-
PDF
- Split View
-
Views
-
Cite
Cite
S.-J. Blakemore, D. Bristow, G. Bird, C. Frith, J. Ward, Somatosensory activations during the observation of touch and a case of vision–touch synaesthesia, Brain, Volume 128, Issue 7, July 2005, Pages 1571–1583, https://doi.org/10.1093/brain/awh500
Close - Share Icon Share
Abstract
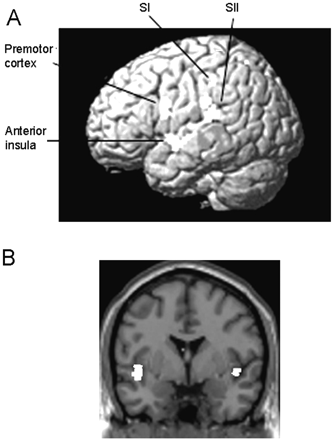
In this study, we describe a new form of synaesthesia in which visual perception of touch elicits conscious tactile experiences in the perceiver. We describe a female subject (C) for whom the observation of another person being touched is experienced as tactile stimulation on the equivalent part of C's own body. Apart from this clearly abnormal synesthetic experience, C is healthy and normal in every other way. In this study, we investigate whether C's ‘mirrored touch’ synesthetic experience is caused by overactivity in the neural system that responds to the observation of touch. A functional MRI experiment was designed to investigate the neural system involved in the perception of touch in a group of 12 non-synesthetic control subjects and in C. We investigated neural activity to the observation of touch to a human face or neck compared with the observation of touch to equivalent regions on an object. Furthermore, to investigate the somatosensory topography of the activations during observation of touch, we compared activations when observing a human face or neck being touched with activations when the subjects themselves were touched on their own face or neck. The results demonstrated that the somatosensory cortex was activated in the non-synesthetic subjects by the mere observation of touch and that this activation was somatotopically organized such that observation of touch to the face activated the head area of primary somatosensory cortex, whereas observation of touch to the neck did not. Moreover, in non-synesthetic subjects, the brain's mirror system—comprising premotor cortex, superior temporal sulcus and parietal cortex—was activated by the observation of touch to another human more than to an object. C's activation patterns differed in three ways from those of the non-synesthetic controls. First, activations in the somatosensory cortex were significantly higher in C when she observed touch. Secondly, an area in left premotor cortex was activated in C to a greater extent than in the non-synesthetic group. Thirdly, the anterior insula cortex bilaterally was activated in C, but there was no evidence of such activation in the non-synesthetic group. The results suggest that, in C, the mirror system for touch is overactive, above the threshold for conscious tactile perception.
Introduction
Synaesthesia, coming from the Greek roots syn, meaning together, and aisthesis, meaning perception, is a condition in which stimulation in one modality results in simultaneous subjective experience of sensation in another modality. The most common form of synaesthesia, in which words or letters are associated with particular colours, is thought to occur in at least 1 in 2000 people, with a female:male ratio of about 6:1 (Baron-Cohen et al., 1996). Here, we describe a new form of synaesthesia in which vision and tactile perception are inextricably linked. We describe a female subject (C) for whom the observation of another person being touched is experienced as tactile stimulation on the equivalent part of her own body. In this neuroimaging study, we investigate whether C's synesthetic experience is associated with overactivity in the neural systems that normally respond to the observation of action (the mirror system) and to the observation of touch.
The mirror system for the observation of action
During the observation of action, a significant proportion of the brain's motor system becomes active (Rizzolatti et al., 2001). In monkeys, neurons in ventral premotor cortex fire both when the monkey executes grasping actions and when it observes another individual (human or monkey) performing the same action (Gallese et al., 1996). There is growing evidence that a similar mirror system also exists in the human brain (Grafton et al., 1996). One functional MRI (fMRI) experiment showed that observing actions leads to somatotopic activation of the premotor cortex, with the mouth represented laterally and the foot medially (Buccino et al., 2001). There is evidence that human mirror areas are selective for biological motion. Tai et al. (2004) measured activity in the left premotor cortex during observation of a grasping movement made either by a human or by a robotic hand. They found that the activity in premotor cortex increased during observation of a human grasp, but not during observation of a robot grasp (relative to static posture baselines).
Recently, a number of brain systems with mirror properties have been described. Common regions are activated by the experience and mere observation of disgust (Wicker et al., 2003), emotional facial expression (Carr et al., 2003), pain (Singer et al., 2004) and touch (Keysers et al., 2004). In the latter study, observing touch to someone else's legs activated similar regions in the secondary somatosensory cortex (SII) in the observer's brain as when the observer's own legs were touched. However, this SII activation was also found during the observation of touch to an object, and no primary somatosensory cortex activity was found in either condition (Keysers et al., 2004).
Mirrored touch synaesthesia
The subject described here (C) has a form of synaesthesia in that she experiences touch from purely visual input. She experiences tactile stimulation on the part of her body that mirrors the body part she observes being touched. C has spent the whole of her life experiencing touch when she observes touch on others, unaware that the vast majority of the population do not experience similar sensations. She was surprised to discover that her perception of touch on observing others being touched is abnormal. Furthermore, since discovering that her experiences were out of the ordinary, C has found that her first cousin (female) reports experiencing the same tactile sensations when observing touch to others. This suggests that, like colour-word synaesthesia, this mirror touch synaesthesia may run in families.
There are various possible causes of C's mirrored touch synaesthesia. One possibility is that it is due to overactivation of somatosensory regions normally activated during the observation of touch (the tactile mirror system; Keysers et al., 2004). Perhaps this system is activated above a threshold for conscious tactile perception in C when she observes touch to another person so that she perceives the touch as if she is the object of it. In most people, this system would be active below a certain threshold, resulting in no conscious perception of tactile stimulation. In the visual system, there is evidence that stimulus processing with awareness is associated with greater activity in ventral visual cortex than processing without awareness (e.g. Beck et al., 2001; for review see Rees et al., 2002). One crucial difference between C's phenomenology and the results of the fMRI described above (Keysers et al., 2004) is that C reports no experience of tactile perception when she observes objects being touched. Therefore, her touch mirror system should not be activated more than normal when she observes objects being touched.
A second explanation of mirrored touch synaesthesia is that it reflects direct connectivity between visual and somatosensory regions that is unique to C. In this account, mirrored-touch synaesthesia would not depend upon the same mechanisms that are believed to be involved in visual–tactile integration in the rest of the population. Analogous cross-modal leakage explanations have been put forward for other types of synaesthesia. For example, it has been proposed that grapheme–colour synaesthesia may be due to direct cross-activation of adjacent colour region (area V4) and grapheme/word form region (e.g. Ramachandran and Hubbard, 2001). Indeed, recent neuroimaging data has demonstrated that V4 is activated by heard words in grapheme–colour synesthetes (Nunn et al., 2002).
A third explanation for mirrored touch synaesthesia is that bimodal cells in the parietal cortex, specifically the intraparietal sulcus, which respond to both visual and tactile stimuli (Bremmer et al., 2001; Macaluso et al., 2003), are activated above the threshold for tactile perception during the observation of touch in C.
Reported cases of vision–touch interaction in patients
There are a number of reported cases of acquired synaesthesia involving touch. D.N., who suffered a stroke resulting in paralysis of and loss of sensation in the left side of his body, was unable to feel any kind of tactile stimulation to the left side of his body if it was hidden from view (Halligan et al., 1996). However, as soon as the tactile stimulation became visible, D.N. was able to feel it. Moreover, if D.N. believed his left arm was being touched (by watching a previously recorded video of his arm being touched but being told that it was a real-time live feedback), he reported being able to feel his arm being touched, even when the experimenter was not in fact touching his arm. This suggests that tactile stimulation is not necessary for D.N. to perceive touch and shows that, in some conditions, vision alone is sufficient. One possibility is that the tactile sensations he feels are due to cross-modal leakage between his primary visual and somatosensory areas as a result of the stroke. Alternatively, the tactile sensations could be due to the output of bimodal visual–somatosensory cells that have been partially deprived of their normal somatosensory input by D.N.'s stroke.
There is one known case in which an interaction between observed and perceived pain has been anecdotally reported. A man who apparently experienced observed pain as pain to himself was posthumously described by his wife (Bradshaw and Mattingley, 2001). The authors suggested this man might, for some reason, have had an abnormal ‘mirror pain’ system. However, this case was described after the patient's death and no information was available about the neural circuitry involved.
The aims of the current study were twofold. First, the experiment was designed to investigate neural interactions between visually perceived touch and tactile perception in the normal, non-synesthetic population. Secondly, we investigated the neural systems underlying C's mirrored touch synaesthesia. We used fMRI to compare brain activity while the synesthetic subject and a group of non-synesthetic, ‘normal control’ subjects observed people being touched and objects being touched.
To investigate whether brain activity during observation of touch follows a somatotopic organization, we compared brain activity during the observation of touch to different areas of the body (human neck and face, and similar regions on an object) and to different sides of the body. There were three reasons why the face and neck were chosen as regions of stimulation in both the videos and the touch conditions. First, the face was chosen because of the known somatotopic representation of this region in primary somatosensory cortex (SI). Although the exact representation of the neck in human SI is unknown, the neck was chosen because it is physically close to the face but does not activate the face area of SI. Secondly, the face and neck were chosen because of the desire to match as closely as possible the human face and neck with an object with face- and neck-like properties (e.g. an electric fan) in the observation conditions. Finally, C reported being particularly sensitive to the observation of touch to another person's face and neck.
Activations during the observation of touch were compared with activations during tactile stimulation to the subject's own face and neck. We made several predictions. First, we predicted that, in the non-synesthetic subjects and in C, observation of touch to another human would activate somatosensory regions more than observation of touch to an object. Secondly, we predicted that SI activity to the observation of touch would be related to the region of the body observed being touched in a somatotopic manner. Finally, we predicted that these observation-related activations would be significantly higher in C than in the control subjects. In addition, there may be additional regions of C's brain that are activated by the observation of touch which are not activated in the control group. This could account for why observed touch is not perceived as touch in most people.
Methods
Subjects
A female, right-handed subject (C, age 41 years), who experienced tactile stimulation on her own body when seeing another person being touched, as well as 12 right-handed control participants (seven females; mean age 28.75 ± 2.66 years), gave informed consent and participated in the study, which was approved by the National Hospital for Neurology and Neurosurgery Ethics Committee.
C claims always to have perceived observed touch on other people as touch to her own body. If she is facing someone, her visual–touch perception is mirrored, such that observing touch on their left cheek, for example, elicits touch on her right cheek. If she is standing next to someone, touch to their right side is felt on her right side. Although always having experienced touch when she observes touch on others, she was unaware that the vast majority of the population do not experience similar sensations until July 2003 when the authors were talking with her about observed touch and the mirror system. She was surprised to discover that her perception of touch on observing others being touched is abnormal. Interestingly, C has 11 family members (all female) with grapheme–colour synaesthesia, which she remembers experiencing herself when she was a child but no longer experiences. One of her family members (female first cousin) also reports having mirrored touch synaesthesia. Unfortunately, it was not possible to include C's cousin in the current study. C's reported perception of touch when observing touch on other people seems to be reliable over time: the words and phrases she uses to describe the observed touch, its intensity and exact location on herself are highly consistent.
The tendency for synaesthesia to run in families has been noted and it has been suggested that the mode of inheritance might be X-linked (Baron-Cohen et al., 1996; Bailey and Johnson, 1997). That C's mirrored touch synaesthesia and the grapheme–colour synaesthesia she experienced as a child occur only within female members of her family suggests a possible genetic link between completely different forms of synaesthesia. One possibility is that the genetic predisposition for synaesthesia is general in nature, and does not code for the modality in which the synaesthesia is experienced. It is possible that C's relatives mainly experience grapheme–colour synaesthesia simply because this is the most common form of synaesthesia. There is similar evidence that people with grapheme–taste synaesthesia have relatives who mainly have grapheme–colour synaesthesia (Ward et al., 2005). This is an intriguing possibility that requires further investigation.
Design
The fMRI experiment was split into three sessions: one ‘touch’ session and two ‘video’ sessions.
Touch session
During the first session, the subject lay on the MRI bed with their eyes shut, while the experimenter applied a tactile stimulus to the subject's neck or face (cheek area). There were four touch conditions defined according to the site of tactile stimulation: Left Neck (LN), Right Neck (RN), Left Face (LF) and Right Face (RF). In addition, there was a baseline condition during which no touch occurred.
The tactile stimulus device consisted of a 2-inch rigid piece of felt attached to the end of a wooden rod (length ∼1 m). The rod reached into the scanner bore and could be positioned by the experimenter such that the felt-tip touched the subject's neck or cheek (or neither in the baseline condition). In each block, the subject was stroked for 20 s on one of the four areas. Over the session, there were five blocks of each tactile stimulus condition and five blocks of the rest baseline. The order of conditions was alternated between Face and Neck on either side.
Video sessions
The touch session was followed by two sessions in which subjects were shown blocks of short video clips. Each video clip lasted 4.5 s. Half the clips (the ‘human’ videos) showed the head and shoulders of a person being touched on their neck or face by the finger of another person. Three different people, one male and two females, featured in these videos and only their head and shoulders were visible. The other half (the ‘object’ videos) showed inanimate objects (a lamp, an electric fan and a loud speaker) being touched on their equivalent ‘neck’ or ‘face’ regions. The object conditions were designed to control for the presence of visual stimulation and movement of the toucher's hand and arm, and for any other non-specific visual factors in the films.
The design was factorial with three factors: (i) focus of the observed touch (human or object); (ii) side of observed touch (left or right); and (iii) location of observed touch (face or neck). This resulted in eight conditions, as shown in Table 1. In addition, a fixation baseline condition was included.
Experimental design for video experiment
. | Human . | Object . | ||
|---|---|---|---|---|
| Right | ||||
| Neck | Human Right Neck (HRN) | Object Right Neck (ORN) | ||
| Face | Human Right Face (HRF) | Object Right Face (ORF) | ||
| Left | ||||
| Neck | Human Left Neck (HLN) | Object Left Neck (OLN) | ||
| Face | Human Left Face (HLF) | Object Left Face (OLF) | ||
. | Human . | Object . | ||
|---|---|---|---|---|
| Right | ||||
| Neck | Human Right Neck (HRN) | Object Right Neck (ORN) | ||
| Face | Human Right Face (HRF) | Object Right Face (ORF) | ||
| Left | ||||
| Neck | Human Left Neck (HLN) | Object Left Neck (OLN) | ||
| Face | Human Left Face (HLF) | Object Left Face (OLF) | ||
Experimental design for video experiment
. | Human . | Object . | ||
|---|---|---|---|---|
| Right | ||||
| Neck | Human Right Neck (HRN) | Object Right Neck (ORN) | ||
| Face | Human Right Face (HRF) | Object Right Face (ORF) | ||
| Left | ||||
| Neck | Human Left Neck (HLN) | Object Left Neck (OLN) | ||
| Face | Human Left Face (HLF) | Object Left Face (OLF) | ||
. | Human . | Object . | ||
|---|---|---|---|---|
| Right | ||||
| Neck | Human Right Neck (HRN) | Object Right Neck (ORN) | ||
| Face | Human Right Face (HRF) | Object Right Face (ORF) | ||
| Left | ||||
| Neck | Human Left Neck (HLN) | Object Left Neck (OLN) | ||
| Face | Human Left Face (HLF) | Object Left Face (OLF) | ||
The videos were presented in 23-second blocks. Each block contained four different video clips from the same condition. The order in which the four video clips were presented within each block was random. At the end of each block, following the four videos, subjects were asked to rate the intensity of the touch applied to the person or object in the most recent video. A screen appeared for 5 s displaying the words ‘hard’, ‘medium’ and ‘soft’. Subjects indicated their answer by pressing one of three buttons on a keypad held in their right hand. The intensity of the touch in the videos was not in fact deliberately varied between the different clips. The question was designed to ensure that the subjects paid attention to the touch stimulus in the videos for its duration.
During each of the video sessions, there was a total of 27 blocks, comprising three repetitions of each of the eight video conditions (see Table 1) and three repetitions of the 23-second fixation baseline block. The order of presentation of the blocks was counterbalanced within and between subjects.
After scanning, subjects were asked whether they felt the observed touch in any of the conditions. If they responded positively, they were asked to watch 32 video clips comprising four exemplars of each of the eight conditions, selected at random from the video clips used in the scanning experiment. The order of presentation of the clips was random. Subjects were asked to rate the intensity of the tactile stimulation they felt on their own face or neck when they watched each video on a scale from 0 (indicating ‘no perceived tactile sensation’) to 5 (indicating ‘very intense tactile sensation’).
Data acquisition
A Siemens ALLEGRA system (Siemens, Erlangen, Germany) operating at 3 T was used to acquire both multi-slice axial gradient-echo, echo-planar T2* weighted image volumes with blood oxygenation level-dependent (BOLD) contrast and axial T1 weighted fast-field echo structural images for anatomical co-registration. Data were acquired in three functional imaging sessions. A total of 205 volumes was acquired in the Touch session, and 250 volumes were acquired in each of the following two Video sessions. Each session began with eight ‘dummy’ volumes, which were subsequently discarded, to allow for T1 equilibration effects. Each functional-image volume comprised 40 × 2 mm axial slices with in-plane resolution of 3 × 3 mm positioned to cover the whole brain. Volumes were acquired continuously every 2.6 s throughout each session [TR (repetition time) = 2.6].
The acquisition of a T1-weighted anatomical image occurred after the three functional sessions and lasted ∼12 min. The total duration of the experiment was ∼45 min.
fMRI data analysis
Functional imaging analysis used the technique of statistical parametric mapping, implemented in SPM2 (Wellcome Department of Imaging Neuroscience, University College London, London, UK; www.fil.ion.ucl.ac.uk/spm). For each subject, the fMRI scans were realigned to correct for inter-scan movement, using sinc interpolation (Friston et al., 1995), and subsequently stereotactically normalized using affine registration followed by non-linear registration. The data were resampled using sinc interpolation, with a resolution of 3 × 3 × 3 mm3, into the standard space of the Montreal Neurological Institute brain. The scans were then smoothed with a Gaussian kernel of 6 mm full-width half maximum.
The analysis of functional imaging data entails the creation of statistical parametric maps that represent a statistical assessment of hypothesized condition specific effects (Friston et al., 1994). Condition-specific effects were estimated with the General Linear Model with a delayed box–car waveform. Low frequency sine and cosine waves modelled and removed subject-specific low frequency drifts in signal, while global changes in activity were removed by proportional scaling. Each component of the model served as a regressor in a multiple regression analysis. For the group of control subjects, the resulting parameter estimates for each regressor at each voxel were then entered into a second level analysis where subject served as a random effect in a within-subjects ANOVA (analysis of variance). For both the group of control subjects (at the second level) and the synesthetic subject (at the first level), the main effects and interactions between conditions were then specified by appropriately weighted linear contrasts and determined using the t-statistic on a voxel-by-voxel basis.
In the Touch session, scans corresponding to the breaks between tactile stimulation, during which time the experimenter was repositioning the stimulus, were excluded from the analysis. Statistical analysis was performed to examine the main effects of tactile stimulation versus baseline [(RN + RF + LN + LF) − baseline]. Because of our specific hypothesis concerning somatotopic representation of observing the face being touched, analysis was performed to examine the main effects of touching the subject's face [(RF + LF) − (RN + LN)], left side [(LF + LN) − (RF + RN)] and right side [(RF + RN) − (LF + LN)]. Analysis of activations associated with touch to the neck relative to touch to the face was not included, as tactile stimulation of the neck is not known to activate a defined area in SI and, as such, this contrast did not form one of our predictions.
In the Video sessions, scans corresponding to the question asked at the end of each block were excluded from the analysis. The data from the video sessions were analysed to examine the main effects for which a priori predictions were made. These were the main effects of watching videos [(HRF + HLF + HRN + HLN + ORF + OLF + ORN + OLN) − baseline] and observing touch to a human compared with touch to an object [(HRF + HLF + HRN + HLN) − (ORF + OLF + ORN + OLN)] (see Table 1 for an explanation of the abbreviations). In addition, analysis of the human video conditions was performed to examine the main effects of observation of touch to the face [(HRF + HLF) − (HRN + HLN)], to the left side [(HLF + HLN) − (HRF + HRN)] and to the right side [(HRF + HRN) − (HLF + HLN)]. For each of these latter contrasts, the resulting images were inclusively masked (at P < 0.05) with the equivalent contrast in the Touch experiment to investigate common activations during tactile stimulation and the observation of tactile stimulation.
These statistical contrasts were used to create an SPM{t}, which was transformed into an SPM{Z} and thresholded at P < 0.05 (corrected on the basis of the theory of random Gaussian fields for multiple comparisons across the whole brain volume examined). We report those regions that survive correction for multiple comparisons over the whole brain at P < 0.05, plus those regions surviving a small volume correction (SVC) of P < 0.05 for which we had an a priori hypothesis for their activation. Specifically, a SVC was applied to activations within a sphere of 6 mm radius in the parietal operculum (SII), 5 mm radius in the postcentral gyrus (SI) and 6 mm radius in the premotor cortex.
Comparison between synesthetic subject and non-synesthetic subjects
At the second level, we made a direct comparison of activity in the synesthetic subject and distribution of activity from the non-synesthetic group for the contrast (observing human–observing object). This analysis was performed to test the hypothesis that there would be significantly greater activity in somatosensory and premotor regions when the synesthetic subject, relative to thenon-synesthetic subjects, observed humans being touched relative to objects being touched. Heuristically, this analysis compares the response of C to the average response of the control subjects, in relation to the control intersubject variability. At each voxel, this analysis tests whether the activity observed in C fell within the same distribution as the activity observed at that voxel in the 12 non-synesthetic subjects, given the mean and variance of this activity across non-synesthetic subjects, or whether it was significantly greater in C.
Results
Perceptual ratings
None of the non-synesthetic subjects reported feeling the observed touch on their own face or neck during any of the video conditions. C was asked to rate the intensity of the tactile stimulation she felt on her own face or neck when she watched each video on a scale from 0 (indicating ‘no perceived tactile sensation’) to 5 (indicating ‘very intense tactile sensation’). Table 2 shows C's perceptual ratings. These indicate that C feels tactile stimulation on her own face or neck when watching another person being touched on their face or neck, but not when watching objects being touched.
C's mean ratings for the perception of touch on her own face or neck during the observation of touch to another person or object's face and neck*
| Observation condition . | C's mean ratings for perception of touch on her own face or neck . |
|---|---|
| HRN | 3.88 |
| HRF | 3.50 |
| HLN | 3.67 |
| HLF | 4.33 |
| Observation condition . | C's mean ratings for perception of touch on her own face or neck . |
|---|---|
| HRN | 3.88 |
| HRF | 3.50 |
| HLN | 3.67 |
| HLF | 4.33 |
C reported feeling no sensation on her own face or neck during the object videos.
C's mean ratings for the perception of touch on her own face or neck during the observation of touch to another person or object's face and neck*
| Observation condition . | C's mean ratings for perception of touch on her own face or neck . |
|---|---|
| HRN | 3.88 |
| HRF | 3.50 |
| HLN | 3.67 |
| HLF | 4.33 |
| Observation condition . | C's mean ratings for perception of touch on her own face or neck . |
|---|---|
| HRN | 3.88 |
| HRF | 3.50 |
| HLN | 3.67 |
| HLF | 4.33 |
C reported feeling no sensation on her own face or neck during the object videos.
fMRI data: touch session
Main effect of touch—baseline: [(RN + RF + LN + LF) − baseline]
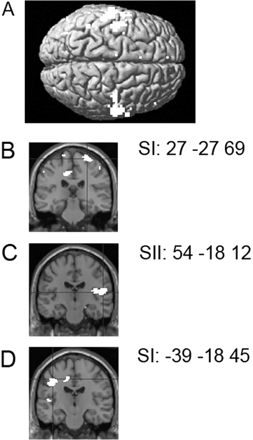
Comparison of the four touch conditions relative to rest in the group of non-synesthetic subjects and in C resulted in activation of a number of somatosensory regions, including SI and SII, and motor and premotor regions (Fig. 1A).
Activations due to tactile stimulation in the non-synesthetic group. (A) Somatosensory activations resulting from the comparison of the four touch conditions relative to rest baseline superimposed on a surface-rendered MR image. (B) SI activation resulting from the comparison of the two touch–face conditions relative to the two touch–neck conditions on a coronal section of a T1 image at y = −27. (C) Right SII activation resulting from the comparison of the two touch–left side conditions relative to the two touch–right side conditions on a coronal section of a T1 image at y = −18. (D) Left SI and SII activation resulting from the comparison of the two touch–right side conditions relative to the two touch–left side conditions shown on a coronal section of a T1 image at y = −18.
Main effect of touch to the face versus neck: [(RF + LF) − (RN + LN)]
Comparison of the two touch–face conditions relative to the two touch–neck conditions in the group of non-synesthetic subjects and in C resulted in activation of regions in SI corresponding to the head area, SII and the parietal cortex (Fig. 1B).
Main effect of touch to the right versus left: left side [(LF + LN) − (RF + RN)] and right side [(RF + RN) − (LF + LN)]
Comparison of the two touch–left side conditions relative to the two touch–right side conditions in the group of non-synesthetic subjects and in C resulted in right-sided activation of SI and SII (Fig. 1C). The contrast of the two touch–right side conditions relative to the two touch–left side conditions resulted in left-sided activation of SI and SII (Fig. 1D).
fMRI data: video sessions
Main effect of observation—baseline: [(HRF + HLF + HRN + HLN + ORF + OLF + ORN + OLN) − baseline]
In the group of non-synesthetic subjects and in C, comparison of the eight observation conditions relative to the baseline fixation resulted in activation of a number of occipital, temporal, parietal, premotor and motor regions. This is as would be expected.
Activations to the observation of touch in the non-synesthetic group
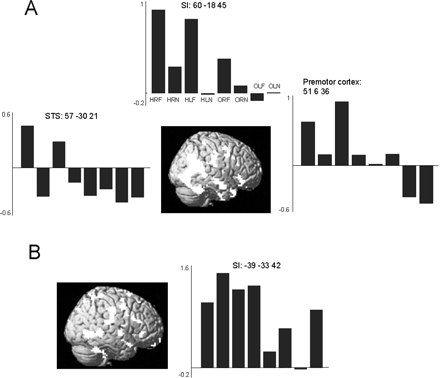
Main effect of observing touching humans relative to observing touching objects: [(HRF + HLF + HRN + HLN) − (ORF +OLF + ORN + OLN)]. In the non-synesthetic subjects, the main effect of observing touch to a human relative to observing touch to an object resulted in activation of the superior temporal sulcus (STS) starting at the temporo-parietal junction and following the STS down the temporal cortex. The STS activation was bilateral, but especially strong on the right. In addition, bilateral fusiform gyrus (including the fusiform face area; Kanwisher et al., 1997), SI, SII and premotor cortex were activated in this contrast (Table 3 and Fig. 2A).
(A) The main effect of observing touch to a human relative to observing touch to an object in the non-synesthetic group resulted in activation of bilateral STS at the temporo-parietal junction, fusiform gyrus, SI, SII and premotor cortex. These activations are shown superimposed on a rendered MR image. Plots show parameter estimates of the relative activation in each of the eight conditions expressed as percentage signal change in right SI, right STS and right premotor cortex. Condition labels as in top plot can be found in Table 1. (B) The main effect of observing touch to a human relative to observing touch to an object in C resulted in activation of the right STS, bilateral SI and SII, insula cortex, left anterior premotor cortex and right cerebellar cortex. These activations are shown superimposed on a rendered image. The plot shows parameter estimates of the relative activation in each of the eight conditions expressed as percentage signal change in left SI.
Activations resulting from the main effect of observing touch to a human relative to observing touch to an object in non-synesthetic group and in C
. | Coordinates . | Z . | Number of voxels . | |||
|---|---|---|---|---|---|---|
| Observing touch to human—observing touch to object in non-synesthetic group | ||||||
| Left occipital gyrus, bordering with superior temporal sulcus | −45 −72 3 | 4.17 | 205 | |||
| Right temporo-parietal junction, intraparietal sulcus and superior temporal sulcus | 60 −57 24 | 4.86 | 989 | |||
| Right fusiform gyrus | 45 −57 −27 | 4.24 | ||||
| Precuneus bordering posterior cingulate | 3 −57 36 | 4.42 | 446 | |||
| Right intraparietal sulcus | 42 −36 60 | 3.11 | 13 | |||
| Left superior temporal sulcus | −60 −21 −12 | 4.03 | 10 | |||
| Right postcentral gyrus (SI) | 66 −18 30 | 3.70 | 90 | |||
| Right parietal operculum (SII) | 63 −15 39 | 3.33 | ||||
| Right inferior frontal gyrus | 63 −18 15 | 3.97 | ||||
| Superior/middle frontal gyrus (premotor cortex) | 54 30 −6 | 3.25 | 27 | |||
| 426 39 | 4.42 | 62 | ||||
| Observing touch to human—observing touch to object in C | ||||||
| Right occipital gyrus | ||||||
| Right superior temporal sulcus | 60 −60 −3 | 6.36 | 76 | |||
| Right intraparietal sulcus | 69 −45 0 | 4.79 | ||||
| Left postcentral gyrus (SI) | 42 −42 66 | 4.41 | 10 | |||
| Right postcentral gyrus (SI) | −39 −33 42 | 6.28 | 31 | |||
| Left parietal operculum (SII) | 54 −21 39 | 5.60 | 31 | |||
| Right parietal operculum (SII) | −57 −30 21 | 5.53 | 75 | |||
| Right cerebellum | 60 −30 18 | 3.96 | 11 | |||
| Left precentral gyrus/premotor cortex | 45 −51 −33 | 5.18 | 17 | |||
| −540 33 | 5.18 | 22 | ||||
. | Coordinates . | Z . | Number of voxels . | |||
|---|---|---|---|---|---|---|
| Observing touch to human—observing touch to object in non-synesthetic group | ||||||
| Left occipital gyrus, bordering with superior temporal sulcus | −45 −72 3 | 4.17 | 205 | |||
| Right temporo-parietal junction, intraparietal sulcus and superior temporal sulcus | 60 −57 24 | 4.86 | 989 | |||
| Right fusiform gyrus | 45 −57 −27 | 4.24 | ||||
| Precuneus bordering posterior cingulate | 3 −57 36 | 4.42 | 446 | |||
| Right intraparietal sulcus | 42 −36 60 | 3.11 | 13 | |||
| Left superior temporal sulcus | −60 −21 −12 | 4.03 | 10 | |||
| Right postcentral gyrus (SI) | 66 −18 30 | 3.70 | 90 | |||
| Right parietal operculum (SII) | 63 −15 39 | 3.33 | ||||
| Right inferior frontal gyrus | 63 −18 15 | 3.97 | ||||
| Superior/middle frontal gyrus (premotor cortex) | 54 30 −6 | 3.25 | 27 | |||
| 426 39 | 4.42 | 62 | ||||
| Observing touch to human—observing touch to object in C | ||||||
| Right occipital gyrus | ||||||
| Right superior temporal sulcus | 60 −60 −3 | 6.36 | 76 | |||
| Right intraparietal sulcus | 69 −45 0 | 4.79 | ||||
| Left postcentral gyrus (SI) | 42 −42 66 | 4.41 | 10 | |||
| Right postcentral gyrus (SI) | −39 −33 42 | 6.28 | 31 | |||
| Left parietal operculum (SII) | 54 −21 39 | 5.60 | 31 | |||
| Right parietal operculum (SII) | −57 −30 21 | 5.53 | 75 | |||
| Right cerebellum | 60 −30 18 | 3.96 | 11 | |||
| Left precentral gyrus/premotor cortex | 45 −51 −33 | 5.18 | 17 | |||
| −540 33 | 5.18 | 22 | ||||
Activations resulting from the main effect of observing touch to a human relative to observing touch to an object in non-synesthetic group and in C
. | Coordinates . | Z . | Number of voxels . | |||
|---|---|---|---|---|---|---|
| Observing touch to human—observing touch to object in non-synesthetic group | ||||||
| Left occipital gyrus, bordering with superior temporal sulcus | −45 −72 3 | 4.17 | 205 | |||
| Right temporo-parietal junction, intraparietal sulcus and superior temporal sulcus | 60 −57 24 | 4.86 | 989 | |||
| Right fusiform gyrus | 45 −57 −27 | 4.24 | ||||
| Precuneus bordering posterior cingulate | 3 −57 36 | 4.42 | 446 | |||
| Right intraparietal sulcus | 42 −36 60 | 3.11 | 13 | |||
| Left superior temporal sulcus | −60 −21 −12 | 4.03 | 10 | |||
| Right postcentral gyrus (SI) | 66 −18 30 | 3.70 | 90 | |||
| Right parietal operculum (SII) | 63 −15 39 | 3.33 | ||||
| Right inferior frontal gyrus | 63 −18 15 | 3.97 | ||||
| Superior/middle frontal gyrus (premotor cortex) | 54 30 −6 | 3.25 | 27 | |||
| 426 39 | 4.42 | 62 | ||||
| Observing touch to human—observing touch to object in C | ||||||
| Right occipital gyrus | ||||||
| Right superior temporal sulcus | 60 −60 −3 | 6.36 | 76 | |||
| Right intraparietal sulcus | 69 −45 0 | 4.79 | ||||
| Left postcentral gyrus (SI) | 42 −42 66 | 4.41 | 10 | |||
| Right postcentral gyrus (SI) | −39 −33 42 | 6.28 | 31 | |||
| Left parietal operculum (SII) | 54 −21 39 | 5.60 | 31 | |||
| Right parietal operculum (SII) | −57 −30 21 | 5.53 | 75 | |||
| Right cerebellum | 60 −30 18 | 3.96 | 11 | |||
| Left precentral gyrus/premotor cortex | 45 −51 −33 | 5.18 | 17 | |||
| −540 33 | 5.18 | 22 | ||||
. | Coordinates . | Z . | Number of voxels . | |||
|---|---|---|---|---|---|---|
| Observing touch to human—observing touch to object in non-synesthetic group | ||||||
| Left occipital gyrus, bordering with superior temporal sulcus | −45 −72 3 | 4.17 | 205 | |||
| Right temporo-parietal junction, intraparietal sulcus and superior temporal sulcus | 60 −57 24 | 4.86 | 989 | |||
| Right fusiform gyrus | 45 −57 −27 | 4.24 | ||||
| Precuneus bordering posterior cingulate | 3 −57 36 | 4.42 | 446 | |||
| Right intraparietal sulcus | 42 −36 60 | 3.11 | 13 | |||
| Left superior temporal sulcus | −60 −21 −12 | 4.03 | 10 | |||
| Right postcentral gyrus (SI) | 66 −18 30 | 3.70 | 90 | |||
| Right parietal operculum (SII) | 63 −15 39 | 3.33 | ||||
| Right inferior frontal gyrus | 63 −18 15 | 3.97 | ||||
| Superior/middle frontal gyrus (premotor cortex) | 54 30 −6 | 3.25 | 27 | |||
| 426 39 | 4.42 | 62 | ||||
| Observing touch to human—observing touch to object in C | ||||||
| Right occipital gyrus | ||||||
| Right superior temporal sulcus | 60 −60 −3 | 6.36 | 76 | |||
| Right intraparietal sulcus | 69 −45 0 | 4.79 | ||||
| Left postcentral gyrus (SI) | 42 −42 66 | 4.41 | 10 | |||
| Right postcentral gyrus (SI) | −39 −33 42 | 6.28 | 31 | |||
| Left parietal operculum (SII) | 54 −21 39 | 5.60 | 31 | |||
| Right parietal operculum (SII) | −57 −30 21 | 5.53 | 75 | |||
| Right cerebellum | 60 −30 18 | 3.96 | 11 | |||
| Left precentral gyrus/premotor cortex | 45 −51 −33 | 5.18 | 17 | |||
| −540 33 | 5.18 | 22 | ||||
Common activations between touch and observation conditions in the non-synesthetic group
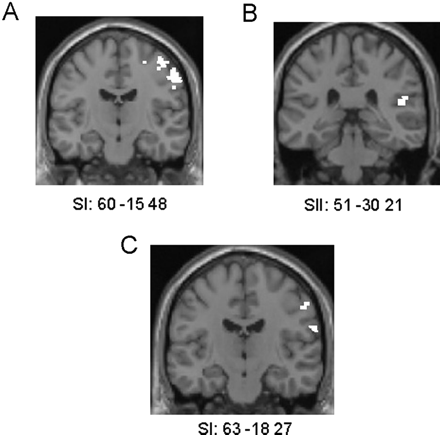
Observing touch to a human face masked with touch to subject's face: [(HRF + HLF) − (HRN + HLN)] inclusively masked with [(RF + LF) − (RN + LN)]. This contrast resulted in activation of the head area of SI located in the anterior wall of the right postcentral gyrus in the non-synesthetic group (Table 4 and Fig. 3A).
(A) Activations in SI head area resulting from the comparison of observing touch to a human face (relative to a human neck) masked with touch to the subject's face (relative to their neck) in the non-synesthetic group, shown on a coronal section of a T1 image at y = −15. (B) Activations in right SII resulting from the comparison of observing touch to the left side of a human (relative the right side) masked with touch to the subject's left side (relative to their right side) in the non-synesthetic group, shown on a coronal section of a T1 image at y = −30. (C) Activations in SI head area resulting from the comparison of observing touch to a human face (relative to a human neck) masked with touch to the subject's face (relative to neck) in C, shown on a coronal section of a T1 image at y = −18.
Observing touch to a human face masked with touch to subject's face: [(HRF + HLF) − (HRN + HLN)] inclusively masked with [(RF + LF) − (RN + LN)]
. | Coordinates . | Z . | Number of voxels . | |||
|---|---|---|---|---|---|---|
| Observing human head—neck masked with touch to head—neck in non-synesthetic group | ||||||
| Right postcentral gyrus (SI head area) | 30 −48 66 | 4.05 | 260 | |||
| Right precentral gyrus | 57 −15 45 | 3.92 | 84 | |||
| Right superior frontal gyrus | 27 −9 69 | 3.44 | 27 | |||
| Observing human head—neck masked with touch to head—neck in C | ||||||
| Right postcentral gyrus (SI) | 63 −18 27 | 2.38 | 10 | |||
| Right parietal operculum (SII) | 51 −24 18 | 1.55 | 3 | |||
| Right precentral gyrus | 57 −15 42 | 2.67 | 4 | |||
. | Coordinates . | Z . | Number of voxels . | |||
|---|---|---|---|---|---|---|
| Observing human head—neck masked with touch to head—neck in non-synesthetic group | ||||||
| Right postcentral gyrus (SI head area) | 30 −48 66 | 4.05 | 260 | |||
| Right precentral gyrus | 57 −15 45 | 3.92 | 84 | |||
| Right superior frontal gyrus | 27 −9 69 | 3.44 | 27 | |||
| Observing human head—neck masked with touch to head—neck in C | ||||||
| Right postcentral gyrus (SI) | 63 −18 27 | 2.38 | 10 | |||
| Right parietal operculum (SII) | 51 −24 18 | 1.55 | 3 | |||
| Right precentral gyrus | 57 −15 42 | 2.67 | 4 | |||
Observing touch to a human face masked with touch to subject's face: [(HRF + HLF) − (HRN + HLN)] inclusively masked with [(RF + LF) − (RN + LN)]
. | Coordinates . | Z . | Number of voxels . | |||
|---|---|---|---|---|---|---|
| Observing human head—neck masked with touch to head—neck in non-synesthetic group | ||||||
| Right postcentral gyrus (SI head area) | 30 −48 66 | 4.05 | 260 | |||
| Right precentral gyrus | 57 −15 45 | 3.92 | 84 | |||
| Right superior frontal gyrus | 27 −9 69 | 3.44 | 27 | |||
| Observing human head—neck masked with touch to head—neck in C | ||||||
| Right postcentral gyrus (SI) | 63 −18 27 | 2.38 | 10 | |||
| Right parietal operculum (SII) | 51 −24 18 | 1.55 | 3 | |||
| Right precentral gyrus | 57 −15 42 | 2.67 | 4 | |||
. | Coordinates . | Z . | Number of voxels . | |||
|---|---|---|---|---|---|---|
| Observing human head—neck masked with touch to head—neck in non-synesthetic group | ||||||
| Right postcentral gyrus (SI head area) | 30 −48 66 | 4.05 | 260 | |||
| Right precentral gyrus | 57 −15 45 | 3.92 | 84 | |||
| Right superior frontal gyrus | 27 −9 69 | 3.44 | 27 | |||
| Observing human head—neck masked with touch to head—neck in C | ||||||
| Right postcentral gyrus (SI) | 63 −18 27 | 2.38 | 10 | |||
| Right parietal operculum (SII) | 51 −24 18 | 1.55 | 3 | |||
| Right precentral gyrus | 57 −15 42 | 2.67 | 4 | |||
Observing touch to the left side of a human masked with touch to the subject's left side
[(HLF + HLN) − (HRF + HRN)] inclusively masked with [(LF + LN) − (RF + RN)]. This contrast resulted in activation of right SII in the non-synesthetic group (98 voxel cluster at 51 −30 21; Z = 3.23) (Fig. 3B). The contrast observing touch to the right side of a human masked with touch to the subject's right side [(HRF + HRN) − (HLF + HLN)] inclusively masked with [(RF + RN) − (LF + LN)] did not reveal any significant activation.
Activations to the observation of touch in C
Main effect of observing touching humans relative to observing touching objects: [(HRF + HLF + HRN + HLN) − (ORF + OLF + ORN + OLN)]. In C, this contrast resulted in activation of the right STS, bilateral SI and SII, bilateral insula cortex, left anterior premotor cortex and right cerebellar cortex (Table 3 and Fig. 2B).
Observing touch to a human face masked with touch to subject's face
[(HRF + HLF) − (HRN + HLN)] inclusively masked with [(RF + LF) − (RN + LN)]. This contrast resulted in activation of the head area of SI located in the anterior wall of the right postcentral gyrus in C (Table 4 and Fig. 3C).
Observing touch to the left or right side of a human masked with touch to the subject's left or right side
[(HLF + HLN) − (HRF + HRN)] inclusively masked with [(LF + LN) − (RF + RN)] and vice versa. There was no significant activation in these masked contrasts for C, possibly because of lack of power.
Comparison between synesthetic and non-synesthetic subjects
There was a significant difference between C and the non-synesthetes for the contrast Observation of touch to a human versus observation of touch to an object in bilateral SI and SII, the anterior insula and the left premotor cortex. These regions were significantly more active in C than in the control group during the observation of touch to a human relative to an object (Table 5 and Fig. 4).
Activations resulting from the comparison between C and the non-synesthetes for the contrast Observation of touch to a human versus observation of touch to an object. (A) Bilateral SI, SII, anterior insula and left premotor cortex were significantly more active in C than in the non-synesthetic group during the observation of touch to a human relative to an object. The left sided-activations are superimposed on a rendered MR image. (B) Bilateral insula activations resulting from this comparison superimposed on a coronal section of a T1 image at y = 0.
Activations resulting from the comparison between C and the non-synesthetes for the contrast Observation of touch to a human versus Observation of touch to an object*
. | Coordinates . | Z . | Number of voxels . | |||
|---|---|---|---|---|---|---|
| C > non-synesthetes for observing human > observing object | ||||||
| Left supramarginal gyrus | −60 −27 30 | 3.52 | 20 | |||
| Left parietal operculum (SII) | −60 −33 18 | 3.55 | 29 | |||
| Right parietal operculum (SII) | 60 −30 18 | 2.95 | 9 | |||
| Left postcentral gyrus (SI) | −39 −33 42 | 3.09 | 11 | |||
| Right postcentral gyrus/central sulcus | 54 −24 39 | 3.29 | 8 | |||
| Left anterior insula cortex | −45 −3 −6 | 3.65 | 67 | |||
| Right anterior insula cortex | 45 0 −3 | 2.95 | 11 | |||
| Left premotor cortex/frontal operculum (Broca's area) | −60 6 18 | 3.31 | 29 | |||
. | Coordinates . | Z . | Number of voxels . | |||
|---|---|---|---|---|---|---|
| C > non-synesthetes for observing human > observing object | ||||||
| Left supramarginal gyrus | −60 −27 30 | 3.52 | 20 | |||
| Left parietal operculum (SII) | −60 −33 18 | 3.55 | 29 | |||
| Right parietal operculum (SII) | 60 −30 18 | 2.95 | 9 | |||
| Left postcentral gyrus (SI) | −39 −33 42 | 3.09 | 11 | |||
| Right postcentral gyrus/central sulcus | 54 −24 39 | 3.29 | 8 | |||
| Left anterior insula cortex | −45 −3 −6 | 3.65 | 67 | |||
| Right anterior insula cortex | 45 0 −3 | 2.95 | 11 | |||
| Left premotor cortex/frontal operculum (Broca's area) | −60 6 18 | 3.31 | 29 | |||
These regions were significantly more active in C than in the non-synesthetic group during the observation of touch to a human relative to an object.
Activations resulting from the comparison between C and the non-synesthetes for the contrast Observation of touch to a human versus Observation of touch to an object*
. | Coordinates . | Z . | Number of voxels . | |||
|---|---|---|---|---|---|---|
| C > non-synesthetes for observing human > observing object | ||||||
| Left supramarginal gyrus | −60 −27 30 | 3.52 | 20 | |||
| Left parietal operculum (SII) | −60 −33 18 | 3.55 | 29 | |||
| Right parietal operculum (SII) | 60 −30 18 | 2.95 | 9 | |||
| Left postcentral gyrus (SI) | −39 −33 42 | 3.09 | 11 | |||
| Right postcentral gyrus/central sulcus | 54 −24 39 | 3.29 | 8 | |||
| Left anterior insula cortex | −45 −3 −6 | 3.65 | 67 | |||
| Right anterior insula cortex | 45 0 −3 | 2.95 | 11 | |||
| Left premotor cortex/frontal operculum (Broca's area) | −60 6 18 | 3.31 | 29 | |||
. | Coordinates . | Z . | Number of voxels . | |||
|---|---|---|---|---|---|---|
| C > non-synesthetes for observing human > observing object | ||||||
| Left supramarginal gyrus | −60 −27 30 | 3.52 | 20 | |||
| Left parietal operculum (SII) | −60 −33 18 | 3.55 | 29 | |||
| Right parietal operculum (SII) | 60 −30 18 | 2.95 | 9 | |||
| Left postcentral gyrus (SI) | −39 −33 42 | 3.09 | 11 | |||
| Right postcentral gyrus/central sulcus | 54 −24 39 | 3.29 | 8 | |||
| Left anterior insula cortex | −45 −3 −6 | 3.65 | 67 | |||
| Right anterior insula cortex | 45 0 −3 | 2.95 | 11 | |||
| Left premotor cortex/frontal operculum (Broca's area) | −60 6 18 | 3.31 | 29 | |||
These regions were significantly more active in C than in the non-synesthetic group during the observation of touch to a human relative to an object.
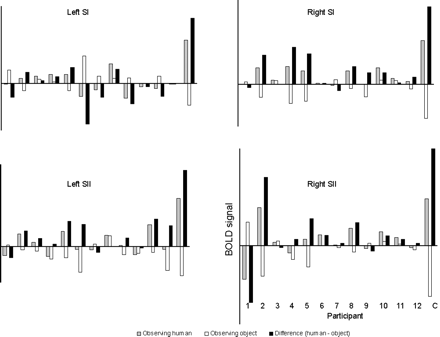
To ensure that C's activations to the observation of touch to a human were not simply significantly higher than the mean of the non-synesthetic group's activations, we plotted individual responses in left and right SI and SII for each subject (Fig. 5). This demonstrated that activity in these regions was higher in C than in any of the non-synesthetic subjects during the observation of touch to a human relative to touch to an object.
Plots showing individual subject neural activity during the conditions in which subjects observed humans being touched (grey bars), the conditions in which they observed objects being touched (white bars), and the difference between activity for the two types of video (Human–Object) (black bars). Activity was mean-corrected for each individual to control for the subject's overall brain activity. Activity is shown for each individual subject in the non-synesthetic group (1–12), and for C, in SI and SII in both hemispheres. The plots indicate that activity in these regions was higher during observation of touch to a human (but not to an object) in C than in all the non-synesthetic individuals.
Discussion
In this study, we investigated the neural systems associated with the observation of touch. The results of the fMRI study demonstrate that, in non-synesthetic subjects, a number of regions including primary and secondary somatosensory cortices are activated by the mere observation of touch to another human (relative to observation of touch to an object). The somatosensory activations to the observation of touch were somatotopically organized, following the classical sensory homunculus in SI (Disbrow et al., 2000). In the non-synesthetic subjects, somatosensory activations were present in the absence of any perception of touch. However, in this study we also investigated a subject, C, who experiences tactile stimulation on her own body when she observes other people being touched. Our fMRI study was designed to investigate the difference in the neural systems that are activated by the observation of touch in this synesthetic subject, who experiences observed touch as tactile stimulation on herself, compared with the control subjects who experience no such synesthetic perception. The somatosensory activation induced by the observation of touch to a human was significantly greater in C, who felt the observed tactile stimulation on her own body, than in the non-synesthetic group. In addition, C showed a higher activation than in the non-synesthetic group in premotor cortex and insula cortex during the observation of touch to a human.
Observation of touch in the brain
Our study demonstrates that the observation of touch to another person's head or neck activates the STS at the temporo-parietal junction, especially on the right, fusiform gyrus (in the region of the fusiform face area; Kanwisher et al., 1997), bilateral SI and SII, and premotor cortex. These regions were activated more by the observation of a human's head or neck being touched than by the observation of a similarly shaped object being touched.
The fusiform gyrus and the STS are typically activated by the visual presentation of faces (Kanwisher et al., 1997). Single-cell studies in the monkey homologue of human STS have identified cells that respond selectively to faces (Baylis et al., 1985; Perrett et al., 1992). In the human brain, the STS is activated by faces, in particular by facial movements (Puce et al., 1998; Wicker et al., 1998). The STS is also part of the brain's mirror system. It is often activated when subjects observe human action and biological motion (Grossman et al., 2000; Grèzes et al., 2001) and, as such, might form part of the mirror system (Rizzolatti et al., 2001). The STS activation in the current study was stronger in the right than in the left hemisphere, which is consistent with previous neuroimaging studies of biological motion (Grossman et al., 2000; Grèzes et al., 2001).
The premotor cortex has similar mirror properties. In monkeys, the premotor cortex contains neurons that respond both to the execution and the observation of action (Gallese et al., 1996). The human premotor cortex has been activated in neuroimaging studies in which subjects observe a range of different actions (Grafton et al., 1996, Rizzolatti et al., 1996, Buccino et al., 2001). It is interesting to note that, in the current experiment, the STS and premotor cortex were activated significantly more by the observation of a human head being touched than by objects being touched. Both the object and human videos contained movement of a human hand (doing the touching), the only difference being the presence of a human face in the human videos. This raises the intriguing possibility that mirror areas are preferentially activated by ‘social’ actions, i.e. actions directed towards other humans. The selectivity of mirror areas for biological agents (rather than inanimate devices) has been suggested (Tai et al., 2004). Our results imply that mirror areas are not only selective for biological actors, but are also preferentially activated when the target of the action is biological.
The intraparietal sulcus contains bimodal cells responsive both to vision and tactile stimulation (Bremmer et al., 1991; Macaluso et al., 2003). The activation of the intraparietal sulcus during the observation of touch to a human might represent responses of these bimodal cells. Alternatively, it is possible that the intraparietal activations reflect activity of neurons that respond to visual stimulation only (Grefkes et al., 2002). SI, comprising areas 1, 2 and 3 in the postcentral sulcus, and SII, located in the parietal operculum in the posterior insula, respond to tactile stimulation (Burton et al., 1993; Del Gratta et al., 2000; Disbrow et al., 2000). It is remarkable that both SI and SII were activated by the mere observation of touch to a human in the current study. This is in line with a recent study demonstrating that observing touch to a person's legs activates SII (Keysers et al., 2004). However, there are several notable differences between the current study and the study by Keysers et al. (2004). First, the videos in the current study depicted touch to the face and neck, whereas the videos in the study by Keysers and colleagues depicted touch to the legs. Secondly, the somatosensory activations here were significantly higher for the observation of touch to a human than to an object. In contrast, SII activation in the study by Keysers and colleagues was found both for observation of touch to human legs and to cylindrical objects.
Furthermore, we found significant activation of SI to the observation of touch to a human. SI activation was somatotopically organized according to which area of the body was observed being touched. The head area of SI, located on the anterior wall of the postcentral gyrus, was activated both by being touched on the face (versus on the neck) and by observing another human being touched on the face (relative to the neck). Keysers et al. (2004) report a non-significant trend towards SI activation to the observation of touch. One possible explanation for the differences between the current study and the one by Keysers and colleagues is that the presence of a human face in the videos used here triggers especially strong and somatotopically organized somatosensory activations (Fig. 2A).
The lateralization that occurred when being touched to one side was also present in SI when observing touch to the same side. SI lateralization to the observation of touch was same-sided rather than being the mirror image of the side being touched. In other words, observing touch to the left side of a human face or neck activated right SI, which is the side of SI activated when being touched on the left side. Such a finding is consistent with studies recording motor evoked potentials (MEPs) evoked by transcranial magnetic stimulation (TMS). MEP threshold is lowered specifically in those muscles that are activated during the observed action (Fadiga et al., 1995). Each hemisphere is more strongly activated when viewing actions conducted by a model's contralateral hand than when viewing actions conducted by an ipsilateral hand (Aziz-Zadeh et al., 2002). Larger MEPs were produced in the right hand when right- rather than left-hand actions were observed, while left-hand MEPs increased only during observation of left-hand movements.
Given the existence of mirror systems in several modalities including action (Rizzolatti et al., 2001), emotion (Carr et al., 2003) and pain (Singer et al., 2004), our data suggest that a similar mirror system exists for the observation of touch. However, one possibility is that the somatosensory activity to the observation of touch observed in our study merely represents tactile imagery. There is neuroimaging evidence that SI and SII are activated by anticipation of touch in the absence of any tactile stimulation (Carlsson et al., 2000). On the other hand, the additional activation in premotor cortex suggests, at least in part, an involvement of the mirror system in this phenomenon. Furthermore, no subject in the non-synesthetic group reported imagining the touch in any of the conditions. Whether the activations to the observation of touch in the current study represent a tactile mirror system or tactile imagery, or whether these systems are one and the same, remains to be investigated.
Mirrored touch synaesthesia
As in the non-synesthetic group, somatosensory, parietal and premotor activation in C was significantly higher during the observation of touch to a human than touch to an object. The somatosensory activation induced by the observation of touch to a human was significantly greater in C than in the non-synesthetic group. Meanwhile, unlike the individuals in the non-synesthetic group, C experienced the observed touch to a human face or neck as tactile stimulation on her own face or neck.
In the Introduction, we outlined three possible explanations for C's synesthetic experiences. First, there could be increased activity in the tactile mirror system, demonstrated in the non-synesthetic subjects in this study and by Keysers et al. (2004), above a threshold for conscious tactile perception. Secondly, the existence of direct connections between C's visual and somatosensory areas could account for the difference between C and the non-synesthetic subjects. This would be akin to the findings that the human visual colour centre is activated by heard words in grapheme-colour synesthetes (Nunn et al., 2002). Thirdly, hyperactivation of bimodal visual-tactile cells could be sufficient to give rise to illusory touch in C.
Although bimodal visual-tactile cells, for example in parietal cortex, may be important in giving rise to mirrored touch synaesthesia, they are unlikely to be the only cells involved because of the changes in activation observed elsewhere in the mirror-touch system, including SI regions. The second account also seems unlikely. If there were direct connections between visual and somatosensory cortices in C, then activity in somatosensory regions would be predicted in C but not necessarily activity in the other areas found here. Nor would we expect to see any somatosensory activation in our normal non-synesthetic subjects when they observe touch, as they would not have the hypothesized direct connections.
The first account is favoured on the basis of the empirical evidence from this study. In most people, it is possible that the somatosensory mirror system, which matches observed and felt touch, is involved in understanding the effect of tactile stimulation on others. This system is normally active below a certain threshold such that no conscious perception of tactile stimulation is experienced. One possibility is that this system is activated above that perceptual threshold in C whenever she observes touch to another person. In this case, rather than simply allowing C to understand the tactile stimulation she is observing, C perceives it as if she were the receiver of it. In support of this supposition, activity in bilateral SI and SII was higher in C's brain than in any of the non-synesthetic subjects during the observation of touch to a human relative to touch to an object (Fig. 5). In other words, SI and SII activity in C was not only significantly higher than the mean activity in these regions in the non-synesthetic population; C's activations were also higher than all individuals within the non-synesthetic group.
In addition to somatosensory activity during the observation of touch to a human, C showed a higher activation in left premotor cortex, in the vicinity of Broca's area in the frontal operculum, in this contrast than did the non-synesthetic group. We propose that this higher activation of premotor cortex represents overactivity of the action mirror system in C. It is possible that, when C observes action, her mirror system is activated to a greater extent than in most people.
This idea of a threshold for conscious perception is supported by several studies showing that consciousness of visual stimuli is associated with greater activity in ventral visual cortex, but that unconscious processing also activates the same region (e.g. Beck et al., 2001; for a review, see Rees et al., 2002). Given the somatotopic activation of SI and SII during the observation of touch in the non-synesthetic group as well as in C, however, this threshold hypothesis may be too simple to account for why the non-synesthetic group, and indeed most people, never perceive observed touch. Presumably the touch mirror system could occasionally be activated above threshold even in normal non-synesthetic subjects. Even though C's somatosensory activations were significantly higher than the activations in the non-synesthetic group, this may not explain why C feels observed touch whereas there was no hint of feeling observed touch in any of the non-synesthetic subjects. It would be surprising if there were no special regions associated exclusively with the conscious experience of touch.
One possible region that mediates the conscious perception of touch on oneself during the observation of touch is the anterior insula. This region was bilaterally activated in C during the observation of touch, but was not activated during the same condition in the non-synesthetic group. The anterior insula contains tactile receptive fields (Burton et al., 1993; Olausson et al., 2002). Furthermore, the insula is associated with self-processing. The anterior insula was activated in neuroimaging studies in which subjects imagined themselves performing actions relative to someone else performing the same action (Ruby and Decety, 2001), looked at pictures of their own face (Kircher et al., 2001) and identified their own memories (Fink et al., 1996). Farrer and Frith (2002) found activation of a very similiar region of the anterior insula cortex, in both hemispheres, when subjects attribute actions to themselves rather than to another person. Given its role in attribution to the self, it is possible that the anterior insula activity found in C in our study, along with overactivation of the touch mirror system, accounts for why she perceives herself as the direct target of the observed touch.
This research was funded by the Wellcome Trust and the Royal Society, UK. The authors are grateful to C for her participation in this study.
References
Aziz-Zadeh L, Maeda F, Zaidel E, Mazziotta J, Iacoboni M. Lateralization in motor facilitation during action observation: a TMS study.
Bailey MES, Johnson KJ. Synaesthesia: is a genetic analysis feasible? In: Baron-Cohen S, Harrison JE, editors. Synaesthesia: classic and contemporary readings. Massachusetts: Blackwell Publishers;
Baron-Cohen S, Burt L, Smith-Laittan F, Harrison J, Bolton P. Synaesthesia: prevalence and familiality.
Baylis GC, Rolls ET, Leonard CM. Selectivity between faces in the responses of a population of neurons in the cortex in the superior temporal sulcus of the monkey.
Beck DM, Rees G, Frith CD, Lavie N. Neural correlates of change detection and change blindness.
Bradshaw JL, Mattingley JB. Allodynia: a sensory analogue of motor mirror neurons in a hyperaesthetic patient reporting instantaneous discomfort to another's perceived sudden minor injury?
Bremmer F, Schlack A, Shah NJ, Zafiris O, Kubischik M, Hoffmann K, et al. Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys.
Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, et al. Action observation activates premotor and parietal areas in somatotopic manner: an fMRI study.
Burton H, Videen TO, Raichle ME. Tactile-vibration-activated foci in insular and parietal-opercular cortex studied with positron emission tomography: mapping the second somatosensory area in humans.
Carlsson K, Petrovic P, Skare S, Petersson KM, Ingvar M. Tickling expectations: neural processing in anticipation of a sensory stimulus.
Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas.
Del Gratta C, Della Penna S, Tartaro A, Ferretti A, Torquati K, Bonomo L, et al. Topographic organization of the human primary and secondary somatosensory areas: an fMRI study.
Disbrow E, Roberts T, Krubitzer L. Somatotopic organization of cortical fields in the lateral sulcus of Homo sapiens: evidence for SII and PV.
Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study.
Farrer C, Frith CD. Experiencing oneself vs another person as being the cause of an action: the neural correlates of the experience of agency.
Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss WD. Cerebral representation of one's own past: neural networks involved in autobiographical memory.
Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach.
Friston KJ, Ashburner J, Frith CD, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images.
Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex.
Grafton ST, Arbib MA, Fadiga L, Rizzolatti G. Localization of grasp representations in humans by positron emission tomography. 2. Observation compared with imagination.
Grefkes C, Weiss PH, Zilles K, Fink GR. Crossmodal processing of object features in human anterior intraparietal cortex: an fMRI study implies equivalencies between humans and monkeys.
Grossman E, Donnelly M, Price R, Pickens D, Morgan V, Neighbor G, et al. Brain areas involved in perception of biological motion.
Grèzes J, Fonlupt P, Bertenthal B, Delon-Martin C, Segebarth C, Decety J. Does perception of biological motion rely on specific brain regions?
Halligan PW, Hunt M, Marshall JC, Wade DT. When seeing is feeling: acquired synaesthesia or phantom touch?
Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception.
Keysers C, Wicker B, Gazzola V, Anton JL, Fogassi L, Gallese V. A touching sight: SII/PV activation during the observation and experience of touch.
Kircher TT, Senior C, Phillips ML, Rabe-Hesketh S, Benson PJ, Bullmore ET, et al. Recognizing one's own face.
Macaluso E, Driver J. Multimodal spatial representations in the human parietal cortex: evidence from functional imaging.
Nunn JA, Gregory LJ, Brammer M, Williams SC, Parslow DM, Morgan MJ, et al. Functional magnetic resonance imaging of synesthesia: activation of V4/V8 by spoken words.
Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, et al. Unmyelinated tactile afferents signal touch and project to insular cortex.
Perrett DI, Hietanen JK, Oram MW, Benson PJ. Organization and functions of cells responsive to faces in the temporal cortex.
Puce A, Allison T, Bentin S, Gore JC, McCarthy G. Temporal cortex activation in humans viewing eye and mouth movements.
Ramachandran VS, Hubbard EM. Psychophysical investigations into the neural basis of synaesthesia.
Rees G, Kreiman G. Koch C. Neural correlates of consciousness in humans.
Rizzolatti, G, Fadiga L, Matelli M, Bettinardi V, Perani D, Fazio F. Localization of grasp representations in humans by PET. 1. Observation versus execution.
Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action.
Ruby P, Decety J. Effect of subjective perspective taking during simulation of action: a PET investigation of agency.
Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain.
Tai YF, Scherfler C, Brooks DJ, Sawamoto N, Castiello U. The human premotor cortex is ‘mirror’ only for biological actions.
Ward J, Simner J, Auyeung V. A comparison of lexical-gustatory and grapheme-colour synesthesia. Cogn Neuropsychology. In press,
Wicker B, Michel F, Henaff MA, Decety J. Brain regions associated with mutual gaze: a PET study.
Author notes
1Institute of Cognitive Neuroscience, 2Wellcome Department of Imaging Neuroscience and 3Department of Psychology, University College London, London, UK