresponds to the time for activity to sweep from anterior piriform to posterior piriform and back again (Wilson and Bower, 1992). Haberly has proposed that this activity represents the olfactory system comparing the odor input to stored representations in posterior piriform. While determination of odor presence and intensity for single molecules may not require this activity, mixture segmentation or complex figure–ground separation may call for such an iterative process. Wilson (2001) suggests that there may be two distinct olfactory systems—one for identification of specific odorants followed by rapid, reliable responses and one for a synthetic memory-based system designed to form perceptual gestalts from complex mixtures. It is unclear if the second type of olfactory discrimination requires a second sniff; however, preliminary results from our laboratory indicate that two sniffs are beneficial in correctly identifying components of binary mixtures (Mainland et al., 2004).
The role of sniffing in our (mis)understanding of olfactory intensity coding
The sniff is an active process, suggesting that passive delivery of odor without sniffing is an unnatural stimulus. Indeed, even in the arena of artificial chemosensory devices, the head of the US Department of Homeland Security's Transportation Security Laboratory notes that “Chemists have been so fixed on detector development [that] that's exactly what we got: very well-developed detectors that have no front ends” (Rouhi, 1997). As the field of fluid dynamics attempts to address this problem in artificial noses (Settles, 2005), it is important to examine the cost of ignoring the sniff in olfactory studies.
Although natural mammalian olfaction is inseparably linked to active sniffing, in most studies of mammalian odor intensity, coding odorants of different concentrations were passively delivered in pulses of identical duration to the olfactory epithelium of an anesthetized animal. In optical imaging experiments, such increases in concentration resulted in increased spatial extent of activity on the surface of the olfactory bulb (Rubin and Katz, 1999; Uchida et al., 2000; Meister and Bonhoeffer, 2001; Spors and Grinvald, 2002; Sugai et al., 2005) and cortex (Sugai et al., 2005). Thus, it is largely agreed that increased concentration is encoded through recruitment of additional glomeruli or cortical space. In awake behaving mammals, however, the olfactomotor system would have prevented continued equal flow rate and duration sampling of a high-concentration odorant. In the awake animal, an odorant would be sampled (sniffed at) for a long duration with a high maximum airflow when at low concentrations but for a short duration with a low maximum airflow when at high concentrations. Thus, the fact that increased odor concentrations recruited additional glomeruli in imaging studies may reflect negation of the olfactomotor system through anesthesia, rather than a realistic mammalian encoding strategy. Indeed, the few studies that directly recorded neural activity in the olfactory system of awake behaving mammals revealed patterns of activity very different from those in the anesthetized preparation (Schoenbaum and Eichenbaum, 1995; Bhalla and Bower, 1997). Most pertinently, increasing odor concentration did not necessarily induce higher rates of activity in the olfactory bulb but rather modulated complex interactions of excitation and suppression in rabbits (Chaput and Lankheet, 1987) and temporally shifted the peak of activity to coincide with an earlier respiratory cycle following odor onset in rats (Chalansonnet and Chaput, 1998). Whereas the differences in results from the anesthetized and unanesthetized animals may reflect direct effects of anesthesia on neural activity, we suggest they reflect anesthetic negation of the olfactomotor system and the resultant sniffing. Indeed, when unanesthetized sniffing rats were studied with 2-deoxy-glucose (Johnson and Leon, 2000), for three of five odorants studied, increasing concentration did not induce different patterns of activity in the olfactory bulb (although such changes were apparent for the remaining two odorants).
The sniff is sufficient for an olfactory percept
Considering all the above evidence, namely, that sniffs 1) are necessary for an olfactory percept, 2) contribute to the representation of odorant intensity and identity, 3) are represented in olfactory cortex, and 4) are controlled by a dedicated sensorimotor subsystem, one may ask whether a sniff alone, without the presence of odorants, is sufficient for an olfactory percept of some sort? Evidence in support of this possibility was first obtained inadvertently during early studies using a method known as “blast olfactometry” (Elsberg, 1937). In this method, an odorant was loaded into a syringe and a fixed-volume bolus of odor was delivered to the nose. This method gave a constant sniff volume; however, it was deemed problematic when Wenzel (1949, 1955) found that subjects responded to the blast pressure as an odor, even when an odorant was not present. Similarly, Leopold (2002), writing about patients with olfactory hallucinations (phantosmia), notes that “Most of the phantosmia patients we have seen can start the odor perception with a small sniff or sneeze and they go to great lengths to avoid nasal airflow.”
The two above examples whereby an olfactory percept was induced by odorless airflow in the nose were consistent with our subjective notion that when humans are asked to imagine an odor, that is, recreate the sensation of smell in the absence of an odorant, they spontaneously sniff. To test this, Bensafi et al. (2003) measured nasal airflow while subjects were trying to imagine various sights, sounds, or smells. They found that olfactory imagery, but not visual or auditory imagery, was accompanied by spontaneous sniffing. Moreover, the properties of the sniff during olfactory imagery resembled those of a sniff during normal olfactory perception. Specifically, just as when perceiving real odorants, when humans were imagining a pleasant odor, they took a large sniff, but when they were imagining an unpleasant odor, they took a small sniff. Moreover, blocking the nasal passage reduced the quality of olfactory imagery, and encouraging sniffing increased the quality of olfactory imagery (Bensafi et al., 2003, 2005). This suggests that sniffs have an important functional role in olfactory imagery and, in fact, generate an olfactory percept of some sort even in the absence of odor.
Conclusion
Sniffs are not merely a stimulus carrier. Here we have shown that sniffs 1) are necessary for the olfactory percept, 2) affect odorant intensity perception and identity perception, 3) drive activity in olfactory cortex, 4) are rapidly modulated in an odorant-dependent fashion by a dedicated olfactomotor system, and 5) are sufficient to generate an olfactory percept of some sort even in the absence of odor. This allows us to conclude that the sniff itself is part of the olfactory percept.
References
Abele, M., Riet, A., Hummel, T., Klockgether, T. and Wullner, U. (
Ache, B.W. (
Adrian, E.D. (
Batsel, H.L. and Lines, A.J. Jr (
Baudoin, C., Feron, C. and Deiss, V. (
Benacka, R. and Tomori, Z. (
Bensafi, M., Porter, J., Pouliot, S., Mainland, J., Johnson, B., Zelano, C., Young, N., Bremner, E., Aframian, D., Khan, R. and Sobel, N. (
Bensafi, M., Pouliot, S. and Sobel, N. (
Bensafi, M., Zelano, C.M., Johnson, B.N., Mainland, J.D., Khan, R. and Sobel, N. (
Berger, A.J. and Mitchell, R.A. (
Bhalla, U.S. and Bower, J.M. (
Bocca, E., Antonelli, A.R. and Mosciaro, O. (
Bojsen-Moller, F. and Fahrenkrug, J. (
Bower, J.M. (
Bressler, S.L. (
Bressler, S.L. and Freeman, W.J. (
Buonviso, N., Amat, C. and Litaudon, P. (
Cerf-Ducastel, B. and Murphy, C. (
Chalansonnet, M. and Chaput, M.A. (
Chaput, M.A. and Lankheet, M.J. (
Connelly, T., Farmer, J.M., Lynch, D.R. and Doty, R.L. (
Decima, E.E. and von Euler, C. (
DeFelipe, J. and Jones, E.G. (
Deiss, V. and Baudoin, C. (
Duchamp-Viret, P., Duchamp, A. and Chaput, M.A. (
Eccles, R., Jawad, M.S. and Morris, S. (
Edwards, D.A., Mather, R.A. and Dodd, G.H. (
Eeckman, F. and Freeman, W. (
Elsberg, C.A. (
Ferdon, S. and Murphy, C. (
Feron, C. and Baudoin, C. (
Feron, C. and Baudoin, C. (
Firestein, S., Shepherd, G.M. and Werblin, F.S. (
Frank, R.A., Dulay, M.F. and Gesteland, R.C. (
Freeman, W.J. (
Freeman, W.J. and Barrie, J. (
Friedrich, R.W. and Laurent, G. (
Frye, R.E. (
Gilbert, A.N. and Rosenwasser, A.M. (
Glasser, R.L., Tippett, J.W. and Davidian, V.A. Jr (
Guthrie, K.M. and Gall, C.M. (
Haberly, L.B. (
Haberly, L.B. (
Haberly, L.B. (
Hahn, I., Scherer, P.W. and Mozell, M.M. (
Heilmann, S. and Hummel, T. (
Holstege, G. (
Hopfield, J.J. (
Hopfield, J.J. and Brody, C.D. (
Hornung, D.E., Chin, C., Kurtz, D.B., Kent, P.F. and Mozell, M.M. (
Hughes, J.R., Hendrix, D.E., Wetzel, N. and Johnston, J.W. (
Hummel, T. and Kobal, G. (
Ikai, Y., Takada, M. and Mizuno, N. (
Ikai, Y., Takada, M., Shinonaga, Y. and Mizuno, N. (
Inaki, K., Takahashi, Y., Nagayama, S. and Mori, K. (
Johnson, B.A., Ho, S.L., Xu, Z., Yihan, J.S., Yip, S., Hingco, E.E. and Leon, M. (
Johnson, B.A. and Leon, M. (
Johnson, B.A., Woo, C.C. and Leon, M. (
Johnson, B.N., Mainland, J.D. and Sobel, N. (
Jourdan, F., Duveau, A., Astic, L. and Holley, A. (
Kareken, D.A., Sabri, M., Radnovich, A.J., Claus, E., Foresman, B., Hector, D. and Hutchins, G.D. (
Kauer, J.S. (
Kent, P.F. and Mozell, M.M. (
Kent, P.F., Mozell, M.M., Youngentob, S.L. and Yurco, P. (
Kepecs, A., Uchida, N. and Mainen, Z.F. (
Ketchum, K.L. and Haberly, L.B. (
Keyhani, K., Scherer, P.W. and Mozell, M.M. (
Laing, D.G. (
Laing, D.G. (
Lancet, D., Greer, C.A., Kauer, J.S. and Shepherd, G.M. (
Laurent, G. (
Le Magnen, J. (
Leopold, D. (
Lisberger, S.G. and Sejnowski, T.J. (
Livermore, A., Hummel, T. and Kobal, G. (
Mackay-Sim, A., Shaman, P. and Moulton, D.G. (
Macrides, F. (
Macrides, F. and Chorover, S.L. (
Mainland, J.D., Johnson, B.N., Khan, R.M., Ivry, R.B. and Sobel, N. (
Mainland, J.D., Khan, R. and Sobel, N. (
Mainland, J.D., Yung, V., Young, N. and Sobel, N. (
Mavlov, L. and Chavdarov, D. (
McLean, J.H. and Shipley, M.T. (
Meister, M. and Bonhoeffer, T. (
Mier-Jedrzejowicz, A. and Green, M. (
Mirza, N., Kroger, H. and Doty, R. (
Mori, K. and Yoshihara, Y. (
Moruzzi, G. (
Moulton, D.G. (
Mozell, M., Kent, P. and Murphy, S. (
Mozell, M.M. and Jagodowicz, M. (
Murphy, B.J. (
Murphy, C., Morgan, C.D., Geisler, M.W., Wetter, S., Covington, J.W., Madowitz, M.D., Nordin, S. and Polich, J.M. (
Mustaparta, H. (
Nail, B.S., Sterling, G.M. and Widdicombe, J.G. (
Palay, S.L. and Chan-Palay, V. (
Paulsen, E. (
Principato, J.J. and Ozenberger, J.M. (
Protopapas, A.D. and Bower, J.M. (
Qureshy, A., Kawashima, R., Imran, M.B., Sugiura, M., Goto, R., Okada, K., Inoue, K., Itoh, M., Schormann, T., Zilles, K. and Fukuda, H. (
Rehn, T. (
Robinson, D.A. (
Rozin, P. (
Rubin, B.D. and Katz, L.C. (
Savic, I., Gulyas, B., Larsson, M. and Roland, P. (
Schoenbaum, G. and Eichenbaum, H. (
Schoenfeld, T.A. and Cleland, T.A. (
Scott, J.W. (
Scott, J.W. and Brierley, T. (
Settles, G.S. (
Shepherd, G.M. (
Small, D.M., Gerber, J.C., Mak, Y.E. and Hummel, T. (
Small, D.M., Jones-Gotman, M., Zatorre, R.J., Petrides, M. and Evans, A.C. (
Sobel, N., Khan, R.M., Hartley, C.A., Sullivan, E.V. and Gabrieli, J.D. (
Sobel, N., Khan, R.M., Saltman, A., Sullivan, E.V. and Gabrieli, J.D. (
Sobel, N., Prabhakaran, V., Desmond, J.E., Glover, G.H., Goode, R.L., Sullivan, E.V. and Gabrieli, J.D. (
Sobel, N., Prabhakaran, V., Hartley, C.A., Desmond, J.E., Zhao, Z., Glover, G.H., Gabrieli, J.D. and Sullivan, E.V. (
Sobel, N., Prabhakaran, V., Zhao, Z., Desmond, J.E., Glover, G.H., Sullivan, E.V. and Gabrieli, J.D. (
Sobel, N., Thomason, M.E., Stappen, I., Tanner, C.M., Tetrud, J.W., Bower, J.M., Sullivan, E.V. and Gabrieli, J.D. (
Spors, H. and Grinvald, A. (
Stewart, W.B., Kauer, J.S. and Shepherd, G.M. (
Sugai, T., Miyazawa, T., Fukuda, M., Yoshimura, H. and Onoda, N. (
Switzer, R.C., DeOlmos, J. and Heimer, L. (
Teghtsoonian, R. and Teghtsoonian, M. (
Teghtsoonian, R. and Teghtsoonian, M. (
Teghtsoonian, R., Teghtsoonian, M., Berglund, B. and Berglund, U. (
Thommesen, G. and Doving, K.B. (
Tomori, Z. (
Tomori, Z., Donic, V., Kurpas, M. and Palenikova, R. (
Uchida, N. and Mainen, Z.F. (
Uchida, N., Takahashi, Y.K., Tanifuji, M. and Mori, K. (
Ueki, S. and Domino, E.F. (
Vanderwolf, C.H. (
Vanderwolf, C.H. (
Veress, E. (
Walker, J.C., Kendal-Reed, M., Hall, S.B., Morgan, W.T., Polyakov, V.V. and Lutz, R.W. (
Wallois, F., Macron, L.J.M., Jounieux, V. and Duron, B. (
Warren, D.W., Walker, J.C., Drake, A.F. and Lutz, R.W. (
Weber, E.H. (
Wenzel, B.M. (
Widdicombe, J.G. (
Wilson, M. and Bower, J.M. (
Youngentob, S.L., Stern, N.M., Mozell, M.M., Leopold, D.A. and Hornung, D.E. (
Yousem, D.M., Williams, S.C., Howard, R.O., Andrew, C., Simmons, A., Allin, M., Geckle, R.J., Suskind, D., Bullmore, E.T., Brammer, M.J. and Doty, R.L. (
Zatorre, R.J., Jones-Gotman, M. and Rouby, C. (
Zelano, C., Bensafi, M., Porter, J., Mainland, J., Johnson, B., Bremner, E., Telles, C., Khan, R. and Sobel, N. (
Zhao, K., Dalton, P., Yang, G.C. and Scherer, P.W. (