-
PDF
- Split View
-
Views
-
Cite
Cite
Gregor Thut, Annika Nietzel, Alvaro Pascual-Leone, Dorsal Posterior Parietal rTMS Affects Voluntary Orienting of Visuospatial Attention, Cerebral Cortex, Volume 15, Issue 5, May 2005, Pages 628–638, https://doi.org/10.1093/cercor/bhh164
Close - Share Icon Share
Abstract
Patients with lesions in posterior parietal cortex (PPC) are relatively unimpaired in voluntarily directing visual attention to different spatial locations, while many neuroimaging studies in healthy subjects suggest dorsal PPC involvement in this function. We used an offline repetitive transcranial magnetic stimulation (rTMS) protocol to study this issue further. Ten healthy participants performed a cue–target paradigm. Cues prompted covert orienting of spatial attention under voluntary control to either a left or right visual field position. Targets were flashed subsequently at the cued or uncued location, or bilaterally. Following rTMS over right dorsal PPC, (i) the benefit for target detection at cued versus uncued positions was preserved irrespective of cueing direction (left- or rightward), but (ii) leftward cueing was associated with a global impairment in target detection, at all target locations. This reveals that leftward orienting was still possible after right dorsal PPC stimulation, albeit at an increased overall cost for target detection. In addition, rTMS (iii) impaired left, but (iv) enhanced right target detection after rightward cueing. The finding of a global drop in target detection during leftward orienting with a spared, relative detection benefit at the cued (left) location (i–ii) suggests that right dorsal PPC plays a subsidiary rather than pivotal role in voluntary spatial orienting. This finding reconciles seemingly conflicting results from patients and neuroimaging studies. The finding of attentional inhibition and enhancement occurring contra- and ipsilaterally to the stimulation site (iii–iv) supports the view that spatial attention bias can be selectively modulated through rTMS, which has proven useful to transiently reduce visual hemispatial neglect.
Introduction
The posterior parietal lobes have long been implicated in visuospatial attention, together with other areas of a frontoparietal network and subcortical structures, based on patient studies (Mesulam, 1981; Posner et al., 1984; Vallar and Perani, 1986; for review, see Halligan et al., 2003) and functional brain imaging in healthy subjects (for review, see Kanwisher and Wojciulik, 2000). Patients with parietal damage can show attentional deficits, generally characterized by an inability to attend or respond to contralesional visual stimuli (hemispatial neglect). Symptoms affect most often the left hemifield, as hemispatial neglect is both more severe and more frequent following right than left parietal damage (Halligan et al., 2003). The evidence for a role of posterior parietal cortex (PPC) in visuospatial attention has been strengthened further by transcranial magnetic stimulation (TMS) studies showing that some of the visuospatial deficits frequently encountered in neglect patients can also be transiently induced in normal volunteers by PPC stimulation (Pascual-Leone et al., 1994; Walsh et al., 1999; Fierro et al., 2000, 2001; Hilgetag et al., 2001; Bjoertomt et al., 2002; Brighina et al., 2002; Müri et al., 2002). These TMS studies generally confirmed the dominance of the right relative to the left parietal cortex in the control of visuospatial attention (Fierro et al., 2000; Brighina et al., 2002; Müri et al., 2002) — and have probed the timing of PPC involvement (Walsh et al., 1999; Fierro et al., 2001; Pourtois et al., 2001; Müri et al., 2002) and the differential implications of distinct cortical areas in different aspects of neglect, i.e. visual versus motor (Brighina et al., 2002) or near versus far space (Bjoertomt et al., 2002). Effects of posterior parietal TMS include impaired detection of visual stimuli in the hemifield contralateral to TMS shown for visual search (Walsh et al., 1999), target detection (Hilgetag et al., 2001) and recognition tasks (Müri et al., 2002), as well as ‘paradoxical positive’ consequences such as enhanced stimulus detection in the visual field ipsilateral to TMS (Walsh et al., 1999; Hilgetag et al., 2001).
Regarding the role of PPC in spatial attention, a distinction has been drawn between ventral PPC, which is a part of the temporo-parietal junction (TPJ), and dorsal PPC areas along the intraparietal sulcus (IPs) (e.g. Corbetta and Shulman, 2002). While the contribution of dorsal PPC/IPs lesions to neglect is still a matter of debate (e.g. Corbetta and Shulman, 2002), there is a general consensus that damage to the ventral PPC and/or other areas of the TPJ is a principle substrate of neglect and other clinical phenomena with visuo-spatial deficits such as extinction (e.g. Vallar, 2001; Corbetta and Shulman, 2002, Karnath et al., 2003). Likewise, while there are contradictory results on the role of dorsal PPC in attentional control when tested within Posner et al.'s influential attention framework (Posner et al., 1984), converging evidence has been provided as to the role of ventral PPC. Posner's framework breaks down the mechanisms of directing visual attention in space into the following three elementary mental operations that can be performed covertly, without overt movements of the eyes or head: (i) disengagement from a current focus of attention (spatial disengagement); (ii) directing attention to a new spatial location (spatial orienting); and (iii) engagement of the target. The framework further distinguishes between attention operations performed under endogenous control (e.g. voluntary orienting) and those driven by a peripheral cue (e.g. reflexive orienting). Within this framework, contradictory results have been obtained regarding dorsal PPC function as many neuroimaging studies have implicated the dorsal PPC (along with areas in the IPs) in voluntary spatial orienting under endogenous control (e.g. Corbetta et al., 1993, 2000; Nobre et al., 1997; Kastner et al., 1999; Hopfinger et al., 2000) but this function appears to be relatively unimpaired in patients with PPC lesions. In fact, patients with PPC damage can use endogenous information to facilitate spatial orienting of attention in any direction (Posner et al., 1984; Friedrich et al., 1998; Smania et al., 1998; Bartolomeo and Chokron, 2002). Converging results, on the other hand, have been obtained for the ventral PPC, suggesting involvement in spatial disengagement along with other areas of the TPJ. Functional imaging in healthy subjects has revealed TPJ activation with disengagement of spatial attention (Corbetta et al., 2000). In agreement with this, PPC lesions have been related to prominent spatial disengagement deficits in contralesional direction (e.g. Posner et al., 1984, 1987; for review, see Losier and Klein, 2001), especially when TPJ damage is involved (Friedrich et al., 1998).
In the present repetitive TMS (rTMS) study, we aimed to shed further light on dorsal PPC involvement in voluntary spatial orienting. In addition, we sought to further investigate whether disruption of this dorsal PPC function might contribute to spatially lateralized attention deficits as evidenced in neglect. For this purpose, rTMS was applied over the right dorsal PPC and a variant of the Posner paradigm was used (Posner et al., 1984). In our study, binaural cues prompted voluntary orienting and maintaining of visual attention at either a left or a right hemifield position (directional cues). This was followed by presentation of a visual target flashed more often at cued locations (valid trials) than at uncued locations or bilaterally (invalid trials). To be able to isolate rTMS-effects on voluntary space exploration, additional neutral cue trials, in which targets were equally likely to appear in either visual field (no spatial cueing but otherwise identical task components), were presented for control purposes. Based on previous evidence of dorsal PPC involvement in voluntary orienting, and given the finding that dorsal PPC/IPs lesions are not related to neglect, we expected that dorsal PPC stimulation would affect voluntary orienting task components to directional cues more than other attentional components tested through neutral cue trials.
We considered two possibilities to explain the conflicting results on the role of dorsal PPC in voluntary spatial orienting. One possible hypothesis is that the dorsal PPC is critically implicated in voluntary spatial orienting, in line with previous neuroimaging studies, and that the lack of deficits in this function in patients with PPC damage is due to advanced functional reorganization at the time of testing. If so, one would expect that right parietal rTMS in healthy subjects would disrupt spatial orienting, because the acute TMS-effects largely avoid the possibility of reorganization (Pascual-Leone et al., 1999). More precisely, the advantage for detecting targets at cued versus uncued locations (Posner et al., 1984) should collapse for left cue conditions, as subjects would be expected to be impaired in voluntarily directing their attention to the left hemifield, contralateral to rTMS. Conversely, one might expect an accentuation of this advantage for right cue conditions, as voluntary orienting to the right (ipsilateral) hemifield might be enhanced. An alternative hypothesis is that the dorsal PPC has a subsidiary rather than a pivotal role in voluntary spatial orienting, hence leading to significant activation of this area during functional imaging, but only to discrete behavioral changes following lesions. In this case, subjects might still benefit from endogenous information to direct visual attention in space, both following PPC damage and in spite of parietal TMS. However, interference with a subsidiary node of the attention-directing network via right parietal TMS would be expected to increase the difficulty of performing the voluntary orienting task, especially in regards to leftward orienting, and should thereby lead to discrete but detectable changes in behavior. Increased difficulty performing voluntary attention shifts to the left has been observed in patients with right parietal damage, which translated into slowing of leftward orienting, although benefits at cued location following completion of the attention shifts were comparable in these patients and a healthy control group (Bartolomeo et al., 2001).
Materials and Methods
Participants
Ten healthy subjects (five women, five men) aged 21–36 years (mean 26 years) participated in the study. Nine were right-handed and one ambidextrous according to the Oldfield Handedness Questionnaire. All had normal or corrected-to-normal vision and no history of neurological or psychiatric disorders. Written informed consent was obtained from all participants prior to participation in the study, which had been approved by the Institutional Review Board of the Beth Israel Deaconess Medical Center.
Experimental Conditions, Stimuli and Paradigm
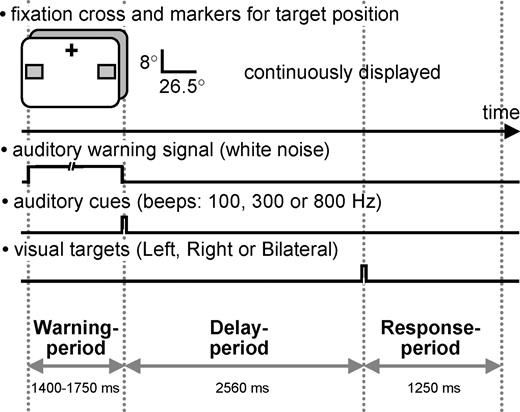
The paradigm was a cued target detection task. We used three different cueing conditions followed by three different target conditions, giving rise to nine cue–target combinations. Cues could be either directional [cueing of visuospatial attention to one of two designated positions in either the left visual field (LVF) or the right visual field (RVF)] or neutral (no spatial cueing). The target appeared subsequently at one of the two designated positions, or bilaterally. Following directional cues, the probability of targets appearing at cued locations was 0.66 (cue L/target L and cue R/target R respectively), and the probability of targets appearing at uncued locations 0.33 (cue L/target R or target B, cue R/target L or target B). Following neutral cues, targets appeared at left or right location markers with equal probability (0.5) but the proportion of targets appearing bilaterally versus unilaterally was 2/1. In order to facilitate covert shifting and maintaining of spatial attention at cued locations, possible target positions were continuously marked by two gray squares. Figure 1 illustrates stimulus configurations, paradigm and the time course of events within a single trial.
Schematic illustration of stimulus configurations, paradigm and time-course of events within a trial. A fixation cross and two lower visual field markers for target position (gray squares) were continuously displayed. Each trial was initiated by a warning period of variable length (1400–1750 ms) characterized by a sustained auditory white noise. The cue which followed on the warning stimulus was a brief auditory tone (50 ms) of either 100, 300 or 800 Hz frequency instructing the subject either to direct their attention covertly to the lower left or lower right marker and to maintain attention at the indicated position (directional cues, 100 and 800 Hz tone respectively), or to covertly attend to both markers simultaneously (neutral cue, 300 Hz tone). After a constant delay of 2560 ms, a target was flashed for 40 ms either in the left or right square or bilaterally. Following a directional cue, targets were more likely to appear at cued position (probability 0.66). Following neutral cues, targets appeared at left or right location markers with equal probability. Subjects were asked to respond with index, middle and ring finger of their right hand as a function of perceived target location.
Fixation Cross and Target Markers
A central fixation cross (0.5° of visual angle) and the gray squares (3° × 3° of visual angle) remained on the screen throughout testing (Fig. 1). The gray squares (red:green:blue phosphors = 155:155:155) were centered on the two possible target positions. The position and distance of the participant's eyes with respect to the screen was stabilized by a head and chin rest (28 cm distance).
Participants were asked to fixate the central cross and to avoid eye movements and saccades at any time point throughout testing, including the period after target presentation. Fixation was monitored via electrooculogram recordings (two bipolar derivations for monitoring horizontal and vertical eye movements respectively). After extensive training (see Procedure), all subjects were able to perform attention shifts without moving their eyes such that none of the trials had to be eliminated.
Auditory Warning Stimulus and Cue
An auditory warning signal — consisting of a burst of white noise lasting 1400–1750 ms – was presented at the beginning of each trial (Fig. 1). The warning signal was followed by the cue, which was also auditory in nature. The cue consisted of a brief sine-wave tone of 50 ms duration that was presented either at 100, 300 or 800 Hz frequency (n = 3 cueing conditions). The warning signal was delivered at 30% of the auditory cue volume; the latter was adjusted by the subjects to the most comfortable intensity level at the beginning of the experiment. Auditory warning signal and cues were both delivered via earphones to both ears.
The burst of white noise indicated the beginning of a new trial. Upon presentation of this auditory warning signal, subjects were instructed to discontinue performance in the previous trial, to resume baseline position (attention on fixation cross) and to prepare for the upcoming auditory cue. The 100 and 800 Hz tones (directional cues) prompted the subjects to covertly shift to and then to maintain visual attention at the designated target positions in the LVF or RVF respectively. The 300 Hz tone (neutral cue) prompted the subjects to covertly attend to both positions simultaneously. The cue–target interval (Fig. 1, delay period) was of relatively long duration (2560 ms) in order to provide sufficient time for cue recognition, disengagement from the auditory mode and voluntary shifts of visual attention without eye movements, if required by a directional cue.
Visual Targets
Visual targets were small black rectangles whose sizes were individually adjusted to peri-threshold levels (on average 0.11° × 0.14° of visual angle, see Procedure). They were flashed for 40 ms in either the lower LVF, the lower RVF, or bilaterally (Fig. 1) in the center of the gray squares (n = 3 target conditions). Target positions corresponded to 8° of vertical and 26.5° of horizontal eccentricity from the central fixation cross. Lower visual field targets were chosen because attentional resolution is greater in the lower than the upper visual field (He et al., 1996; Intriligator and Cavanagh, 2001, Mounts and Gavett, 2004), and because left visual hemispatial neglect is more severe in the lower as compared to the upper visual field (e.g. Halligan and Marshall, 1989; Ladavas et al., 1994; Pitzalis et al., 1997).
Subjects were required to respond with either index, middle or ring finger of the right hand (buttons b, n, m of keyboard) according to perceived target locations (left, bilateral, right). Participants were explicitly told that targets would be presented at peri-threshold size such that they would perceive only ∼50% of the stimuli, to respond following perception of a target only, and to withhold responses or guesses if no target was detected. Independent of whether targets were perceived or missed, subjects were asked to resume baseline position (attention on fixation cross, see above) promptly following the beginning of a new trial, which was signaled by the auditory warning stimulus.
Procedure
The experiment was performed over two successive days. On the first day, subjects completed a training session that also served for target titration but did not include TMS. The experimental session took place on the second day.
Target Titration and Training Session
Subjects took part in a training session that served to familiarize them with the task and to determine the optimal target size for each subject separately via target titration. We aimed to present targets at peri-threshold size in order to avoid floor or ceiling effects. Subjects performed the target detection task in three runs of 120 trials each, in which targets of five different sizes were presented [dimensions of targets in pixels: 1 × 2, 2 × 2, 2 × 3, 3 × 3 or 3 × 4, pixel size ∼0.2 mm × ∼0.2 mm (∼0.05° × ∼0.05°), longer axis horizontal if target rectangular]. Target dimensions were adapted after the first run if the largest target was not perceived in at least 85% of trials. After each run, subjects were given feedback. Each run lasted ∼10 min. Out of the five targets, two adjacent peri-threshold targets were selected per subject for presentation during the following day.
Experimental Session
Subjects performed the target detection task in a total of 288 trials over 30 min, both prior to and immediately following a single, continuous, 25 min train of 1 Hz rTMS to the right parietal cortex. Performance was interspersed with regular 1 min breaks. The experiment lasted 1 h 30 min (30 min task, 25 min rTMS, 30 min task). The design gave rise to 96 trials per condition if collapsed over cue or target location conditions (left, bilateral, right) and allowed the time-course of rTMS effects to be investigated.
TMS Protocol
TMS pulses were applied in trains in an offline rTMS protocol at stimulation parameters shown to reduce visual (Boroojerdi et al., 2000) and motor cortex excitability (e.g. Chen et al., 1997; Muellbacher et al., 2000; Touge et al., 2001; Romero et al., 2002) as well as to disrupt cognitive brain function (e.g. Kosslyn et al., 1999; Hilgetag et al., 2001; Robertson et al., 2001; Shapiro et al., 2001; Mottaghy et al., 2002; Sack et al., 2002) beyond the period of rTMS itself. Accordingly, TMS-effects were assessed by comparing task performance prior (baseline) and following rTMS (pre versus post).
rTMS Frequency, Duration and Apparatus
A single, continuous train of rTMS was administered for 25 min at 1 Hz using a 70 mm figure-of-eight coil and a Magstim Super Rapid Transcranial Magnetic Stimulator (Magstim Company, Dyfed, UK).
rTMS Site
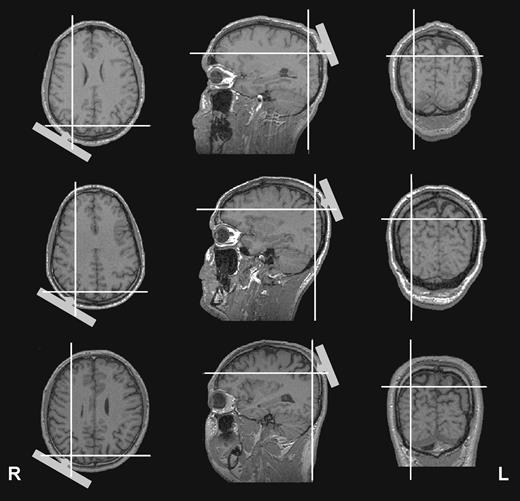
The stimulation site was defined for each subject individually within the 10–20 electroencephalogram (EEG) coordinate system and corresponded to position P4, localized over right parietal cortex. The anatomical site of stimulation was determined offline in three subjects using their magnetic resonance images and optical tracking via a frameless stereotaxic system (Brainsight, Rogue Research, Montreal). TMS sites were localized to areas over right IPs as illustrated in Figure 2.
TMS sites relative to individual magnetic resonance images of three subjects as extrapolated using a frameless stereotaxic system. The cross-hairs highlight the cortical point located radially inward from the center of the coil (positioned on P4 of the 10–20 international electrode system). The figure suggests right dorsal PPC (around the intraparietal sulcus) as the site of TMS for all three exemplar subjects.
rTMS intensity
We chose to set TMS intensity to a fixed level for all subjects (80% maximum stimulator output), instead of tuning to individual phosphene or motor thresholds, as there is no evidence that phosphene or motor thresholds correlate with the effects of rTMS outside visual and motor cortex respectively (Stewart et al., 2001; Boroojerdi et al., 2002; Robertson et al., 2003). In our subjects, 80% rTMS intensity corresponded on average to 129% of resting motor threshold of the right hand (range 107–145%).
Analysis
Data were subjected to overall repeated-measure ANOVAs with the factors TMS (Pre versus Post), Cue (L, R, Neutral) and Target Position (L, B, R). In order to assess the time-course of TMS effects and not to miss potential TMS effects of short duration (<30 min), data were broken down into three blocks covering a period of ∼10 min each. This gave rise to a fourth within-subject factor: Time (1st, 2nd, 3rd block). Statistically significant main effects or interactions were further explored by conducting simple effects. We chose 10 min epochs for evaluating the time-course of TMS effects because previous 1 Hz rTMS studies have found that offline rTMS-effects on cognition outlast the magnetic stimulation by at least half of its duration (reviewed by Robertson et al., 2003). With 25 min of magnetic stimulation, 10 min epochs thus minimize the risk for missing short-term offline effects and still lead to a satisfactory number of repetitions per condition in each block, as opposed to shorter periods.
Although target detection rate was the primary (dependent) variable of interest, reaction times are also reported. Detection rates reflect a perception measure that is influenced by the allocation of attention to spatial positions through cueing, as is reaction time (RT). Due to the design of our task, however, RT might have been influenced by additional motor factors. Because subjects responded with one of three designated fingers as a function of perceived target configuration (L, B or R) and because targets were more likely to appear at cued locations, some effects on RT may have been influenced by motor preparation for the most probable response (effects of Cue × Target) and by dexterity differences between the three fingers (effects of Target).
Results
Target Titration
Detection rate during the last run of the training session is illustrated in Figure 3A for left, bilateral and right targets respectively, as well as for the three cue conditions and five target sizes (mean titration curves over all subjects). The mean titration curves have been calculated after aligning individual curves to the two adjacent targets (targets 1 and 2) that were selected for the experimental runs. Targets 1 and 2 were correctly detected with mean frequencies of 0.30 and 0.66 respectively. Frequency of target detection significantly increased with target size from a detection rate of near 0 (sub-threshold targets) to near 1 (supra-threshold targets) [F(5,45) = 31.8, P < 0.0001, see Fig. 3A line drawings]. This shows that, as instructed, subjects responded only when they truly perceived the targets and did not attempt to guess if targets were not detected. Guessing through anticipation of target onset and position, which would have been possible because of the constant cue–target interval and probabilistic cue–target relationship inherent in our design, would be associated with relatively high detection rates (at least 0.33) for sub-threshold targets, which was clearly not the case (see Fig. 3A, line drawings). Figure 3B, illustrating false alarm rates at cued positions for each of the three target conditions, further emphasizes that subjects did not guess when sub-threshold targets were presented. If subjects were guessing, the false alarm rate would be expected to be much higher than zero for sub-threshold targets, especially for the illustrated false alarms at cued positions, because of the probabilistic cue–target relationship. However, this was clearly not the case as the false alarm rate in sub-threshold trials was near zero in all conditions (Fig. 3B).
Performance during training session. (A) Average detection rate of correctly perceived targets (±SE) during the last training run as a function of target position (L: left, B: bilateral, R: right), cue (L: left, N: neutral, R: right) and target size (five different sizes tested per subject). The curves are aligned on the peri-threshold targets [Target 1 (T1) and 2 (T2)] that were chosen for the experimental session. Box plots to the right of each line drawing represent average detection rates over all target sizes. Note that subjects showed an advantage for target detection at cued positions (see box plots), suggesting that subjects performed the covert attention shifts correctly. Asterisks indicate significant (*P < 0.05, **P < 0.01), and the symbol t a trend (P < 0.08) for statistical differences between conditions. (B) Average false alarm rates at cued locations (±SE) during the last training run as a function of target position and size. Box plots to the right represent average false alarm rates over all target sizes. Note that the false alarm rates at cued locations were low, except regarding false left- or right-target alarms to bilateral targets following left or right cueing respectively (middle panel), which would be expected if subjects adequately allocate their attention to cued positions (‘neglect’ of unattended locations). Asterisks indicate significant (**P < 0.01) or the symbol t a trend (P < 0.08) for statistical difference from zero as revealed by one-sample t-tests.
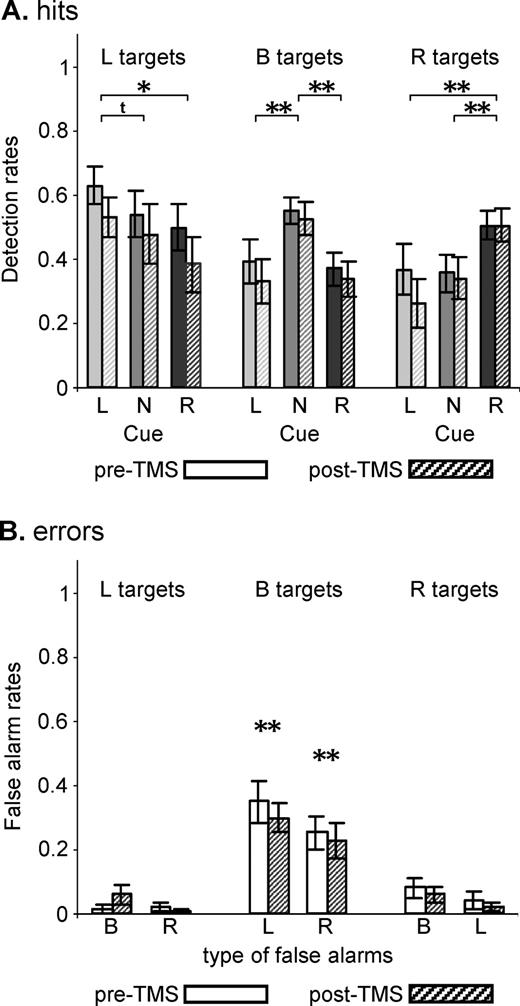
Importantly, detection rates varied as a function of target and cue [significant interaction Cue × Target: F(4,36) = 6.7, P < 0.001], reflecting an advantage for target detection at cued locations (see Fig. 3A, box plots representing mean detection rates). Subjects showed an advantage for detecting left targets (Fig. 3A, left panels) following left cueing as compared to neutral [F(1,9) = 5.6, P = 0.04] or right cueing [F(1,9) = 4.2, P = 0 0.07]. Right targets (Fig. 3A, right panels) were detected more easily following right cueing than neutral [F(1,9) = 4.4, P = 0.066] or left cueing [F(1,9) = 5.1, P = 0.05]. Bilateral targets (Fig. 3A, middle panels) were detected more accurately following neutral cueing than left [F(1,9) = 6.1, P = 0.036] or right cueing [F(1,9) = 10.8, P = 0.009]. The low detection rate for bilateral targets following left or right cueing (Fig. 3A, middle panel) was due to a high number of ‘false unilateral alarms’ in these conditions for targets presented at peri-threshold size (Fig. 3B, middle panel), which would be expected if attention shifts were performed adequately (‘neglect’ at unattended locations). False left-target alarms were significantly increased in bilateral target trials when peri-threshold targets were preceded by a left cue (Fig. 3B, middle panel, one-sample t-test versus zero: t = 3.3, P = .009). Conversely, false right-target alarms were increased in bilateral target trials when peri-threshold targets were preceded by a right cue (Fig. 3B, middle panel, one-sample t-test versus zero: t = 2.0, P = 0.08). There were no false alarms for bilateral targets presented at sub-threshold level (Fig. 3B, middle panel) because these targets were invisible even at cued location, and there were fewer false alarms at supra-threshold size probably because targets became more salient and tended to be perceived bilaterally even if attention was shifted to one side.
Both the advantage for target detection at cued versus uncued location (Fig. 3A, left and right panels), as well as the increased number of ‘false unilateral alarms’ in bilateral target trials following directional cueing (Fig. 3B, middle panel), strongly suggest that subjects performed the spatial orienting task correctly. In other words, the results indicate that the advantage for target detection at cued locations as well as the high number of ‘false unilateral alarms’ in bilateral target trials at cued positions are a consequence of spatial orienting components of the task, in the absence of any evidence for confounding effects due to guessing of target onset or position through anticipation.
Finally, note that subjects showed an advantage for detecting left targets over right targets [F(1,9) = 5.4, P = 0.046; Fig. 3A] and produced more false left- than right-target alarms in bilateral target trials following left or right cueing respectively (Fig. 3B, middle panel). An identical leftward bias has been reported previously by Hilgetag et al. (2001), who tested healthy subjects in a similar target detection paradigm at peri-threshold levels. This left bias is consistent with ‘pseudoneglect’, the natural tendency of neurologically normal subjects to attend more easily to the left than to the right visual hemifield (e.g. Jewell and McCourt, 2000; Fierro et al., 2000, 2001; Bjoertomt et al., 2002; Brighina et al., 2002).
Experimental Session: rTMS-independent Effects
The proportion of correctly detected targets (detection rate, Fig. 4A) for the different target locations was influenced by cue [overall ANOVA: interactions Cue × Target: F(4,36) = 10.3, P < 0.0001], independently of TMS (TMS × Cue × Target: F < 1, n.s.). Subjects showed an advantage for detection of left targets (Fig. 4A, left box plots) following left as compared to neutral [F(1,9) = 4.2, P = 0.07] or right cueing [F(1,9) = 7.3, P = 0.023]. Right-target hits (Fig. 4A, right box plots) were significantly increased after right as compared to neutral [F(1,9) = 17.5, P = 0.002] or left cueing [F(1,9) = 20.2, P = 0.0015]. Finally, detection rate was increased in bilateral target conditions (Fig. 4A, middle box plots) following neutral with respect to left [F(1,9) = 14.5, P = 0.004] or right cueing [F(1,9) = 11.3, P = 0.008]. As in the training session, the reduced detection rate for bilateral targets following left or right cueing relative to neutral cueing was explained by a high proportion of ‘false unilateral alarms’ at cued positions (Fig. 4B, middle box plots, one sample t-tests versus zero: L false alarms: t = 6.2, P < 0.001/R false alarms: t = 5.7, P < 0.001), again independent of TMS (no significant effect of TMS). These data reproduce the results of the training session and strongly suggest that subjects performed the required task, including attention shifts to cued locations, correctly.
Effect of cue on target detection in the experimental session. (A) Average detection rates (±SE) as a function of target location and cue conditions prior and following rTMS. Data are collapsed over the two peri-threshold targets T1 and T2. Participants showed a significant advantage for target detection at cued positions, replicating the results of the training session (see Fig. 3A, box plots). Asterisks or the symbol t refer to significant (*P < 0.05, **P < 0.01) or to a trend (P < 0.08) for statistical differences (data collapsed over pre- and post-TMS values). (B) Corresponding false alarm rates at cued positions (average ± SE) as a function of target position. As in the training session (see Fig. 3B, box plots), false unilateral alarm rates to bilateral targets following directional cueing (middle panel) were significantly different from zero as revealed by one-sample t-tests.
Furthermore, replicating the left bias observed in the training session (see Target titration), there was a significant advantage for detection of left over right targets [Fig. 4A, left versus right box plots, F(1,9) = 6.5, P = 0.03] as well as a tendency for producing more false left- than right-target alarms in bilateral target trials following directional cueing (Fig. 4B, middle box plot).
In analogy to detection rate, reaction times to the different target locations depended on cue [overall ANOVA: interactions Cue × Target: F(4,36) = 27.2, P < 0.0001], irrespective of TMS (three-way interaction TMS × Cue × Target: F < 1, n.s.). Participants responded faster to left targets following left (466 ± 20 ms) than neutral [571 ± 30 ms; F(1,9) = 21.4, P = 0.001] or right cueing [585 ± 33 ms; F(1,9) = 24.6, P < 0.001]. Similarly, participants responded faster to right targets following right (496 ± 23 ms) than after neutral [603 ± 30 ms; F(1,9) = 27.3, P < 0.001] or left cueing [648 ± 32 ms; F(1,9) = 26.4, P < 0.001]. Finally, responses to bilateral targets were faster after neutral (512 ± 17 ms) than left [613 ± 27 ms; F(1,9) = 50.4, P < 0.0001] or right cueing [622 ± 23 ms; F(1,9) = 61.4, P < 0.0001]. Subjects also showed a RT advantage for left over right targets [F(1,9) = 8.2, P = 0.02].
Experimental Session: Effects of Right Posterior Parietal rTMS
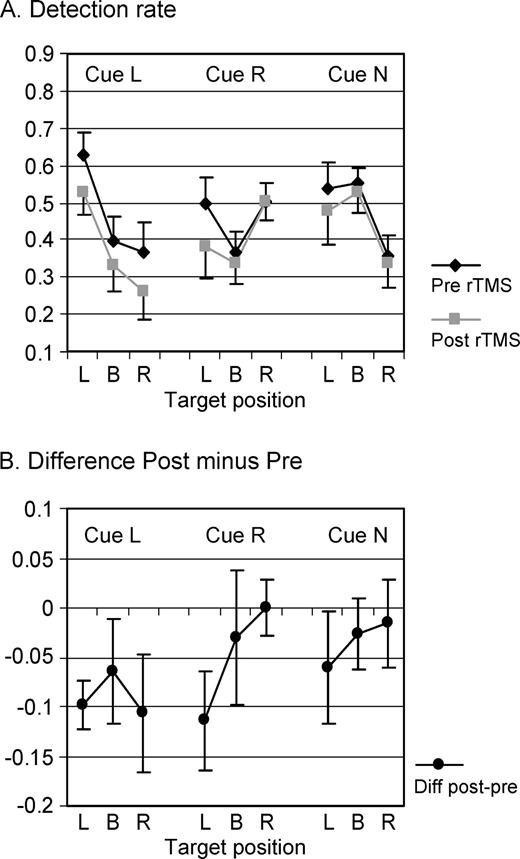
While rTMS did not affect the advantage for target detection at cued location (Cue × Target interaction independent of TMS, see above), the overall ANOVA on detection rate revealed a TMS-effect depending on cue [interaction TMS × Cue: F(2,18) = 3.8, P = 0.04; see Fig. 5]. As compared to baseline (Pre rTMS; see Fig. 5B for difference plots), the number of correctly detected targets was significantly decreased following rTMS in conditions where cues instructed voluntary leftward orienting [F(1,9) = 12.8, P = 0.006]. The decrease in detection rate following left cues was in the order of 10% (Fig. 5B, Cue L) and was independent of target position (simple test: TMS × Target; F < 1, n.s.). This global rTMS effect was not observed for right [F(1,9) = 2.2, P = 0.17, Fig. 5B, Cue R] or neutral cue conditions [F(1,9) = 1.5, P = 0.25, Fig. 5B, Cue N].
Effects of right posterior parietal rTMS on target detection with no significant change over time of testing. Average detection rate and standard errors in all nine conditions (three Cues × three Targets) and collapsed over 30 min of testing (A) during baseline (pre-rTMS) and following 25 min of 1 Hz rTMS (post-rTMS) and (B) represented as changes in correct target detection after rTMS (difference values Post minus Pre, negative values correspond to impaired detection rate). The data shown are identical to Figure 4A, except for reordering to better represent the effects of TMS. rTMS affected target detection in left-cue conditions irrespective of target position (Cue L, P < 0.01) and impaired left-target detection following right cueing (Cue R/Target L, P < 0.05). No significant effects were observed for neutral cues (Cue N).
Overall, TMS-effects did not depend on target position [interaction TMS × Target: F(2,18) = 1.1, n.s.], i.e. target detection was not significantly impaired in the left relative to the right or full visual field by TMS. This was due to the globally reduced detection rate in left-cue conditions and the relatively spared detection of left targets to neutral cues (see Fig. 5B). Separate ANOVAs performed on detection rate in each left-target condition (cue L, R or N) indeed revealed TMS-induced impairment of left-target detection only in the left-cue [F(1,9) = 17.9, P = 0.002] and right-cue conditions [F(1,9) = 5.8, P = 0.04], but no effect of TMS on left-target detection in the neutral-cue trials [F(1,9) = 1.3, n.s.]. The reduction in detection rate for left targets in the right-cue condition (Fig. 5B, Cue R–Target L) was also ∼10%.
Repetitive, parietal TMS had no effect on reaction times as the overall ANOVA on RT showed neither significant main effects nor interactions of factor TMS (all F < 1, n.s.).
Experimental Session: Time-dependency of Right Parietal rTMS-effects
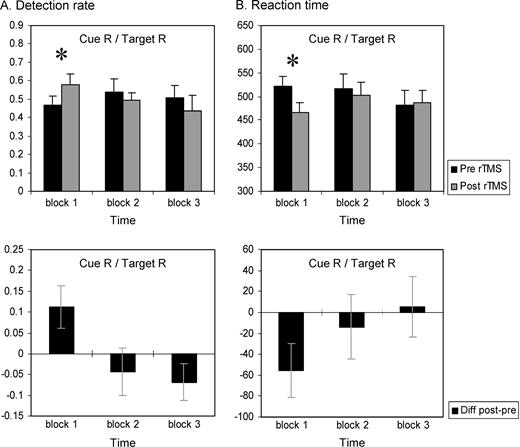
Evaluation of TMS-effects as a function of time after TMS showed that only in one out of the nine conditions did rTMS induce effects that changed significantly over the 30 min testing period, as revealed by TMS × Time interactions. The overall ANOVA on detection rate revealed a significant four-way interaction TMS × Cue × Target × Time [F(8,72) = 2.3, P = 0.03]. Breaking down this interaction showed a significant three-way interaction TMS × Target × Time for the right-cue condition only [F(4,36) = 3.7, P = 0.01], as well as a significant two-way interaction TMS × Time for the right-cue/right-target condition only [F(2,18) = 3.9, P = 0.038]. In this condition (cue R/Target R, Fig. 6A), participants showed an advantage for target detection (increased detection rate) following rTMS as compared with baseline. This effect was observed in the first block after rTMS [F(1,9) = 5.4, P = 0.04] but was washed out in the two subsequent blocks [block 2: F < 1, P = n.s.; block 3: F(1,9) = 2.7, P = 0.14]. The increase in detection rate was paralleled by an advantage in terms of reaction time (decreased RT, Fig. 6B), again only present during the first 10 min immediately after rTMS [block 1: F(1,9) = 5.3, P = 0.047; blocks 2 and 3: F < 1, n.s.]. It might be argued that comparison between the first pre-TMS block and the first post-TMS block might isolate a practice effect. However, there was no significant performance change across the pre-TMS blocks in terms of either detection rate [F(2,18) < 1, n.s.] or reaction time [F(2,18) = 2.4, n.s.]. Moreover, the performance enhancement during the first 10 min following rTMS was also significant when values of the first post-rTMS block were compared with the average of all baseline trials [block 1: F(1,9) = 5.7, P = 0.041 for detection rate, F(1,9) = 4.6, P = 0.06 for reaction time, block 2 and 3: n.s.]. Altogether, both detection rate and reaction time data point toward a TMS-induced enhancement of target detection in the right visual field after rightward cueing that is observed in the first 10 min following rTMS.
Time-dependent effects of right posterior parietal rTMS on target detection. (A) Average detection rate and (B) reaction time to right targets following right cueing (Cue R/Target R) over time (three blocks of 10 min each) during pre- (baseline) versus post-rTMS (upper panels) and represented as difference values (lower panels, see legend Fig. 5). The data show an advantage for target detection within the first 10 min after rTMS that was observed for both detection rate (significantly increased) and RT (significantly decreased) but was washed out in the subsequent 20 min of testing.
The rTMS-effects observed in the overall ANOVA (impaired detection rate in cue L and cue R/target L conditions, see above) did not reveal a significant change over the three successive 10 min blocks, as rTMS-effects in these conditions did not show a significant interaction with Time. However, simple tests between baseline (pre-TMS) and post-TMS blocks of these two conditions showed that significant TMS-effects were restricted to the first 10–20 min after rTMS (blocks 1 and 2: F-values ranging from 11.5 to 3.6, P-values ranging from 0.008 to 0.091). In the last block, i.e. 20–30 min after magnetic stimulation, TMS-effects did not reach significance [block 3: all F(1,9) < 2.2, P > 0.17].
Discussion
In the present study, we probed the implication of the right dorsal PPC in voluntary visuospatial attention control using an offline rTMS protocol and a variant of Posner et al.'s (Posner et al., 1984) cue–target paradigm. Our main findings and conclusions are the following:
The advantage for target detection at cued versus uncued locations, in particular in the left-cue condition (voluntary orienting to the left), was preserved following right parietal rTMS. This does not support the hypothesis that dorsal PPC is essential for voluntary orienting of visuospatial attention, because in this case the benefit provided by the directional cue would be expected to collapse after parietal rTMS. However, we found a global, TMS-induced deficit in target detection for left-cue conditions independent of target position that lasted for at least 20 min post-rTMS. This suggests that voluntary orienting to the left is still possible following right parietal rTMS, albeit at an increased, overall cost for target detection, and favors the hypothesis that dorsal PPC plays a subsidiary rather than pivotal role in voluntary spatial orienting. The general impairment for target detection when subjects voluntarily orient and maintain their attention to the left (cue L trials) is interpreted to reflect an increased load on general attention resources that results from an increased difficulty in performing the leftward orienting task correctly. Because general attention resources can be assumed to be limited, a TMS-induced difficulty in orienting and maintaining attention leftwards is likely to affect concurrent target detection globally. The global reduction is reminiscent of non-lateralized attentional deficits, which are associated with neglect and have been related to right PPC damage (for review, see Husain and Rorden, 2003). Non-lateralized deficits include deficient timing of attention allocation or impaired sustained attention. However, the global TMS-effect is not observed across all cue and target conditions, but is specific to leftward orienting components of the task. Therefore, we believe that this TMS effect is unlikely to reflect interference with non-lateralized attentional components, and propose instead that TMS affected the spatially lateralized process of voluntary orienting to the left.
Left-target detection was significantly impaired by TMS when targets appeared at unattended locations (invalid trials, Cue R/Target L). This condition requires disengagement from a current (right) focus of attention to reorient to the left. One possible interpretation of the deficit in these trials is that the magnetically stimulated site is involved in disengagement of attention and reorienting in the direction contralateral to the rTMS-induced ‘virtual’ lesion. However, an alternative explanation is that the deficit is a consequence of facilitated voluntary rightward orienting, as evidenced in the initial 10 min following rTMS (enhanced target detection in Cue R/Target R trials). We tentatively favor the second interpretation (see below).
Left-target detection was not consistently affected across all cueing conditions by TMS, i.e. was significantly impaired following directional cueing but relatively spared in neutral cue trials. This suggests that dorsal PPC stimulation interfered with spatial orienting of attention, related by design to directional cues, rather than with other attentional task components tested through neutral cue trials. For example, the relatively spared left-target detection in neutral cue trials speaks against a TMS-induced deficit in engaging attention. Our findings also rule out non-specific effects of TMS (unrelated to interference with the target brain tissue), hence obviating the need for a control TMS site or sham condition in our design. Non-specific effects are inconsistent with the differential effects of rTMS we observed across conditions (lateralized deficits, no change for neutral cues).
PPC and Voluntary Spatial Orienting
Our results are in agreement with previous neuroimaging studies in healthy subjects revealing PPC activation during voluntary attention shifts (Kastner et al., 1999; Corbetta et al., 2000; Hopfinger et al., 2000). These studies identified the dorsal PPC (along the IPs) to be involved in the voluntary control of visuospatial attention through the isolation of cue-related signals from target-related activity (Corbetta and Shulman, 2002). In the present study, we show that leftward spatial orienting under endogenous control is affected by right posterior parietal TMS (over P4). Perceptual deficits similar to hemispatial neglect can be induced by TMS over both P4 (Pascual-Leone et al., 1994; Hilgetag et al., 2001; Müri et al., 2002) as well as P6 (Fierro et al., 2000, 2001; Brighina et al., 2002), with P4 being the more dorsal parietal position. Individual MRI scans co-registered with scalp coordinates indeed suggest dorsal PPC areas surrounding the IPs as our most probable target site (Fig. 2).
In applying TMS over the right dorsal PPC, we interfered with spatial orienting to the left (cue L trials), but also facilitated rightward orienting (cue R/target R trials). These findings replicate and further clarify the results of Hilgetag et al. (2001) who reported TMS-induced attentional deficits contralateral to magnetic stimulation (P3/P4) as well as ipsilateral enhancement in a target detection task not isolating spatial orienting from other components of attentional space representation. Our results thus extend those of Hilgetag et al. (2001) in suggesting that rTMS over P4 predominantly affects spatial orienting. In analogy to the present study, Hilgetag et al. (2001) applied rTMS at 1 Hz frequency and over P4 and investigated offline rTMS effects on target detection at peri-threshold levels. Hilgetag et al. used shorter rTMS trains (10 min) at lesser intensity (90% motor threshold) than employed in the present study. Despite these differences, maximum rTMS-effects on detection rate are of similar magnitude in the two studies (10% change from baseline). The duration of effects cannot be compared, as Hilgetag et al. discontinued the behavioral task after 5 min of testing and did not assess the timing of their rTMS-effects. As to effect-size and -duration, comparisons with other parietal TMS studies on spatial attention are difficult because these previous experiments applied TMS either online to task performance (Pascual-Leone et al., 1994; Walsh et al., 1999; Fierro et al., 2000, 2001; Oliveri et al., 2001; Pourtois et al., 2001; Bjoertomt et al., 2002; Brighina et al., 2002; Müri et al., 2002) or investigated the offline effects of 1 Hz rTMS in neglect patients (e.g. Brighina et al., 2003). Previously reported effects on other cognitive functions following 1 Hz rTMS in healthy subjects (5–15 min application, 60–130% motor threshold; for review, see Robertson et al., 2003) range in magnitude between 10% and 20% changes from baseline (e.g. Kosslyn et al., 1999; Robertson et al., 2001; Mottaghy et al., 2002; Sack et al., 2002) and outlast the rTMS stimulation by at least half the duration of the stimulation train itself (for review, see Robertson et al., 2003). Our results are in line with these findings in the literature regarding effect size and duration of the rTMS-effects.
Behaviorally similar phenomena with contralesional impairment and ipsilesional ‘hyperattention’ have also been demonstrated in patients with parietal damage (Smania et al., 1998). The pattern of rTMS-effects we observed is consistent with the idea that some aspects of neglect might be due to a disinhibition of attention processors within the unimpaired (left) hemisphere caused by damage to structures with equivalent function in the opposite (right) hemisphere (interhemispheric competition model: Kinsbourne 1977, 1987). According to this model of neglect, the net attention vector that results from interhemispheric competition is tilted away from the impaired (left) hemifield towards the ‘intact’ (right) hemifield predicting functional inhibition and release. In our case, the net attentional bias would result from dynamic rather than static attention vectors corresponding to the ability to shift attention left- or rightwards respectively. A similar phenomenology can also be explained by changes in rotation of attention space around the vertical body axis through altered transformation of multisensory afferent inputs into a body-centered reference frame (Karnath, 1997), particularly with respect to target detection at the eccentricity studied by our design, i.e. ∼25° (Karnath, 1997, Karnath et al., 1998). Hence, our data cannot discriminate between Kinsbourne's and Karnath's model of neglect.
Although performance during leftward orienting was impaired by rTMS, subjects showed a preserved advantage for target detection at cued versus uncued locations. This indicates that rTMS interfered with but did not completely disrupt spatial orienting. Even patients with large parietal lesions can make use of cue information to voluntarily direct attention towards spatial locations independent of visual field, suggesting that these patients are relatively unimpaired regarding this function (Posner et al., 1984; Friedrich et al., 1998; Smania et al., 1998). Patients with parietal damage show a gradual increase in the advantage for target detection at cued location with prolonged cue–target interval (as the focus of attention is converging gradually towards target location), which is symmetrical for both right- and leftward cueing conditions (Posner et al., 1984). Furthermore, the advantage for target detection increases when the predictability of the side of target presentation is raised and the patients rely more on endogenously controlled attention shifts, an effect that is comparable between the two visual fields (Smania et al., 1998) even for patients with dorsal PPC lesions (Friedrich et al., 1998). Finally, free, exploratory saccades under voluntary control do not show any direction-specific deficit in patients with parietal lesion, in contrast to reflexive saccades that are driven by a visual target, i.e. that are not under endogenous control (Niemeier and Karnath, 2000, 2003). This is of interest as psychological and neuroimaging studies indicate that covert attention shifts and eye movements (i.e. overt visuospatial orienting) are tightly linked (e.g. Corbetta, 1998). Taken together, our findings are thus in line with both the neuroimaging studies suggesting PPC involvement in voluntary covert spatial orienting, as well as the clinical evidence that this function is relatively preserved following PPC damage. Our data reconcile these seemingly contradictory results in suggesting that the right PPC is involved, but does not play a pivotal role, in spatial orienting under voluntary control. One possible explanation for the relative preservation is the bilateral organization of the dorsal parts of the frontoparietal network for spatial orienting, which consist of the frontal eye field (FEF) and the IPs (Corbetta and Shulman, 2002). This bilateral organization might facilitate functional reorganization or compensation following unilateral damage/interference. Another possibility is that there might be a functional specialization within this dorsal network, for instance for displacement of the attention focus (FEF) and the online integration of multisensory afferent inputs to map spatial coordinates for covert orienting (IPs).
PPC and Disengagement of Attention
Finally, we found a rTMS-induced impairment of target detection when participants had to reorient from a right focus of attention to the left (invalid trials, cue R/target L). This is the condition in which patients with right parietal damage show disproportional deficits, interpreted to reflect a difficulty in the disengagement of attention in contralesional direction (Posner et al., 1984, 1987; Friedrich et al., 1998; Losier and Klein, 2001). The difficulty is also regarded as being extinction-like as it occurs only for contralesional targets when an event captures attention in the ipsilesional visual field (Posner et al., 1984; Friedrich et al., 1998). However, the finding is generally associated with lesions in the ventral part of the PPC (TPJ) only (Friedrich et al., 1998). Similarly, stronger TPJ responses are evoked by invalid than valid cue–target trials as shown by functional magnetic resonance imaging, suggesting that the TPJ is preferentially involved in attentional disengagement and/or reorienting towards unattended locations, while the IPs does not show such differential responses (Corbetta et al., 2000). Hence, rTMS over our target region surrounding the right IPs (dorsal PPC) would not be expected to affect attentional disengagement processes directly. We thus interpret the rTMS-induced deficit in left-target detection following right cueing to be a consequence of facilitated rightward attention shifts evidenced in the cue R/target R condition, rather than to reflect a disengagement deficit. However, ‘distant’ rTMS-effects on the TPJ through anatomical connections between the dorsal and ventral parts of the PPC or through direct spread of the rTMS impact cannot be ruled out completely. A result speaking against ‘distant’ effects on TPJ function, however, is our finding that bilateral target detection was not affected by TMS, thus showing absent left visual extinction with simultaneous, double stimulation in both visual fields. This is relevant because visual extinction has been attributed to circumscribed TPJ damage (Karnath et al., 2003). In summary, we conclude that our data set is best explained by rTMS interference with voluntary spatial orienting per se.
Concluding Remarks
Although current models implicate the right temporo-parietal junction and/or the right superior and middle temporal gyrus in visuospatial neglect or extinction (e.g. Vallar, 2001; Corbetta and Shulman, 2002; Karnath et al., 2001, 2003), our data suggest that neglect-like phenomena with attentional inhibition and release occurring contra- and ipsi-‘lesionally’ can also be induced through rTMS over regions surrounding the right intraparietal sulcus. These phenomena concern the ability to direct attention left- or rightwards respectively. This is consistent with the view that dysfunction of the dorsal, frontoparietal network for attention (FEF-IPs) might possibly contribute to some aspects of neglect (Perry and Zeki, 2000; Corbetta and Shulman, 2002). It also provides further support to the findings that spatial attention biases can be modulated through rTMS, which has been proven useful to transiently reduce visual hemispatial neglect (Oliveri et al., 2001; Brighina et al., 2003) and suggests dorsal PPC as a possible candidate for stimulation.
The work was supported by a grant of the Swiss National Science Foundation (no 823A-061230) and grants from the National Institutes of Health (RO1-NS47754, RO1-EY12091 and K24 RR018875).
References
Bartolomeo P, Chokron S (
Bartolomeo P, Sieroff E, Decaix C, Chokron S (
Bjoertomt O, Cowey A, Walsh V (
Boroojerdi B, Prager A, Muellbacher W, Cohen LG (
Boroojerdi B, Meister IG, Foltys H, Sparing R, Cohen LG, Topper R (
Brighina F, Bisiach E, Piazza A, Oliveri M, La Bua V, Daniele O, Fierro B (
Brighina F, Bisiach E, Oliveri M, Piazza A, La Bua V, Daniele O, Fierro B (
Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG (
Corbetta M (
Corbetta M, Shulman GL (
Corbetta M, Miezin FM, Shulman GL, Petersen SE (
Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL (
Fierro B, Brighina F, Oliveri M, Piazza A, La Bua V, Buffa D, Bisiach E (
Fierro B, Brighina F, Piazza A, Oliveri M, Bisiach E (
Friedrich FJ, Egly R, Rafal RD, Beck D (
Halligan PW, Marshall JC (
Halligan PW, Fink GR, Marshall JC, Vallar G (
He S, Cavanagh P, Intriligator J (
Hilgetag CC, Theoret H, Pascual-Leone A (
Hopfinger JB, Buonocore MH, Mangun GR (
Husain M, Rorden C (
Intriligator J, Cavanagh P (
Jewell G, McCourt ME (
Kanwisher N, Wojciulik E (
Karnath HO (
Karnath HO, Ferber S, Himmelbach M (
Karnath HO, Himmelbach M, Kuker W (
Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG (
Kinsbourne M (
Kosslyn SM, Pascual-Leone A, Felician O, Camposano S, Keenan JP, Thompson WL, Ganis G, Sukel KE, Alpert NM (
Ladavas E, Carletti M, Gori G (
Losier BJ, Klein RM (
Mesulam MM (
Mottaghy FM, Gangitano M, Sparing R, Krause BJ, Pascual-Leone A (
Mounts JRW, Gavett BE (
Müri RM, Buhler R, Heinemann D, Mosimann UP, Felblinger J, Schlaepfer TE, Hess CW (
Muellbacher W, Ziemann U, Boroojerdi B, Hallett M (
Niemeier M, Karnath HO (
Niemeier M, Karnath HO (
Nobre AC, Sebestyen GN, Gitelman DR, Mesulam MM, Frackowiak RS, Frith CD (
Oliveri M, Bisiach E, Brighina F, Piazza A, La Bua V, Buffa D, Fierro B (
Pascual-Leone A, Gomez-Tortosa E, Grafman J, Alway D, Nichelli P, Hallett M (
Pascual-Leone A, Bartres-Faz D, Keenan JP (
Perry RJ, Zeki S (
Pitzalis S, Spinelli D, Zoccolotti P (
Pourtois G, Vandermeeren Y, Olivier E, de Gelder B (
Posner MI, Walker JA, Friedrich FJ, Rafal RD (
Posner MI, Walker JA, Friedrich FA, Rafal RD (
Robertson EM, Tormos JM, Maeda F, Pascual-Leone A (
Robertson EM, Theoret H, Pascual-Leone A (
Romero JR, Anschel D, Sparing R, Gangitano M, Pascual-Leone A (
Sack AT, Sperling JM, Prvulovic D, Formisano E, Goebel R, Di Salle F, Dierks T, Linden DE (
Shapiro KA, Pascual-Leone A, Mottaghy FM, Gangitano M, Caramazza A (
Smania N, Martini MC, Gambina G, Tomelleri G, Palamara A, Natale E, Marzi CA (
Stewart LM, Walsh V, Rothwell JC (
Touge T, Gerschlager W, Brown P, Rothwell JC (
Vallar G (
Vallar G, Perani D (
Author notes
1Center for Non-invasive Brain Stimulation, Harvard Medical School and Beth Israel Deaconess Medical Center, Boston, MA, USA, 2Functional Brain Mapping Laboratory, Department of Neurology, University Hospital Geneva, Switzerland, 3Neurology Department, Charité, Humboldt University, Berlin, Germany and 4Institut Guttman, Barcelona, Spain

![Performance during training session. (A) Average detection rate of correctly perceived targets (±SE) during the last training run as a function of target position (L: left, B: bilateral, R: right), cue (L: left, N: neutral, R: right) and target size (five different sizes tested per subject). The curves are aligned on the peri-threshold targets [Target 1 (T1) and 2 (T2)] that were chosen for the experimental session. Box plots to the right of each line drawing represent average detection rates over all target sizes. Note that subjects showed an advantage for target detection at cued positions (see box plots), suggesting that subjects performed the covert attention shifts correctly. Asterisks indicate significant (*P < 0.05, **P < 0.01), and the symbol t a trend (P < 0.08) for statistical differences between conditions. (B) Average false alarm rates at cued locations (±SE) during the last training run as a function of target position and size. Box plots to the right represent average false alarm rates over all target sizes. Note that the false alarm rates at cued locations were low, except regarding false left- or right-target alarms to bilateral targets following left or right cueing respectively (middle panel), which would be expected if subjects adequately allocate their attention to cued positions (‘neglect’ of unattended locations). Asterisks indicate significant (**P < 0.01) or the symbol t a trend (P < 0.08) for statistical difference from zero as revealed by one-sample t-tests.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cercor/15/5/10.1093/cercor/bhh164/2/m_cercorbhh164f03_ht.jpeg?Expires=1716490493&Signature=qlYnmPf6S05vP8mjURbGBhIB8MxjJYSn70zKXX~Zx4PEObiXOLusJMc87XZRmt1BEpqowp65hP~dhbB8PLHskRYPc7weerlBZMpN4G75pSzdaFrn7FFnuu8ewTVLd42Exnoc4BEPMbcDscq3hzyt8AJSP3-7fD8XSwhXWEq8~h717cpszZwLvQo82Z4jp9BI9oKtIcXKvBoHG2pSkoJLmm5wEqgWBxdqDUj941m4yqEjUA-MOcV7PjxnjevWtTY~1t7TVK2mmPksYS0BBaSfHbHSJfXz5OukmU8onfmYMmFCBJ4S8jbq-LOmLIhaiisASPAGYVom-NjmLw2ZpcqCQQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)