-
PDF
- Split View
-
Views
-
Cite
Cite
Thomas Brandt, Franz Schautzer, Derek A. Hamilton, Roland Brüning, Hans J. Markowitsch, Roger Kalla, Cynthia Darlington, Paul Smith, Michael Strupp, Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans, Brain, Volume 128, Issue 11, November 2005, Pages 2732–2741, https://doi.org/10.1093/brain/awh617
Close - Share Icon Share
Abstract
The human hippocampal formation plays a crucial role in various aspects of memory processing. Most literature on the human hippocampus stresses its non-spatial memory functions, but older work in rodents and some other species emphasized the role of the hippocampus in spatial learning and memory as well. A few human studies also point to a direct relation between hippocampal size, navigation and spatial memory. Conversely, the importance of the vestibular system for navigation and spatial memory was until now convincingly demonstrated only in animals. Using magnetic resonance imaging volumetry, we found that patients (n = 10) with acquired chronic bilateral vestibular loss (BVL) develop a significant selective atrophy of the hippocampus (16.9% decrease relative to controls). When tested with a virtual variant (on a PC) of the Morris water task these patients exhibited significant spatial memory and navigation deficits that closely matched the pattern of hippocampal atrophy. These spatial memory deficits were not associated with general memory deficits. The current data on BVL patients and bilateral hippocampal atrophy revive the idea that a major—and probably phylogenetically ancient—function of the archicortical hippocampal tissue is still evident in spatial aspects of memory processing for navigation. Furthermore, these data demonstrate for the first time in humans that spatial navigation critically depends on preserved vestibular function, even when the subjects are stationary, e.g. without any actual vestibular or somatosensory stimulation.
Introduction
Input from the vestibular system is important for navigation and spatial memory in animals; however, controversy still surrounds the role of the hippocampus. The human hippocampal formation is widely agreed to be important for memory processing aspects, such as early encoding, consolidation and retrieval (Scoville and Milner, 1957; Manns et al., 2003), but it is also thought to be involved in spatial memory functions (e.g. see McNaughton et al., 1996; Mumby, 2001 for reviews). Earlier research in rodents highlighted a hippocampal role in spatial functions (Becker and Olton, 1981; Jarrard, 1993), while more recent research de-emphasized this role and likewise argued for a principally non-spatial role in memory processing as well (Bunsey and Eichenbaum, 1995; McEchron and Disterhoft, 1999; Eichenbaum, 2003). Wood et al. (2000), for instance, argued that the rat hippocampus, like the human one, encodes what occurred earlier and what will occur next, even in a spatial T-maze situation, and therefore plays a role similar to that of the human hippocampus in episodic memory formation. Currently, some authors do not consider the human hippocampus essential for maintaining or retrieving remotely formed spatial representations of major landmarks, routes, distances and directions (Rosenbaum et al., 2000). Others continue to emphasize the direct relation between hippocampal size, navigation and spatial memory in humans (Maguire et al., 2000; Biegler et al., 2001). Especially the right human hippocampus may be related to allocentric spatial memory (Feigenbaum and Morris, 2004; Parslow et al., 2005) and proximity judgements (Maguire et al., 1996), the left hippocampus may be engaged in topokinetic memory (mental navigation) (Berthoz, 1997).
Several electrophysiological studies from the 1990s demonstrated that vestibular stimulation could modulate the activity of ‘head direction cells’ in the thalamus (e.g. Blair and Sharp, 1996; see Taube et al., 1996 for a review) and ‘place cells’ in the hippocampus (O'Mara et al., 1994; Gavrilov et al., 1995; Wiener et al., 1995). Using functional MRI, Vitte et al. (1996) demonstrated in humans that caloric stimulation could activate the hippocampal formation. Various anatomical connections were proposed to join the vestibular nuclei to the hippocampus via the thalamus, the dorsal tegmental nucleus (i.e. the ‘head direction pathway’) or the pedunculopontine tegmental nucleus (i.e. the ‘theta pathway’) (Smith, 1997; Cuthbert et al., 2000; Horii et al., 2004). Several reports suggested that spatial learning deficits in animals after vestibular lesions or stimulation are linked to changes in circuitry critical for place learning and navigation. For example, vestibular stimulation modulated ‘head direction cells’ in the anterior thalamic nuclei (Stackman and Taube, 1997), and location-related firing of ‘place cells’ in the hippocampus was abolished in rats after bilateral labyrinthectomy (Stackman et al., 2002; Russell et al., 2003a). Thus, vestibular signals are necessary for location-specific ‘place cell’ activity in the hippocampus, which provides a putative neural substrate for the spatial representation also involved in human navigation (Ekstrom et al., 2003).
Patients with bilateral vestibular deficits can execute goal-directed linear locomotion without impairment, but they fail to accurately negotiate corners when walking along a triangular path (Glasauer et al., 2002). In a pilot study we found spatial memory deficits in patients with chronic bilateral vestibular loss (BVL; Schautzer et al., 2003). This prompted us to test spatial and non-spatial memory [using a virtual Morris water task (MWT) and the Wechsler Memory Scale, respectively] and to determine the volume of the hippocampus in 10 patients with chronic BVL due to neurofibromatosis 2, who had undergone bilateral vestibular neurectomy. We addressed the following questions:
Can MRI volumetry show morphological changes in the hippocampus of BVL patients?
Are the BVL-related deficits in spatial memory and navigation selective or associated with general memory deficits? Since males had outperformed females in place learning but not cued-navigation in previous studies (Astur et al., 1998; Driscoll et al., 2003), sex was included as a between-subject factor in the analysis.
Methods
Subjects
Ten patients (four women, six men; mean ± SD = 38.0 ± 6.7 years) with BVL and ten sex- and age-matched controls (mean ± SD = 38.7 ± 5.4 years) with no known neurological history participated in the study. All patients had undergone a bilateral vestibular nerve section 5–10 years before the test and subsequently had a complete BVL. Only one patient had a total postoperative hearing loss. Patients and control subjects were also matched for years of school education (patients: 10.9 ± 1.8 years and controls: 11.1 ± 1.7 years). Neither patients nor controls had professions requiring pronounced spatial memory or a history of extensive experience in navigation.
MRI volumetry
Acquisition protocol
Patients and controls were examined using a superconducting 1.5-T scanner and the circular polarized head coil (Magnetom Vision®, Siemens Medical Systems, Germany). T2-weighted images (TR/TE = 2300/20/85 ms, field of view 220 mm, 3 mm slice, 1 average) and matched FLAIR images (TR/TE = 5000/110 ms, TI = 1850 ms) were obtained in the oblique coronal orientation perpendicular to the long axis of the hippocampus. Additionally, a 3D gradient echo sequence was measured (MPRAGE, TR/TE = 9.7/4 ms, 15 degree flip angle with a pixel size of 1.02 × 1.02 mm). The total measurement time took ∼25 min per patient.
MRI data post-processing
Image data processing for the hippocampal volume was performed on a remote Linux workstation, using FSL and MedX 3.4 (Medical Numerics, Inc. Sterling, VA, USA). Both the T1-weighted and the T2-weighted datasets were computed by manually tracing the outlines of the hippocampus on reformatted successive coronal 1-mm slices. The hippocampal head, body and tail were segmented manually according to recently described state-of-the-art protocols (Bernasconi et al., 2003). The ento- and the perirhinal cortex (Bernasconi et al., 1999) and the parahippocampal cortex (Insausti et al., 1998) were not included. The resulting data were adapted to the imaging parameters and documented in a protocol file.
The Sienax protocol (Version 2.2) was used on the 3D dataset for total brain volume estimation (Smith et al., 2002). Total brain tissue volume was estimated from a single image, after being registered to standard (Talairach) space. The protocol runs tissue segmentation to estimate the volume of grey matter (GM) and white matter (WM) tissue and CSF separately, and multiplies this by the estimated scaling factor to reduce head-size-related variability between subjects.
Memory and memory-influencing functions
Intelligence
To estimate pre-morbid intelligence, a German-language adaptation of the National Adult Reading Test of Nelson was selected.
Memory and attention/concentration
The Wechsler Memory Scale–Revised was used in full, since it constitutes the most universally employed memory test battery and allows the calculation of several indices (general memory, attention/concentration, visual memory, verbal memory and delayed recall). Furthermore, the doors sub-test of the doors and people test of Baddeley et al. (1994) was used. It allows measurement of visual recognition memory. Colour photographs of doors have to be memorized and then each of the presented doors has to be identified from an array of four doors, two on the upper and two on the lower half of a sheet of paper. All tests were administered to 9 of the 10 patients using computer-screen based visual instructions. One patient refused to undergo the testing.
Virtual MWT
The MWT (Morris, 1981) is the gold standard for testing spatial learning, spatial memory and navigation in rodents. Rats are trained to navigate to an escape platform in a circular pool of water. The escape platform is made invisible by submerging it just below the surface of the water; however, normal rats can directly navigate to the platform from several release points. Such behaviour is generally agreed to be related to ambient visual cues in the extra-maze environment which remain in a fixed spatial location to the platform throughout training.
The virtual MWT (VMWT) is a purely visual task that is devoid of many forms of stimulus control involved in spatial navigation. Accurate navigation to a hidden platform depends upon a constellation of visual cues in the extra-maze environment, whereas other ambiguous cues provided in the room and pool geometry appear to be utilized for non-spatial strategies. The virtual environment used here for all patients and 10 controls was described in detail elsewhere (Hamilton et al., 2002). In brief, it consisted of a circular pool located in the centre of a room with a square floor plan. Four conspicuous distal cues of equal size were placed around the distal walls. The cues were positioned so that one cue was on each of the four walls, and the platform could not be encountered by simply moving towards a single cue from any release point. The platform occupied ∼2% of the pool area and was in the centre of one quadrant (N/E). A first-person view of the virtual environment was displayed on a 17-inch PC laptop monitor with a 45° field of view. The observer's position was always slightly above the surface of the water. Forward movement was controlled by the UP (↑) arrow key on the keyboard, and rotation by the LEFT (←) and RIGHT (→) arrow keys. Backward navigation or up–down movement within the pool was not possible. A full 360° rotation in the absence of forward movement required ∼2.5 s to complete and the direct path from a release point to the opposite side of the pool took ∼4 s.
Design and procedure
Training and testing were done in three phases that required a total of ∼30 min to complete. During Phase I, participants completed five hidden platform training blocks, each consisting of four trials. Starting locations during Phase I were sampled pseudo-randomly without replacement from the four locations corresponding to the cardinal compass points. The latency and path length required to navigate to the hidden platform were measured for each trial (latency was measured from the time the first movement was made until the platform was found; path length was measured as the total distance travelled to find the platform, divided by the diameter of the virtual pool). A maximum of 60 s was allotted to locate the platform during Phase I trials, after which the platform was made visible by raising it above the surface of the water. Phase II consisted of a single 45 s probe trial during which the platform was removed from the environment. The starting location for the probe trial was selected pseudo-randomly from the two starting locations furthest from the platform location. Two dependent measures were recorded for the probe trial: (i) initial heading error (the angular deviation from a straight trajectory to the platform measured 1 s after movement was initiated) and (ii) percentage of time spent in the platform quadrant. During Phase III the platform was slightly raised above the water surface to make it visible for two blocks of four trials. Starting locations were determined as in Phase I, and the latency and path length required to navigate to the visible platform were measured for each trial. The Phase III condition provided a control task that did not require spatial processing, which is intact in individuals with spatial navigation impairment (Driscoll et al., 2003; Hamilton et al., 2003).
Results
MRI volumetry
MRI volumetry revealed a 16.91% decrease in total hippocampal volume in the BVL patients relative to controls (see Table 1; Fig. 1). An analysis of variance (ANOVA) with BVL status and sex as between-subject factors confirmed a significant difference in hippocampal volume between BVL patients and controls [F(1, 17) = 13.08, P < 0.01]. Males had significantly greater total hippocampal volume than females [F(1, 17) = 5.25, P < 0.05]; however, the BVL status by sex interaction term was not significant (P = 0.92), indicating that BVL did not differentially affect males or females in the current sample. Separate ANOVAs for each hemisphere indicated that BVL was associated with smaller hippocampi in both the left [F(1, 17) = 10.36, P < 0.01] and right [F(1, 17) = 13.77, P < 0.01] hemispheres, and neither of the BVL × sex interactions reached statistical significance (both P > 0.46).
In BVL patients, a 16.91% volume loss in the hippocampus (arrows) was observed in comparison to age- and sex-matched controls (normal hippocampus: dotted arrows). Volume loss was similar for the left and right hippocampus. Analysis of variance (ANOVA) with BVL status and sex confirmed a significant difference in hippocampal volume between BVL patients and controls [F(1, 17) = 13.08]. Shown are examples of coronal 3 mm MRI T2-weighted images with a distance of 6 mm. (A) 39-year-old female volunteer. (B) 40-year-old female BVL patient (for methodological details, see Methods). This patient had a total volume of 3.9 ml (left hippocampus 1939.18 mm3, right hippocampus 2002.82 mm3).
Mean hippocampal, GM, WM, CSF and whole-brain volume (± SD) measured by MRI volumetry in NF2 patients and controls*
. | Control (male) . | Control (female) . | BVL (male) . | BVL (female) . |
|---|---|---|---|---|
| Right HPC (cm3) | 2.67 (0.14) | 2.55 (0.23) | 2.31 (0.40) | 2.02 (0.24) |
| Left HPC (cm3) | 2.91 (0.26) | 2.42 (0.31) | 2.33 (0.47) | 2.06 (0.15) |
| Total HPC (cm3) | 5.59 (0.38) | 4.98 (0.52) | 4.64 (0.83) | 4.08 (0.38) |
| Grey matter (cm3) | 594.32 (27.38) | 577.48 (30.94) | 648.79 (58.64) | 503.64 (60.01) |
| White matter (cm3) | 687.33 (101.07) | 596.53 (40.47) | 690.90 (83.22) | 532.29 (48.26) |
| Whole brain (GM + WM, cm3) | 1281.65 (103.1) | 1174.01 (65.60) | 1339.69 (139.8) | 1035.94 (99.94) |
| CSF (cm3) | 170.16 (27.11) | 168.25 (20.20) | 240.34 (42.81) | 197.65 (30.80) |
| Right HPC/whole brain | 0.209 (0.013) | 0.211 (0.021) | 0.174 (0.06) | 0.195 (0.015) |
| Left HPC/whole brain | 0.228 (0.019) | 0.198 (0.020) | 0.177 (0.024) | 0.199 (0.015) |
| Total HPC/whole brain | 0.437 (0.032) | 0.408 (0.036) | 0.351 (0.051) | 0.394 (0.027) |
. | Control (male) . | Control (female) . | BVL (male) . | BVL (female) . |
|---|---|---|---|---|
| Right HPC (cm3) | 2.67 (0.14) | 2.55 (0.23) | 2.31 (0.40) | 2.02 (0.24) |
| Left HPC (cm3) | 2.91 (0.26) | 2.42 (0.31) | 2.33 (0.47) | 2.06 (0.15) |
| Total HPC (cm3) | 5.59 (0.38) | 4.98 (0.52) | 4.64 (0.83) | 4.08 (0.38) |
| Grey matter (cm3) | 594.32 (27.38) | 577.48 (30.94) | 648.79 (58.64) | 503.64 (60.01) |
| White matter (cm3) | 687.33 (101.07) | 596.53 (40.47) | 690.90 (83.22) | 532.29 (48.26) |
| Whole brain (GM + WM, cm3) | 1281.65 (103.1) | 1174.01 (65.60) | 1339.69 (139.8) | 1035.94 (99.94) |
| CSF (cm3) | 170.16 (27.11) | 168.25 (20.20) | 240.34 (42.81) | 197.65 (30.80) |
| Right HPC/whole brain | 0.209 (0.013) | 0.211 (0.021) | 0.174 (0.06) | 0.195 (0.015) |
| Left HPC/whole brain | 0.228 (0.019) | 0.198 (0.020) | 0.177 (0.024) | 0.199 (0.015) |
| Total HPC/whole brain | 0.437 (0.032) | 0.408 (0.036) | 0.351 (0.051) | 0.394 (0.027) |
HPC, hippocampus.
Measures were not obtained or could not be computed for four participants (3 BVL patients and 1 control).
Mean hippocampal, GM, WM, CSF and whole-brain volume (± SD) measured by MRI volumetry in NF2 patients and controls*
. | Control (male) . | Control (female) . | BVL (male) . | BVL (female) . |
|---|---|---|---|---|
| Right HPC (cm3) | 2.67 (0.14) | 2.55 (0.23) | 2.31 (0.40) | 2.02 (0.24) |
| Left HPC (cm3) | 2.91 (0.26) | 2.42 (0.31) | 2.33 (0.47) | 2.06 (0.15) |
| Total HPC (cm3) | 5.59 (0.38) | 4.98 (0.52) | 4.64 (0.83) | 4.08 (0.38) |
| Grey matter (cm3) | 594.32 (27.38) | 577.48 (30.94) | 648.79 (58.64) | 503.64 (60.01) |
| White matter (cm3) | 687.33 (101.07) | 596.53 (40.47) | 690.90 (83.22) | 532.29 (48.26) |
| Whole brain (GM + WM, cm3) | 1281.65 (103.1) | 1174.01 (65.60) | 1339.69 (139.8) | 1035.94 (99.94) |
| CSF (cm3) | 170.16 (27.11) | 168.25 (20.20) | 240.34 (42.81) | 197.65 (30.80) |
| Right HPC/whole brain | 0.209 (0.013) | 0.211 (0.021) | 0.174 (0.06) | 0.195 (0.015) |
| Left HPC/whole brain | 0.228 (0.019) | 0.198 (0.020) | 0.177 (0.024) | 0.199 (0.015) |
| Total HPC/whole brain | 0.437 (0.032) | 0.408 (0.036) | 0.351 (0.051) | 0.394 (0.027) |
. | Control (male) . | Control (female) . | BVL (male) . | BVL (female) . |
|---|---|---|---|---|
| Right HPC (cm3) | 2.67 (0.14) | 2.55 (0.23) | 2.31 (0.40) | 2.02 (0.24) |
| Left HPC (cm3) | 2.91 (0.26) | 2.42 (0.31) | 2.33 (0.47) | 2.06 (0.15) |
| Total HPC (cm3) | 5.59 (0.38) | 4.98 (0.52) | 4.64 (0.83) | 4.08 (0.38) |
| Grey matter (cm3) | 594.32 (27.38) | 577.48 (30.94) | 648.79 (58.64) | 503.64 (60.01) |
| White matter (cm3) | 687.33 (101.07) | 596.53 (40.47) | 690.90 (83.22) | 532.29 (48.26) |
| Whole brain (GM + WM, cm3) | 1281.65 (103.1) | 1174.01 (65.60) | 1339.69 (139.8) | 1035.94 (99.94) |
| CSF (cm3) | 170.16 (27.11) | 168.25 (20.20) | 240.34 (42.81) | 197.65 (30.80) |
| Right HPC/whole brain | 0.209 (0.013) | 0.211 (0.021) | 0.174 (0.06) | 0.195 (0.015) |
| Left HPC/whole brain | 0.228 (0.019) | 0.198 (0.020) | 0.177 (0.024) | 0.199 (0.015) |
| Total HPC/whole brain | 0.437 (0.032) | 0.408 (0.036) | 0.351 (0.051) | 0.394 (0.027) |
HPC, hippocampus.
Measures were not obtained or could not be computed for four participants (3 BVL patients and 1 control).
Total GM, WM, CSF and an estimate of total parenchyma (whole-brain volume; GM + WM) are shown in Table 1. These measures were not obtained or could not be computed for four participants (3 BVL patients and 1 control). There were no significant BVL main effects for whole-brain GM (P = 0.66), WM (P = 0.43) or whole-brain volume (P = 0.44); however, BVL was associated with increased CSF [F(1, 14) = 11.43, P < 0.01]. Significant effects of sex were found for WM, GM, and whole-brain volume (males > females, all P < .005), but not for CSF (P = 0.15). The sex × BVL interaction was significant only for GM [F(1, 14) = 9.06, P < 0.01; all other interactions P > 0.19] and was due to a greater sex difference (males > females) in the BVL group.
To control for group differences in whole-brain volume, analyses of covariance (ANCOVAs) were also performed for the hippocampal measures with whole-brain volume (GM + WM) as a single covariate. BVL patients still showed a significant decrease relative to controls for right hippocampal volume [F(1, 13) = 9.98, P < 0.01], left hippocampal volume [F(1, 13) = 6.56, P < 0.05] and total hippocampal volume [F(1, 13) = 8.93, P < 0.05]. None of the sex main effects or sex × BVL interactions were significant for the ANCOVAs (all P > 0.12). Although the sex × BVL interactions were not significant, an interesting pattern was observed among sex and BVL status. Table 1 suggests that BVL may have had a larger impact on hippocampal volume in female BVL patients; however, this group had only four patients. While the control subjects showed a non-significant negative correlation (r = −0.165) between hippocampal volume and age, which was within the range of values in normal ageing (Raz, 2000), BVL patients had a positive, non-significant correlation between age and hippocampal volume (r = 0.12). All but one of the control subjects was under 54 years of age; thus, these correlations must be evaluated cautiously, given the restricted age ranges (see the Methods section).
An analysis of zero-order correlations between performance in the VMWT (see below) and hippocampal volume in the BVL patients failed to reveal a significant correlation [r(9) = −0.26 (P = 0.50) for percent time in the platform quadrant on the probe trial and total hippocampal volume]. It is important to note that people either solve the VMWT efficiently or they apply a non-efficient strategy. Thus, while a continuous relationship between hippocampal volume and spatial learning may be expected, the measure of spatial learning used here only provides meaningful continuous information if individuals are actually learning by using a spatial strategy. If not, any variability in behaviour does not reflect variability in spatial learning and should not be expected to correlate with factors related to spatial learning performance. At the group level, performance differences in the VMWT largely reflected differences in the proportion of individuals in each group who used a spatial strategy.
Pre-morbid intelligence and non-spatial memory
Quantitative data on memory and attention/concentration of the patients as assessed using the Wechsler Memory Scale–Revised are presented in Table 2. The pre-morbid intelligence level of all patients was average to above average. Only one patient was significantly impaired in all measures of memory. Most of the other patients were in the normal or even above-average range. Only visual recognition memory, as measured using the doors test, was impaired in four patients, and the attention/concentration index was below average in two patients.
Results for behavioural tests of memory and intelligence
| Subject . | Age (years) . | Education . | Intelligence (IQ; MWT-B) . | Wechsler memory scale-revised . | . | . | . | . | Doors test (percentile) . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | . | GMI . | VisM . | VerbM . | Att/Conc . | Delayed recall . | . | ||||
| B.B. | 40 | 9 | 112 | 66 | <50 | 81 | 77 | 57 | <1 | ||||
| S.E. | 38 | 13 | 124 | 136 | 105 | 138 | 129 | 110 | 25 | ||||
| A.F. | 34 | 9 | 104 | 124 | 105 | 126 | 117 | 115 | 15–25 | ||||
| A.H. | 62 | 12 | 118 | 116 | 109 | 117 | 86 | 105 | 15 | ||||
| C.H. | 38 | 12 | 136 | 106 | 112 | 102 | 111 | 113 | 90 | ||||
| G.H. | 55 | 12 | 130 | 108 | 116 | 101 | 83 | 111 | 75 | ||||
| H.J. | 40 | 9 | 101 | 117 | 110 | 116 | 91 | 103 | 75 | ||||
| G.K. | 43 | 12 | 107 | 114 | 106 | 114 | 99 | 99 | 90 | ||||
| B.P. | 40 | 13 | 104 | 98 | 117 | 90 | 93 | 101 | 5 | ||||
| Subject . | Age (years) . | Education . | Intelligence (IQ; MWT-B) . | Wechsler memory scale-revised . | . | . | . | . | Doors test (percentile) . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | . | GMI . | VisM . | VerbM . | Att/Conc . | Delayed recall . | . | ||||
| B.B. | 40 | 9 | 112 | 66 | <50 | 81 | 77 | 57 | <1 | ||||
| S.E. | 38 | 13 | 124 | 136 | 105 | 138 | 129 | 110 | 25 | ||||
| A.F. | 34 | 9 | 104 | 124 | 105 | 126 | 117 | 115 | 15–25 | ||||
| A.H. | 62 | 12 | 118 | 116 | 109 | 117 | 86 | 105 | 15 | ||||
| C.H. | 38 | 12 | 136 | 106 | 112 | 102 | 111 | 113 | 90 | ||||
| G.H. | 55 | 12 | 130 | 108 | 116 | 101 | 83 | 111 | 75 | ||||
| H.J. | 40 | 9 | 101 | 117 | 110 | 116 | 91 | 103 | 75 | ||||
| G.K. | 43 | 12 | 107 | 114 | 106 | 114 | 99 | 99 | 90 | ||||
| B.P. | 40 | 13 | 104 | 98 | 117 | 90 | 93 | 101 | 5 | ||||
Test results below average are marked in bold. Att/Conc, attention/concentration index; GMI, general memory index; IQ, intelligence quotient; MWT-B, Mehrfach-Wahl-Wortschatztest B; VisM, visual memory index; VerbM, verbal memory index.
Results for behavioural tests of memory and intelligence
| Subject . | Age (years) . | Education . | Intelligence (IQ; MWT-B) . | Wechsler memory scale-revised . | . | . | . | . | Doors test (percentile) . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | . | GMI . | VisM . | VerbM . | Att/Conc . | Delayed recall . | . | ||||
| B.B. | 40 | 9 | 112 | 66 | <50 | 81 | 77 | 57 | <1 | ||||
| S.E. | 38 | 13 | 124 | 136 | 105 | 138 | 129 | 110 | 25 | ||||
| A.F. | 34 | 9 | 104 | 124 | 105 | 126 | 117 | 115 | 15–25 | ||||
| A.H. | 62 | 12 | 118 | 116 | 109 | 117 | 86 | 105 | 15 | ||||
| C.H. | 38 | 12 | 136 | 106 | 112 | 102 | 111 | 113 | 90 | ||||
| G.H. | 55 | 12 | 130 | 108 | 116 | 101 | 83 | 111 | 75 | ||||
| H.J. | 40 | 9 | 101 | 117 | 110 | 116 | 91 | 103 | 75 | ||||
| G.K. | 43 | 12 | 107 | 114 | 106 | 114 | 99 | 99 | 90 | ||||
| B.P. | 40 | 13 | 104 | 98 | 117 | 90 | 93 | 101 | 5 | ||||
| Subject . | Age (years) . | Education . | Intelligence (IQ; MWT-B) . | Wechsler memory scale-revised . | . | . | . | . | Doors test (percentile) . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | . | GMI . | VisM . | VerbM . | Att/Conc . | Delayed recall . | . | ||||
| B.B. | 40 | 9 | 112 | 66 | <50 | 81 | 77 | 57 | <1 | ||||
| S.E. | 38 | 13 | 124 | 136 | 105 | 138 | 129 | 110 | 25 | ||||
| A.F. | 34 | 9 | 104 | 124 | 105 | 126 | 117 | 115 | 15–25 | ||||
| A.H. | 62 | 12 | 118 | 116 | 109 | 117 | 86 | 105 | 15 | ||||
| C.H. | 38 | 12 | 136 | 106 | 112 | 102 | 111 | 113 | 90 | ||||
| G.H. | 55 | 12 | 130 | 108 | 116 | 101 | 83 | 111 | 75 | ||||
| H.J. | 40 | 9 | 101 | 117 | 110 | 116 | 91 | 103 | 75 | ||||
| G.K. | 43 | 12 | 107 | 114 | 106 | 114 | 99 | 99 | 90 | ||||
| B.P. | 40 | 13 | 104 | 98 | 117 | 90 | 93 | 101 | 5 | ||||
Test results below average are marked in bold. Att/Conc, attention/concentration index; GMI, general memory index; IQ, intelligence quotient; MWT-B, Mehrfach-Wahl-Wortschatztest B; VisM, visual memory index; VerbM, verbal memory index.
Spatial memory
Tests of spatial memory and place learning, however, revealed deficits in the BVL patients.
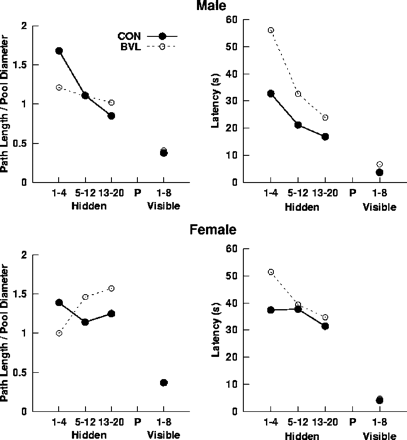
Figure 2 shows the performance of BVL patients and controls during the hidden platform place learning trial blocks (hidden platform training). Hidden platform trials were blocked into three levels (trials 1–4, 5–12 and 13–20), which were included as a within-subject factor in a repeated measures ANOVA. Overall, BVL patients took more time and longer paths to navigate to the platform than controls. For the path length measure, the only effects to reach statistical significance were the Trial block by BVL status interaction [F(2, 36) = 4.30, P < 0.05] and the Trial block × sex interaction [F(2, 36) = 4.33, P < 0.05]. These effects were attributable to a significant linear decrease in path length to navigate to the platform in male controls [F(1, 5) = 10.71, P < 0.05], which was not detected in the BVL patients or female controls [all P > 0.167]. For the latency measure, only the main effects of Trial block [F(2, 36) = 11.28, P < 0.01] and BVL status [BVL > control; F(1, 18) = 4.67, P < 0.05] reached statistical significance. The Trial block effect was due to a linear decrease in latency to navigate to the platform across the three Trial blocks [F(1, 18) = 15.12, P < 0.01].
Mean latency and path length for male and female patients and controls to navigate to the platform during the 20 hidden platform (place learning) and eight visible platform (cued-navigation) trials. Average values were computed for hidden platform trials 1–4, 5–12 and 13–20, and the eight visible platform trials.
Since the path length measure is independent of speed, it provides a relatively pure measure of navigation accuracy. In contrast, a BVL-related increase in latency might have several causes (e.g. general slowing). The group differences observed in the first Trial block were because some patients did not find the platform within 60 s, but were able to navigate to a visible platform (resulting in artificial reduction in path length). Importantly, a statistical comparison of BVL patients and controls on path length and latency during the first trial failed to yield significant differences (both P > 0.13). The BVL patients showed some improvement, apparently by adopting a better, but suboptimal, strategy; however, they still failed to reach the level of controls. Most patients did not navigate directly to the platform at the end of training but used circuitous or otherwise random strategies. If they had been given additional training, it is doubtful that they would have reached the level of controls. Normal adults achieve this task within 12 trials, and additional training did not appear to improve performance in impaired individuals.
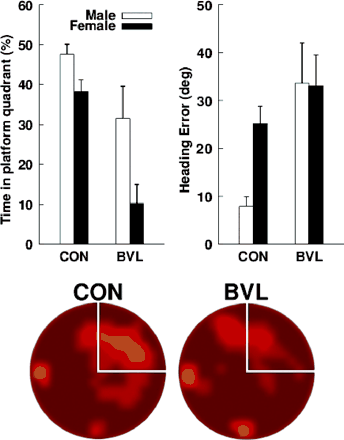
Behavioural data from the no-platform probe trial are shown in Fig. 3. There were significant main effects of BVL status [control > BVL; F(1, 18) = 18.07, P < 0.01] and Sex [males > females; F(1, 18) = 8.55, P < 0.01] for percent time spent in the platform quadrant during the probe trial. The interaction term was not significant [P = 0.261]. There was also a significant main effect of BVL status [BVL > control; F(1, 18) = 8.75, P < 0.01] for initial heading error. Although male controls had lower heading error values than female controls, the main effects of sex and the interaction term did not reach statistical significance [both P > 0.13]. The pattern of means obtained during the no-platform probe trial followed that obtained for hippocampal volume, shown in Table 1.
(Top, left) Mean percentage of search time that each group (female and male controls and NF2 patients) spent in the platform quadrant during the no-platform probe trial of phase II. (Top, right) Mean initial heading error for each group during the no-platform probe trial of phase II. Error bars are ±1 SEM. (Bottom) Dwell time for BVL patients and controls during the no-platform probe trial. Light yellow areas indicate regions where a relatively large amount of time was spent; dark red areas indicate regions where a relatively small amount of time was spent. The platform quadrant is demarcated by white lines.
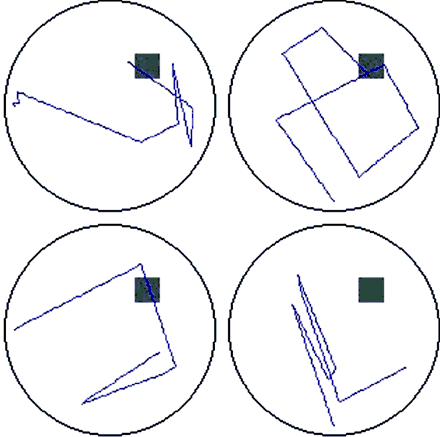
Female BVL patients spent significantly less time than controls or male BVL patients in searching in the platform quadrant during the probe trial (Table 3). If one assumes that ∼25% is chance performance, then the female patients could have had a preference for some other region of the pool. Figure 4 shows the swim paths for all four female BVL patients: clearly there is no systematic preference for a quadrant of the pool other than the platform quadrant, and the female BVL patients did not have a preference for the platform quadrant. The amount of time spent in the release quadrants tended to increase because subjects started at one of the locations far from the platform. BVL females apparently preferred one of the quadrants adjacent to the release quadrants as indicated by the 41.5% value for the SE quadrant; however, this is not clearly evident in the swim paths. A simple explanation of this observation is that the BVL females spent more time near the release point. In fact, all other groups had values >30% for some quadrant other than the platform quadrant (SE for BVL males and control males, and NW for control females). Due to the small sample sizes and pseudo-random selection of the release point in the probe trial, these apparent preferences can be attributed to group differences in the release location. Since the release points were equidistant from the platform quadrant, a valid comparison of percent search values for each of the groups can be made, whereas direct comparisons for the other quadrants are not unbiased (they are influenced by whether the release locations are sampled equally often for all groups). Nonetheless, several additional analyses were carried out to address the possibility that there were group preferences for regions other than the target quadrant. Due to the apparent preference of BVL females for the SE quadrant, we also computed the percentage of the overall path length spent navigating in each quadrant. The means (SEMs) were as follows: NE (platform quadrant) = 16.6% (6.4), SE = 30.6% (5.1), SW = 22.9% (4.1) and NW = 25.8% (8.3). The percentage of the overall path was only 30.6% for the SE, indicating that the 41.5% value obtained for search time is related to the time spent in the quadrant without moving.
Original recordings of the swim paths during the VMWT probe trial for female BVL patients. Swim paths do not indicate any systematic bias towards a particular spatial location (grey square represents the hidden escape platform).
Data for VMWT probe trial as percent time spent in the four platform quadrants (means, SEM)
. | Control (male) . | Control (female) . | BVL (male) . | BVL (female) . |
|---|---|---|---|---|
| NE(plat) | 44.4% (2.7) | 40.9% (6.0) | 35.6% (5.3) | 12.8% (4.9) |
| SE | 31.1% (9.2) | 17.3% (8.9) | 30.2% (7.3) | 41.5% (5.4) |
| SW | 11.3% (5.2) | 7.6% (5.9) | 17.1% (6.6) | 21.9% (5.5) |
| NW | 13.3% (7.5) | 34.2% (11.3) | 17.1% (4.0) | 23.9% (6.3) |
. | Control (male) . | Control (female) . | BVL (male) . | BVL (female) . |
|---|---|---|---|---|
| NE(plat) | 44.4% (2.7) | 40.9% (6.0) | 35.6% (5.3) | 12.8% (4.9) |
| SE | 31.1% (9.2) | 17.3% (8.9) | 30.2% (7.3) | 41.5% (5.4) |
| SW | 11.3% (5.2) | 7.6% (5.9) | 17.1% (6.6) | 21.9% (5.5) |
| NW | 13.3% (7.5) | 34.2% (11.3) | 17.1% (4.0) | 23.9% (6.3) |
NE, northeast; SE, southeast; SW, southwest; NW, northwest.
Data for VMWT probe trial as percent time spent in the four platform quadrants (means, SEM)
. | Control (male) . | Control (female) . | BVL (male) . | BVL (female) . |
|---|---|---|---|---|
| NE(plat) | 44.4% (2.7) | 40.9% (6.0) | 35.6% (5.3) | 12.8% (4.9) |
| SE | 31.1% (9.2) | 17.3% (8.9) | 30.2% (7.3) | 41.5% (5.4) |
| SW | 11.3% (5.2) | 7.6% (5.9) | 17.1% (6.6) | 21.9% (5.5) |
| NW | 13.3% (7.5) | 34.2% (11.3) | 17.1% (4.0) | 23.9% (6.3) |
. | Control (male) . | Control (female) . | BVL (male) . | BVL (female) . |
|---|---|---|---|---|
| NE(plat) | 44.4% (2.7) | 40.9% (6.0) | 35.6% (5.3) | 12.8% (4.9) |
| SE | 31.1% (9.2) | 17.3% (8.9) | 30.2% (7.3) | 41.5% (5.4) |
| SW | 11.3% (5.2) | 7.6% (5.9) | 17.1% (6.6) | 21.9% (5.5) |
| NW | 13.3% (7.5) | 34.2% (11.3) | 17.1% (4.0) | 23.9% (6.3) |
NE, northeast; SE, southeast; SW, southwest; NW, northwest.
To address this issue in more detail a comparison of BVL and Sex effects for the non-target quadrants is also provided along with effect sizes. For the target quadrant the effect sizes for the BVL and Sex effects were 0.18 and 0.1, respectively (power was 0.92 and 0.85, respectively). For the remaining quadrants the effect sizes (power) for the BVL effect were as follows: NW = 0.02 (0.085) [F(1, 18) < 1], SW = 0.024 (0.12) [F(1, 18) < 1] and SE = 0.04 (0.15) [F(1, 18) < 1]. Thus, the group effect sizes and power were the greatest for the target quadrant. An identical pattern was observed for the Sex effect with effect sizes (power): NW = 0.07 (0.37) [F(1, 18) = 2.90, P > 0.1], SW = 0.03 (0.20) [F(1, 18) = 1.11, P > 0.30] and SE = 0.04 (0.15) [F(1, 18) < 1]. Again, the greatest effect sizes were observed for the target quadrant. All in all, these observations support the view that apparent preferences for quadrants other than the target quadrant were not systematically related to BVL status or sex. Instead these apparent preferences only reflected variation in search time that was due to differences in release locations.
Mean latency and path length to navigate to the visible platform during the eight cued-navigation trials were computed for each participant (see Fig. 2), and analysed with BVL status and sex as factors. None of the main effects or interaction terms for the path length measure reached statistical significance; however, the BVL status main effect approached significance [BVL > control; F(1, 18) = 3.91, P = 0.064; all other P > 0.14]. Compared with the training and probe trial data, the BVL patients did not appear to be substantially impaired in navigating to a distinct cue. Although the paths for BVL patients during the visible platform trials were direct and the latencies to navigate to the platform were substantially lower than the training latencies, there was still a main effect of BVL status for latency [BVL > control; F(1, 18) = 4.70, P < 0.05], which was in part due to the low variability in the controls. The sex main effect and interaction were not significant for the latency measure (for both P > 0.18).
Discussion
The main findings of our investigation are 2-fold: (i) BVL patients had normal to superior memory performance on standardized tests, but were deficient in the virtual maze situation and (ii) the bilateral hippocampal volume of the patients was reduced compared with that of sex- and age-matched controls, who also matched the patients in years of schooling and personal experience in navigation. Thus, BVL patients were impaired relative to the controls in hippocampus-dependent spatial learning in a virtual task (on a PC) that did not require vestibular information.
It is almost a natural law that the hippocampus as the core structure of the medial temporal lobe is the most important brain structure for learning and memory (Scoville and Milner, 1957; McNaughton et al., 1996; Maguire et al., 2000; Biegler et al., 2001; Mumby, 2001; Whishaw et al., 2001; Etienne and Jeffery, 2004; Ergorul and Eichenbaum, 2004). Consequently, one would expect the patients to have considerable memory deficits: first, because of their deficits in the virtual maze and secondly, because of their hippocampal pathology. However, only one of the nine patients tested had moderate amnesia. The others performed average or even above the memory level expected on the basis of their educational background (six patients had at least one sub-test score significantly above average in the WMS-R), although the WMS-R includes various visual and verbal sub-tests and measures of recognition memory, cued recall and free recall. Only one patient had attention deficits. Four of the nine patients scored significantly below average on the visual recognition test (doors test) (Table 2). This more sensitive test—which as a recognition memory test initially seems easier than most WMS-R tests—has a spatial component as well, since there are always four spatially arranged doors during the recognition condition, and the remembrance of spatially distributed details helps to find the correct door. At least two patients performed even above average on this test, probably because they used non-spatial cues for recognition as well.
The discrepancy between spatial versus non-spatial memory deficits could be due to the special role of the hippocampus in spatial functions (McNaughton et al., 1996; Bohbot et al., 1998; Biegler et al., 2001; Mumby, 2001; Whishaw et al., 2001; Maguire et al., 2003; Etienne and Jeffery, 2004). Although this role may in general be attributed more to the right (which was more severely affected in our study) than to the left hippocampus (Curtis et al., 2000; de Toledo-Morrell et al., 2000), deficits in topographical orientation and spatial memory functions have been observed in patients after damage to either right or left hemisphere (Kessels et al., 2001, 2004), possibly depending on tasks and strategies (Maguire et al., 2000, 2003; Lambrey et al., 2002). Astur et al. (2002) tested patients with unilateral hippocampus resections in a virtual MWT. They found that when these patients are required to use spatial cues to navigate to a hidden escape platform in a pool, they exhibit severe impairments in spatial navigation regardless of the side of surgery. Driscoll et al. (2003) found that normal ageing was associated with decreases in left and right hippocampal volume, which correlated with age-related deficits in place learning in the VMWT.
The existence of a close link between spatial memory and superior memory in general is also supported by the finding that outstanding memorizers usually apply spatial learning strategies (Maguire et al., 2003). Furthermore, hippocampal processing of spatial memory seems to rely primarily on vestibular input (see Smith, 1997 for a review). In an elegant single-unit recording study in rats, Stackman et al. (2002) showed that temporary inactivation of the vestibular system resulted in the disruption of location-specific firing in hippocampal place cells. They concluded that vestibular signals have an important influence on hippocampal spatial representations. Consequently this may explain the navigational deficits in human patients with vestibular dysfunction. Similarly, Russell et al. (2003a) showed that permanent bilateral vestibular lesions resulted in place cell dysfunction that lasted even 6 weeks following the lesions. FMRI studies demonstrated that vestibular stimulation (Vitte et al., 1996) and imagined locomotion (Jahn et al., 2004) activated the hippocampal formation. Functional imaging studies, in which subjects navigated in virtual environments during PET (Maguire et al., 1997, 1998) or fMRI scanning (Grön et al., 2000; Hartley et al., 2003), showed activation of especially the right hippocampus in wayfinding, a navigation task defined as ‘finding novel paths between locations’, mainly when the subjects used spatial landmarks to navigate in the early phase of a place-learning task in a computer-generated virtual environment (Iaria et al., 2003). In a recent fMRI study, hippocampal activation was most prominent during initial navigational learning, pointing to its role in incorporating new information into an emerging memory representation (Wolbers et al., 2005). While limited data in rats indicate that bilateral peripheral vestibular lesions produce long-term changes in spatial learning (Russell et al., 2003b), patient data illustrating the relation between hippocampal-based amnesia and vestibular dysfunctions have until now been lacking.
Explanations for why bilateral vestibular damage results in hippocampal atrophy have included chronic stress, which is associated with vestibular dysfunction (Maclennan et al., 1998). Several lines of evidence suggest that this explanation is unlikely. First, our patients received their BVLs 5–10 years previously, and although vestibular compensation for bilateral vestibular damage is limited (for example, the vestibulo-ocular reflex never recovers its normal function (Curthoys and Halmagyi, 1995), they would have achieved a steady state of compensation within the first year post-op. Secondly, animal studies show that elevated salivary cortisol (in guinea pigs; Gliddon et al., 2003) and blood corticosterone levels (in rats; Lindsay et al., 2005) decrease rapidly following vestibular damage and are normal within 2 weeks post-op. These results suggest that animals subjected to BVL are not chronically stressed.
Another explanation is that hippocampal atrophy is possibly a direct or indirect result of BVL. Bilateral input of one vestibular organ to both hippocampi has been demonstrated by electrophysiological experiments in guinea pigs. In one study, the right labyrinth was electrically stimulated and evoked field potentials were recorded over the hippocampal formation bilaterally (Cuthbert et al., 2000). Although bilateral vestibular lesions abolish the tonic input from the peripheral vestibular system (resting discharge is ∼100 Hz) as well as its modulation by head movements, spontaneous resting activity regenerates bilaterally in the vestibular nuclei over time (e.g. Ris and Godaux, 1998). However, these neurons no longer respond normally to head movement because of the permanent loss of dynamic vestibular information (Ris and Godaux, 1998). Whether this leads to cell death in the hippocampus or to changes in neuronal cytoarchitecture remains to be seen. We are currently investigating these possibilities in BVL rats. Studies of hippocampal slices, removed from rats 5–6 months following a unilateral vestibular lesion, found that CA1 neurons exhibited a marked decrease in electrical excitability in response to stimulation of the Schaffer collateral pathway, and this effect was evident both ipsilateral and contralateral to the lesion (Zheng et al., 2003).
Thus, there is evidence that acquired chronic loss of vestibular function can cause hippocampal atrophy. The pattern of means for the probe trial measures in the VMWT closely matched the pattern of hippocampal volumes observed in the patient and control groups. Spatial navigation requires a continuous representation of the location and motion of the individual within a 3-dimensional environment, whose coordinates are provided mainly by vestibular and visual cues. Hippocampal atrophy may consequently impair complex forms of spatial memory processing, while non-spatial functions remain well preserved. Perhaps the ancient phylogenetic role of the hippocampus in spatial memory processing (Kessels et al., 2001), which is based on intact vestibular input, is more sensitive to hippocampal atrophy than more advanced, non-spatial roles that rely additionally on the surrounding medial temporal lobe and prefrontal tissue (Dolan and Fletcher, 1997; Gaffan, 2002; Markowitsch et al., 2003; Owen et al., 1996).
The authors thank Kirsten Labudda for administering most of the psychological tests (WMS-R, Doors Test, MWT-B). The authors also thank Dr M. Holtmannspötter and Dr Jun Ma for their help in calculating brain volumes, and Judy Benson for critically reading the manuscript.
References
Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water task: a large and reliable sex difference.
Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task.
Baddeley A, Emslie H, Nimmo-Smith I. Doors and People Test. Bury St. Edmunds, UK: Thames Valley Test Company;
Becker JT, Olton DS. Cognitive mapping and hippocampal system function.
Bernasconi N, Bernasconi A, Andermann F, Dubeau F, Feindel W, Reutens DC. Entorhinal cortex in temporal lobe epilepsy: a quantitative MRI study.
Bernasconi N, Bernasconi A, Caramanos Z, Antel SB, Andermann F, Arnold DL. Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region.
Berthoz A. Parietal and hippocampal contribution to topokinetic and topographic memory.
Biegler R, McGregor A, Krebs JR, Healy SD. A larger hippocampus is associated with longer-lasting spatial memory.
Blair HT, Sharp PE. Visual and vestibular influences on head-direction cells in the anterior thalamus of the rat.
Bohbot VD, Kalina M, Stepankova K, Spackova N, Petrides M, Nadel L. Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex.
Bunsey M, Eichenbaum H. Selective damage to the hippocampal region blocks long-term retention of a natural and nonspatial stimulus– stimulus association.
Curthoys IS, Halmagyi GM. Vestibular compensation: a review of the oculomotor, neural, and clinical consequences of unilateral vestibular loss.
Curtis CE, Zald DH, Lee JT, Pardo JV. Object and spatial alternation tasks with minimal delays activate the right anterior hippocampus proper in humans.
Cuthbert PC, Gilchrist DP, Hicks SL, MacDougall HG, Curthoys IS. Electrophysiological evidence for vestibular activation of the guinea pig hippocampus.
de Toledo-Morrell L, Dickerson B, Sullivan MP, Spanovic C, Wilson R, Bennett DA. Hemispheric differences in hippocampal volume predict verbal and spatial memory performance in patients with Alzheimer's disease.
Dolan RJ, Fletcher PC. Dissociating prefrontal and hippocampal function in episodic memory encoding.
Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, et al. The aging hippocampus: cognitive, biochemical and structural findings.
Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, Newman EL, et al. Cellular networks underlying human spatial navigation.
Ergorul C, Eichenbaum H. The Hippocampus and memory for “what”, “where”, and “when”.
Feigenbaum JD, Morris RG. Allocentric versus egocentric spatial memory after unilateral temporal lobectomy in humans.
Gavrilov VV, Wiener SI, Berthoz A. Enhanced hippocampal theta EEG during whole body rotations in awake restrained rats.
Glasauer S, Amorim MA, Viaud-Delmon I, Berthoz A. Differential effects of labyrinthine dysfunction on distance and direction during blindfolded walking of a triangular path.
Gliddon C, Darlington CL, Smith PF. Activation of the hypothalamic–pituitary–adrenal axis following vestibular deafferentation in pigmented guinea pig.
Gron G, Wunderlich AP, Spitzer M, Tomczak R, Riepe MW. Brain activation during human navigation: gender-different neural networks as substrate of performance.
Hamilton DA, Driscoll I, Sutherland RJ. Human place learning in a virtual Morris water task: some important constraints on the flexibility of place navigation.
Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD. Children with fetal alcohol syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task.
Hartley T, Maguire EA, Spiers HJ, Burgess N. The well-worn route and the path less traveled: distinct neural bases of route following and wayfinding in humans.
Horii A, Russell NA, Smith PF, Darlington CL, Bilkey DK. Vestibular influences on CA1 neurons in the rat hippocampus: an electrophysiological study in vivo.
Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: variability and change with practice.
Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, et al. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices.
Jahn K, Deutschlander A, Stephan T, Strupp M, Wiesmann M, Brandt T. Brain activation patterns during imagined stance and locomotion in functional magnetic resonance imaging.
Jarrard LE. On the role of the hippocampus in learning and memory in the rat.
Kessels RP, de Haan EH, Kappelle LJ, Postma A. Varieties of human spatial memory: a meta-analysis on the effects of hippocampal lesions.
Kessels RP, Hendriks M, Schouten J, Van Asselen M, Postma A. Spatial memory deficits in patients after unilateral selective amygdalohippocampectomy.
Lambrey S, Viaud-Delmon I, Berthoz A. Influence of a sensorimotor conflict on the memorization of a path traveled in virtual reality.
Lindsay L, Liu P, Gliddon C, Zheng Y, Smith PF, Darlington CL. Cytosolic glucocorticoid receptor expression in the rat vestibular nucleus and hippocampus following unilateral vestibular deafferentation.
Maclennan KM, Smith PF, Darlington CL. Adrenalectomy-induced neuronal degeneration.
Maguire EA, Burke T, Phillips J, Staunton H. Topographical disorientation following unilateral temporal lobe lesions in humans.
Maguire EA, Frackowiak RS, Frith CD. Recalling routes around London: activation of the right hippocampus in taxi drivers.
Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O'Keefe J. Knowing where and getting there: a human navigation network.
Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, et al. Navigation-related structural change in the hippocampi of taxi drivers.
Maguire EA, Valentine ER, Wilding JM, Kapur N. Routes to remembering: the brains behind superior memory.
Manns JR, Hopkins RO, Reed JM, Kitchener EG, Squire LR. Recognition memory and the human hippocampus.
Markowitsch HJ, Vandekerckhovel MMP, Lanfermann H, Russ MO. Engagement of lateral and medial prefrontal areas in the ecphory of sad and happy autobiographical memories.
McEchron MD, Disterhoft JF. Hippocampal encoding of non-spatial trace conditioning.
McNaughton BL, Barnes CA, Gerrard JL, Gothard K, Jung MW, Knierim JJ, et al. Deciphering the hippocampal polyglot: the hippocampus as a path integration system.
Morris RGM. Spacial localization does not require the presence of local cues.
Mumby DG. Perspectives on object recognition memory following hippocampal damage: lessons from studies in rats.
O'Mara SM, Rolls ET, Berthoz A, Kesner RP. Neurons responding to whole body motion in the primate hippocampus.
Owen AM, Milner B, Petrides M, Evans AC. A specific role for the right parahippocampal gyrus in the retrieval of object-location: a positron emission tomography study.
Parslow DM, Morris RG, Fleminger S, Rahman Q, Abrahams S, Recce M. Allocentric spatial memory in humans with hippocampal lesions.
Raz N. Aging of the brain and its impact on cognitive performance: integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. Handbook of ageing and cognition—II. Mahwah, NJ: Erlbaum;
Ris L, Godaux E. Neuronal activity in the vestibular nuclei after contralateral or bilateral labyrinthectomy in the alert guinea pig.
Rosenbaum RS, Priselac S, Kohler S, Black SE, Gao F, Nadel L, et al. Remote spatial memory in an amnesic person with extensive bilateral hippocampal lesions.
Russel NA, Horii A, Smith PF. The long-term effects of permanent vestibular lesions on hippocampal spatial firing.
Russell NA, Horii A, Smith PF, Darlington CL, Bilkey DK. Bilateral peripheral vestibular lesions produce long-term changes in spatial learning in the rat.
Schautzer F, Hamilton D, Kalla R, Strupp M, Brandt T. Spatial memory deficits in patients with chronic bilateral vestibular failure.
Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions.
Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis.
Stackman RW, Taube JS. Firing properties of head direction cells in the rat anterior thalamic nucleus: dependence upon vestibular input.
Stackman RW, Clark AS, Taube JS. Hippocampal spatial representations require vestibular input.
Taube JS, Goodridge JP, Golob EJ, Dudchenko PA, Stackman RW. Processing the head direction signal: a review and commentary.
Vitte E, Derosier C, Caritu Y, Berthoz A, Hasboun D, Soulie D. Activation of the hippocampal formation by vestibular stimulation: a functional magnetic resonance imaging study.
Whishaw IQ, Hines DJ, Wallace DG. Dead reckoning (path integration) requires the hippocampal formation: evidence from spontaneous exploration and spatial learning tasks in light (allothetic) and dark (idiothetic) tests.
Wiener SI, Korshunov VA, Garcia R, Berthoz A. Interial, substratal and landmark cue control of hippocampal CA1 place cell activity.
Wolbers T, Buchel C. Dissociable retrosplenial and hippocampal contributions to successful formation of survey representations.
Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location.
Author notes
1Department of Neurology, Ludwig-Maximilians University, Munich, Germany, 2Department of Neurology and Psychosomatics, LKH Villach, Austria, 3Department of Psychology, University of New Mexico, Albuquerque, USA, 4Department of Neuroradiology, Ludwig-Maximilians University, Munich, Germany, 5Physiological Psychology, University of Bielefeld, Bielefeld, Germany and 6Department of Pharmacology and Toxicology, School of Medical Sciences, University of Otago Medical School, Dunedin, New Zealand


![In BVL patients, a 16.91% volume loss in the hippocampus (arrows) was observed in comparison to age- and sex-matched controls (normal hippocampus: dotted arrows). Volume loss was similar for the left and right hippocampus. Analysis of variance (ANOVA) with BVL status and sex confirmed a significant difference in hippocampal volume between BVL patients and controls [F(1, 17) = 13.08]. Shown are examples of coronal 3 mm MRI T2-weighted images with a distance of 6 mm. (A) 39-year-old female volunteer. (B) 40-year-old female BVL patient (for methodological details, see Methods). This patient had a total volume of 3.9 ml (left hippocampus 1939.18 mm3, right hippocampus 2002.82 mm3).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/brain/128/11/10.1093/brain/awh617/2/m_awh617f1.gif?Expires=1716441010&Signature=Lz2pmcF~i3DR6TSdBqcv3nlI95HFI04dUC8Xc12EsnM1pDdyijrGlKXVBLFeD770MSPp9kWyS82CnNO3n8btsB-YqZATxOKU8KhscvmJVzo-E~P8ciQX3OQgIyofnEL627mHCNn~JSLHk6wL6B26HXM2M4rmt3x-yrt8vmO5oxGeYNpoF2Ag55hNMPz1AjmTnRFR~FNiFkdpvAGbWUc-X2AOh4EdF-TnIT3eiCXoWSFisSsMPCGYBLlU1UeTxr6Is5wzxmQje~hw1UqurLhxApMEsmcgOWPv3g8jX4ufCF1yi9qDPDhvH4ojHNI9KccYejrnt~9G4~yyMimZ-T9NAA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)