Abstract
We aimed to define the chronically altered gene expression signature of traumatic brain injury (TBI-sig) to discover novel treatments to reverse pathologic gene expression or reinforce the expression of recovery-related genes. Genome-wide RNA-sequencing was performed at 3 months post-TBI induced by lateral fluid-percussion injury in rats. We found 4964 regulated genes in the perilesional cortex and 1966 in the thalamus (FDR < 0.05). TBI-sig was used for a LINCS analysis which identified 11 compounds that showed a strong connectivity with the TBI-sig in neuronal cell lines. Of these, celecoxib and sirolimus were recently reported to have a disease-modifying effect in in vivo animal models of epilepsy. Other compounds revealed by the analysis were BRD-K91844626, BRD-A11009626, NO-ASA, BRD-K55260239, SDZ-NKT-343, STK-661558, BRD-K75971499, ionomycin and desmethylclomipramine. Network analysis of overlapping genes revealed the effects on tubulins (Tubb2a, Tubb3, Tubb4b), Nfe2l2, S100a4, Cd44 and Nfkb2, all of which are linked to TBI-relevant outcomes, including epileptogenesis and tissue repair. Desmethylclomipramine modulated most of the gene targets considered favorable for TBI outcome. Our data demonstrate long-lasting transcriptomics changes after TBI. LINCS analysis predicted that these changes could be modulated by various compounds, some of which are already in clinical use but never tested in TBI.
Similar content being viewed by others
Introduction
Traumatic brain injury (TBI) affects 2.5 million people in Europe and USA and leads to chronic disabilities in over 40% of affected patients1,2,3. Despite a large number of preclinical and clinical studies, there are currently no pharmacotherapies that improve post-TBI outcome4,5,6 and treatment of TBI remains a major unmet clinical need. The current lack of pharmacotherapies relates to our limited understanding of the molecular changes occurring at the acute, subacute and chronic phases that could serve as stage-dependent treatment targets for this heterogeneous condition7.
Genome-wide gene expression studies performed to date have largely focused on analysis of the perilesional cortex and ipsilateral hippocampus8,9,10 and only one study has reported findings from the thalamus11. Almost all studies have exclusively assessed the transcriptomics changes at the acute post-TBI phase, i.e., within 24–48 h post-injury8,10,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29 and therefore provide limited information about the mechanisms that underlie functional recovery and epileptogenesis, which occur weeks to months post-TBI30. Some studies demonstrated changes in the genes involved in inflammation8,10,16 and apoptosis9, most likely injury-related molecular changes. In silico analyses of the transcriptomics data, a strategy used to evaluate and identify new drug candidates, e.g., for the treatment of cancer31,32, have not yet been performed to reveal novel treatment targets for TBI.
To gain a better understanding of the chronic post-TBI molecular changes that could serve as treatment targets to combat post-TBI impairment, we first analyzed the transcriptomics signature of the TBI (TBI-sig) in the perilesional cortex, as well as in the ipsilateral thalamus and hippocampus in a clinically relevant animal model of closed-head TBI at 3 months after trauma. The datasets were applied to identify novel drug candidates by performing a Library of Integrated Cellular Signatures (LINCS) analysis on the TBI-sig.
Results
GW transcriptomics profile of the perilesional cortex, thalamus and hippocampus after TBI
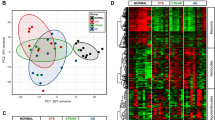
Three months after TBI, differential expression of 4964 genes in the perilesional cortex (2583 upregulated and 2381 downregulated) and 1966 in the thalamus (1144 upregulated and 822 downregulated) was observed compared to sham-operated animals (Fig. 1A). In the hippocampus, only one gene, Npy2r, was downregulated. In the perilesional cortex and thalamus, we found 480 commonly upregulated (Fig. 1B) and 841 commonly downregulated genes (Fig. 1C). The hippocampus shared no common alterations with the other brain areas.
Transcriptomics profile of the perilesional cortex, ipsilateral thalamus and ipsilateral hippocampus at 3 months after lateral fluid-percussion-induced traumatic brain injury (TBI) in rat.
(A) A circos plot showing the chromosomal location of differentially expressed genes in the cortex (outermost circle), thalamus (middle) and hippocampus (innermost). We found 4964 differentially expressed genes in the cortex, 1966 in the thalamus and only 1 in the hippocampus. Red lines indicate upregulated and blue lines downregulated genes. (B) Venn-diagram showing the number of common downregulated genes in the perilesional cortex, thalamus and hippocampus. Altogether, 480 genes were downregulated in both the perilesional cortex and ipsilateral thalamus. (C) Venn-diagram showing the number of common upregulated genes. Altogether, 841 genes were upregulated in both the perilesional cortex and ipsilateral thalamus. Note the absence of any upregulated genes in the ipsilateral hippocampus. (D) Hierarchically clustered heatmap of differentially expressed genes in the perilesional cortex, thalamus and hippocampus. Sham-operated and injured animals clustered into their own clusters. Also, the perilesional cortex, thalamus and hippocampus clustered into their own clusters. Thalamic expression in one rat with TBI, however, clustered into its own branch. Abbreviations: Cx-s, cortex sham-operated animal; Cx-t, cortex TBI; HC-s, hippocampus sham-operated animals; HC-t, hippocampus TBI; Tha-s, thalamus sham-operated animal; Tha-t, thalamus TBI.
Unsupervised hierarchal clustering of the gene expression profile in the perilesional cortex and thalamus, but not in the hippocampus, differentiated the injured rats from the control animals. Clustering also differentiated the gene expression profile of the three brain areas from each other in both controls and rats with TBI (Fig. 1D). Moreover, Spearman correlation of the fold-change in the expression of all genes between the brain areas revealed a similar direction in the gene expression between the cortex and thalamus (rs = 0.4556922, p < 2.2e–16), the cortex and hippocampus (rs = 0.254917, p < 2.2e–16) and the thalamus and hippocampus (rs = 0.216593, p < 2.2e–16).
Gene ontology analysis indicated 57 enriched gene sets in the perilesional cortex (Fig. 2A; Supplementary Table S1), 37 in the thalamus (Fig. 2B; Supplementary Table S2) and 1 in the hippocampus. In the perilesional cortex and thalamus, ion-channel and mitochondrial gene sets were significantly downregulated and immunity and inflammatory gene sets were upregulated. In the hippocampus, only a ligand-gated channel activity gene set was downregulated (note that the GSEA analysis was done with normal p-values to rank all genes involved, also those which were not significantly regulated after TBI).
Cytoscape interaction maps of significantly enriched gene ontology terms in the (A) perilesional cortex and (B) ipsilateral thalamus at 3 months after traumatic brain injury (TBI). Interaction map from the cortex highlighted upregulation of immunity and inflammatory gene sets and downregulation of an ion channel gene set. The same gene sets were also highlighted in the thalamus, which had enriched downregulated mitochondrion-related gene sets. Color codes: upregulated gene sets are shown in red and downregulated gene sets are shown in blue.
Generation of gene lists for network analysis
Parallel and statistically significant gene expression alterations of the perilesional cortex and thalamus were used to construct the TBI-sig. The hippocampal data were not included due to the scarcity of expression changes. Consequently, a total of 874 upregulated and 464 downregulated genes were included in the TBI-sig.
The COMPOUND-sig of nine novel top hits and two previously tested compounds in NEU cell lines were downloaded with LINCS API (http://api.lincscloud.org/). Table 1 summarizes the analysis of the overlap between the TBI-sig and COMPOUND-sig. The number of genes with significant expression changes varied from 10 to 28. All 11 overlapping gene lists were included in the IPA gene network analysis (see below).
IPA gene network analysis of overlapping gene lists
Compounds that have demonstrated disease-modifying effects on epileptogenesis
Comparison of TBI-sig and COMPOUND-sig with LINCS analysis revealed two compounds, sirolimus and celecoxib, which were previously investigated in in vivo epileptogenesis models (see Pitkänen et al.30). The sirolimus gene networks in the IPA was generated from 20 genes (Table 1) and resulted in the generation of two networks (Supplementary Fig. S9). Many of the genes in the sirolimus network connected to the Ywhaz gene, which is upregulated by sirolimus (downregulated in the perilesional cortex and thalamus by TBI, as expected based on the negative LINCS connectivity score, Table 1). IPA gene network analysis also suggested a strong link to the Esr1and Ubc genes in the sirolimus network, even though their transcription is not regulated by sirolimus or by TBI.
The celecoxib network was generated from 23 genes (Table 1), from which IPA generated 3 gene networks (Supplementary Fig. S10). Celecoxib network 1 highlighted Nfe2l2 and S100a4. Celecoxib network 2 indicated a direct connection between Tubb2a and Tubb3. Both were downregulated by celecoxib in the NEU cell line, indicating a parallel effect of TBI as predicted by the positive LINCS connectivity score. Celecoxib network 3 highlighted the chromosome 16 open reading frame 45 protein-coding gene (C16of45).
Novel compounds revealed by LINCS analysis
Overlapping gene lists between the nine “novel compounds” and the TBI-sig are shown in Table 1. IPA gene network analysis of gene lists related to the six “novel compounds” with a positive connectivity score in the NEU cell line (BRD-K91844626, BRD-A11009626, NO-ASA, BRD-K75971499, ionomycin and desmethylclomipramine) indicated tubulins in five of six networks. Interestingly, Tubb2a and Tubb3 were present in five networks [BRD-K91844626, BRD-A11009626, NO-ASA, ionomycin (Supplementary Fig. S1–S4) and desmethylclomipramine (Fig. 3)]. Moreover, Tubb2a and Tubb3 were directly connected with each other and both were downregulated by the five compounds. The desmethylclomipramine and NO-ASA overlapping networks contained an even larger number of tubulin-related nodes [Tubb2a, Tubb3, Tubb4b, tubulin (complex), β-tubulin and tubulin (family)] and all these nodes were downregulated in the NEU cell line by desmethylclomipramine and NO-ASA. Desmethylclomipramine also upregulated Nfkb2, Nfe2l2 and S100a4. BRD-K75971499 was the only positively connected “novel compound” without any tubulin nodes in the overlapping network (Supplementary Fig. S5). BRD-K75971499 downregulated Vim and upregulated Bag3, both of which also had a large number of connections to other genes in the overlapping network.
IPA gene networks generated from overlapping genes in the desmethylclomipramine signature and TBI-sig.
(A) Network 1 highlighted tubulin downregulation and NFE2L2 and NFKB2 upregulation by desmethylclomipramine. (B) Network 2 highlighted S100A4 upregulation by desmethylclomipramine. Color codes: red, upregulated genes; green, downregulated genes by desmethylclomipramine.
IPA gene network analysis of gene lists related to the nine “novel compounds” revealed that three of these compounds had a negative connectivity score in the NEU cell line (BRD-K55260239, SDZ-NKT-343 and STK-661558). All of these compounds downregulated Cd44, which was upregulated by TBI. The compounds exhibited no common patterns regarding gene expression upregulation (Supplementary Fig. S6-S8).
Discussion
The present study was designed to test the hypothesis that a bioinformatics comparison of the post-TBI transcriptomic signature to a signature induced by a given drug could be used to identify novel pharmacotherapies to modify post-TBI morbidities, including epileptogenesis.
We induced TBI with lateral fluid percussion injury (FPI) in rats and performed GW mRNA-sequencing from the perilesional cortex, thalamus and hippocampus at 3 months post-TBI. We performed the analysis at a chronic time-point to eliminate transcriptomics changes related to the acute post-injury phase and to reveal expression changes relevant to mechanisms related to chronic evolution of post-TBI morbidities, including epileptogenesis. We found major similarities in the gene expression patterns in the perilesional cortex and thalamus after TBI, but not in the hippocampus. In particular, we found negative enrichment of ion-channel and mitochondrial membrane gene sets and positive enrichment of inflammation-related gene sets in the perilesional cortex and ipsilateral thalamus. Similar gene ontology enrichments, particularly those related to inflammation, have been reported at acute time-points after lateral FPI as well as in other experimental models of TBI8,10,16,33,34; thus, our data indicate that these functional gene sets remain activated over a wide post-TBI time window. Our data also revealed a strong downregulation of ion channel-related gene sets. Only two previous transcriptomics studies have reported regulation of ion channels at an acute time-point (<24 h) after experimental TBI16,34, showing changes in potassium and sodium channels. Our data show a strong downregulation in a large number of ion-channel gene sets, providing a molecular basis for the long-lasting changes in excitability observed after TBI. Taken together, perilesional cortex and thalamus, which are anatomically heavily interconnected, maintain similar transcriptomics changes in inflammatory and ion channel pathways. Changes in the expression levels are chronic, lasting up to 3 months, which suggests a wide therapeutic time window for treatments targeting these gene networks.
Next we performed a LINCS analysis to determine compounds that modulate the TBI-sig. Comparison of the TBI-sig with the COMPOUND-sig of compounds listed in the LINCS database pinpointed novel TBI-sig modifying compounds, but also celecoxib and sirolimus, two treatments previously shown to have disease-modifying effects in proof-of-concept studies using in vivo models35, including post-TBI epileptogenesis36. In particular, we found celecoxib to “strengthen” the expression of 23 genes regulated by TBI, whereas sirolimus had an opposing (“normalizing”) effect on the expression of 20 genes regulated by TBI.
Celecoxib upregulated transcription factor Nfe2l2 (or Nrf2), which is translocated to the nucleus after brain injuries37,38 to promote the expression of numerous antioxidant, anti-inflammatory and neuroprotective proteins37,39,40. Moreover, a recent study reported that mice injected with AAV Nrf2 displayed significantly fewer generalized seizures, with a profound reduction in microglia activation41. Celecoxib also upregulated S100a4, which is normally expressed in the brain at low levels42. S100a4 induces neuroprotection in models of brain injury43. Moreover, our analysis revealed that celecoxib downregulated Tubb3 and Tubb2a. This is of interest as inactivating mutations in genes encoding tubulins were recently reported to associate with epilepsy and abnormal neuronal migration and organization44,45. While the effects of celecoxib on Nfe2l2 and S100a4 support the endogenous chronic repair mechanisms of the brain, it remains to be explored whether or not the net effect of tubulin downregulation on post-TBI recovery is favorable.
The LINCS analysis also highlighted an mTOR inhibitor, sirolimus (rapamycin), as a drug with a high negative connectivity score. Sirolimus is the most investigated compound in different genetic and acquired models of epileptogenesis, showing favorable antiepileptogenic and co-morbidity modifying effects (for review, see Pitkänen et al.30). Its gene expression profile, however, opposed the changes induced by TBI. Also, the gene networks regulated by sirolimus showed little overlap with those regulated by celecoxib, supporting the concept that post-injury outcome can be modified by affecting complementary non-overlapping molecular networks.
Next we investigated whether the compounds affecting the TBI-sig had any common gene targets and whether the targets are the same as those modulated by known antiepileptogenic compounds. From the 1064 novel drug candidates found by the LINCS analysis, desmethylchlomipramine and NO-ASA showed the most remarkable modulatory effects on the post-TBI transcriptomic changes. Similar to celecoxib, desmethylclomipramine upregulated Nfe2l2, Nfkb2 and S100a4 and downregulated tubulins. NO-ASA (3-nitrooxyphenyl acetylsalicylate, NCX-4016), an anti-inflammatory and antithrombotic compound46, modulated the expression of 28 genes in the TBI-sig, including downregulation of tubulin expression. Ionomycin, an ionophore known to increase intracellular Ca2+ 47, modulated both Nfe2l2 and tubulins, whereas BRD-K91844626 and BRD-A11009626 modulated only tubulins. The four remaining compounds with unknown mechanisms of action, BRD-K55260239, SDZ-NKT343, STK-661558 and BRD-K75971499 all downregulated Cd44, which encodes a cell-surface glycoprotein involved in cell adhesion and migration48 and remains upregulated for up to 2 months in a mouse model of stab brain injury49. These compounds exhibited no overlap with the gene networks modulated by celecoxib, sirolimus, desmethylchlomipramine, or ionomycin.
Taken together, our in silico data highlighted tubulins, Nfe2l2, Nfkb2 and S100a4 as target genes modulated by compounds with a high LINCS connectivity score relative to the TBI-sig. Moreover, our data suggested that desmethylclomipramine, an active metabolite of the commonly used antidepressant clomipramine, is a promising candidate to be explored as a recovery-enhancing treatment after brain injury.
Materials and Methods
Animals
TBI was induced in five adult male Sprague-Dawley rats (330–370 g at the time of TBI or sham operation) with lateral FPI having an impact pressure 3.30 ± 0.01 atm (duration of post-impact apnea 15–25 sec) as previously described50,51. Five sham-operated rats served as controls. At 3 months after TBI, rats were deeply anesthetized with isoflurane and decapitated. The brain was removed from the skull, flushed with 0.9% cold (4 °C) sodium chloride and two 2-mm-thick coronal slices (between −2.2 to −6.2 from the bregma) were cut with a slicing matrix (#15007, Rodent Brain Matrix, Ted Pella, Inc, Redding, CA, USA). The perilesional cortex, thalamus and dentate gyrus (including CA3c-b) were then dissected under a magnifying glass on top of the light table, moved to a microcentrifuge tube, snap frozen in liquid nitrogen and stored at −70 °C until RNA extraction. The remaining tissue pieces were immersion-fixed in 10% formalin and thionin-stained to verify the occurrence of lesion (data not shown).
All animal procedures were approved by The Animal Ethics Committee of Provincial Government of Southern Finland and performed in accordance with the guidelines of the European Community Council Directives 2010/63/EU.
Preparation of the sequencing library and RNA-sequencing
RNA was extracted from the perilesional cortex, thalamus and hippocampus DNaesy Blood & Tissue kit (#69504, Qiagen, Hilden, Germany) followed by DNase digestion. mRNA from 2 μg of total RNA was enriched using Dynabeads Oligo (dT)25 beads (#61002, Invitrogen, Carlsbad, CA, USA). The sequencing library was prepared with the NEBNext mRNA Library Prep Reagent Set (#E6100S, New England Biolabs, Ipswich, MA, USA). Quality control of the total RNA and sequencing libraries was performed using a MultiNA electrophoresis device (Shimazu, Kyoto, Japan). Then, sequencing of the mRNA library (RNA-Seq) for the perilesional cortex and hippocampus was carried out with an Illumina Genome Analyzer IIx (San Diego, CA, USA) with 36 cycles and for the thalamus using Illumina HiSeq 2000 (San Diego, CA, USA) with 50 cycles. Base-calling was performed using an Illumina Off-Line Basecaller v1.8. RNA-Seq raw data were saved to the NCBI Gene Expression Omnibus (GEO; series accession number GSE80174).
Bioinformatics
Quality control of the sequencing raw reads was performed using FastQC52. Sequencing reads were aligned to the Ensemble RN5 genome with Spliced Transcripts Alignment to Reference (STAR) software (version 2.3.0e_r291)53. Two hippocampal samples were discarded from further analysis due to low alignment percentage (32.9% and 35.5%). From the remaining 28 samples, 64.3 ± 7.9% of the perilesional cortical (5 control, 5 TBI), 66.9 ± 3.7% hippocampal (3 control, 5 TBI) and 80.5 ± 4.6% of thalamic (5 control, 5 TBI) raw reads were aligned to the RN5 reference genome. Differentially expressed genes were identified with DEseq2 R package (R version 3.1.0)54. The adjusted p-value was calculated with a Benjamini–Hochberg false discovery rate (FDR). FDR < 0.05 was considered a statistically significant difference in the gene expression. Hierarchal clustering was performed by heatmap.2 function in gplots R package to genes that had FDR < 0.05 in at least one brain area. Direction of the fold-change between the brain areas was compared with Spearman’s correlation by corr.test R function.
Enrichment of gene ontology terms was analyzed with Gene Set Enrichment Analysis (GSEA)55 with C5-catogory gene sets in Molecular Signatures Database (MSigDB). Ranked-gene lists for GSEA of the perilesional cortex, hippocampus and thalamus were prepared by ranking the genes in order by p-value and then multiplying with the sign of the fold-change. Upregulated genes were assigned with positive and downregulated genes with a negative rank. The GSEA gene set was considered significantly enriched when the q-value was <0.01. GSEA results were visualized with a enrichment map v.1.3 plugin with P-value Cutoff 0.005; q-value Cutoff 0.01 and Similarity Cutoff 0.5 in Cytoscape 2.8.356.
Evaluation of drug candidates
We first generated the “TBI gene expression signature” (TBI-sig) from our TBI dataset, which included genes with significantly altered expression in both the cortex and thalamus and submitted it to the LINCS web query. This TBI-sig was compared to the expression signature of each test compound (COMPOUND-sig) separately in three cell lines: in terminally differentiated neurons (NEU), terminally differentiated neurons treated with KCl (NEU.KCL) and iPS-derived neural progenitor cells (NPC), which were available in the LINCS database. To filter reproducible gene expression signatures from all query results and fetch differentially expressing genes by compounds, we used the Lincscloud Application Programming Interface (API) through a custom R-script. This resulted in a total of 3358 hits with a connectivity score between 1.0 to −1.0. The positive connectivity score in the LINCS analysis indicated that the direction (either upregulated or downregulated) of the TBI-sig was similar to that of the COMPOUND-sig; a negative connectivity score indicated a reverse direction and a score of 0 indicated no association between the expression signatures. Drug candidates with a connectivity score from 0.1 to −0.1 were filtered out from the final list as their connectivity to the TBI-sig was considered weak. This resulted in data from 1064 compounds, of which sirolimus and celecoxib demonstrate favorable disease-modifying effects on epileptogenesis in in vivo animal models. Carbamazapine, with no documented effect on epileptogenesis, was also on the list35. We also found tacrolimus and zonisamide, included in in vivo epileptogenesis studies, on the original list of 3358 compounds, but their connectivity scores did not survive the filtering criterion.
Next, we prepared a list of 18 “novel top hits”, which included 3 compounds with the highest positive and 3 compounds with the highest negative connectivity scores from each of the 3 cell lines (Supplementary Table S3). Another list included the three compounds that have already been assessed in in vivo epileptogenesis models, i.e., sirolimus (positive effect), celecoxib (positive effect) and carbamazepine (no effect). Next, we generated the list of overlapping genes between the TBI-sig and the COMPOUND-sig induced in NEU cell lines only by the a) novel top hits (n = 9) and b) previously tested compounds (n = 2) (Table 1). As summarized in Table 1, the overlapping gene lists were available from 9 of 18 “novel compounds” and from 2 already known disease-modifying compounds, which were then used to generate gene networks with IPA.
Additional Information
How to cite this article: Lipponen, A. et al. Analysis of Post-Traumatic Brain Injury Gene Expression Signature Reveals Tubulins, Nfe2l2, Nfkb, Cd44 and S100a4 as Treatment Targets. Sci. Rep. 6, 31570; doi: 10.1038/srep31570 (2016).
References
Maas, A. I. R. et al. Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery 76, 67–80 (2015).
Faul, M., Xu, L. & Traumatic, W. M. Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Centers Dis. Control Prev. Natl. Cent. Inj. Prev. Control (2010).
Corrigan, J. D., Selassie, A. W. & Orman, J. A. L. The epidemiology of traumatic brain injury. J. Head Trauma Rehabil. 25, 72–80 (2010).
Dougall, D., Poole, N. & Agrawal, N. Pharmacotherapy for chronic cognitive impairment in traumatic brain injury. Cochrane database Syst. Rev. 12, CD009221 (2015).
Diaz-Arrastia, R. et al. Pharmacotherapy of traumatic brain injury: state of the science and the road forward: report of the Department of Defense Neurotrauma Pharmacology Workgroup. J. Neurotrauma 31, 135–158 (2014).
Marklund, N., Bakshi, A., Castelbuono, D. J., Conte, V. & McIntosh, T. K. Evaluation of pharmacological treatment strategies in traumatic brain injury. Curr. Pharm. Des. 12, 1645–1680 (2006).
Saatman, K. E. et al. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma 25, 719–738 (2008).
Israelsson, C. et al. Distinct cellular patterns of upregulated chemokine expression supporting a prominent inflammatory role in traumatic brain injury. J. Neurotrauma 25, 959–74 (2008).
Hellmich, H. L. et al. Pathway analysis reveals common pro-survival mechanisms of metyrapone and carbenoxolone after traumatic brain injury. PLoS One 8, e53230 (2013).
Samal, B. B. et al. Acute Response of the Hippocampal Transcriptome Following Mild Traumatic Brain Injury After Controlled Cortical Impact in the Rat. J. Mol. Neurosci. 57, 282–303 (2015).
Dolenec, P., Pilipović, K., Rajič, J. & Župan, G. Temporal pattern of neurodegeneration, programmed cell death and neuroplastic responses in the thalamus after lateral fluid percussion brain injury in the rat. J. Neuropathol. Exp. Neurol. 74, 512–26 (2015).
White, T. E. et al. Bilateral gene interaction hierarchy analysis of the cell death gene response emphasizes the significance of cell cycle genes following unilateral traumatic brain injury. BMC Genomics 17, 130 (2016).
Wong, Y.-H. et al. Temporal Genetic Modifications after Controlled Cortical Impact-Understanding Traumatic Brain Injury through a Systematic Network Approach. Int. J. Mol. Sci. 17 (2016).
Zhang, X.-Y. et al. Analysis of key genes and modules during the courses of traumatic brain injury with microarray technology. Genet. Mol. Res. 13, 9220–9228 (2014).
Vonder Haar, C. et al. Comparison of the effect of minocycline and simvastatin on functional recovery and gene expression in a rat traumatic brain injury model. J. Neurotrauma 31, 961–75 (2014).
White, T. E. et al. Gene expression patterns following unilateral traumatic brain injury reveals a local pro-inflammatory and remote anti-inflammatory response. BMC Genomics 14, 282 (2013).
Hellmich, H. L. et al. Pathway analysis reveals common pro-survival mechanisms of metyrapone and carbenoxolone after traumatic brain injury. PLoS One 8, e53230 (2013).
Valiyaveettil, M. et al. Modulation of cholinergic pathways and inflammatory mediators in blast-induced traumatic brain injury. Chem. Biol. Interact. 203, 371–375 (2013).
Colak, T. et al. Microarray-based gene expression analysis of an animal model for closed head injury. Injury 43, 1264–1270 (2012).
Risling, M. et al. Mechanisms of blast induced brain injuries, experimental studies in rats. Neuroimage 54 Suppl 1, S89–97 (2011).
Crawford, F. et al. Apolipoprotein E-genotype dependent hippocampal and cortical responses to traumatic brain injury. Neuroscience 159, 1349–1362 (2009).
Goodman, J. C., Cherian, L. & Robertson, C. S. Cortical expression of prolactin (PRL), growth hormone (GH) and adrenocorticotrophic hormone (ACTH) is not increased in experimental traumatic brain injury. Acta Neurochir. Suppl. 102, 389–390 (2008).
Kukacka, J. et al. Blood metallothionein, neuron specific enolase and protein S100B in patients with traumatic brain injury. Neuro Endocrinol. Lett. 27 Suppl 2, 116–120 (2006).
Ishikawa, Y. et al. Search for novel gene markers of traumatic brain injury by time differential microarray analysis. Acta Neurochir. Suppl. 96, 163–167 (2006).
von Gertten, C., Flores Morales, A., Holmin, S., Mathiesen, T. & Nordqvist, A.-C. S. Genomic responses in rat cerebral cortex after traumatic brain injury. BMC Neurosci. 6, 69 (2005).
Michael, D. B., Byers, D. M. & Irwin, L. N. Gene expression following traumatic brain injury in humans: analysis by microarray. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 12, 284–290 (2005).
Rall, J. M., Matzilevich, D. A. & Dash, P. K. Comparative analysis of mRNA levels in the frontal cortex and the hippocampus in the basal state and in response to experimental brain injury. Neuropathol. Appl. Neurobiol. 29, 118–131 (2003).
Kobori, N., Clifton, G. L. & Dash, P. Altered expression of novel genes in the cerebral cortex following experimental brain injury. Brain Res. Mol. Brain Res. 104, 148–158 (2002).
Matzilevich, D. A., Rall, J. M., Moore, A. N., Grill, R. J. & Dash, P. K. High-density microarray analysis of hippocampal gene expression following experimental brain injury. J. Neurosci. Res. 67, 646–663 (2002).
Pitkänen, A. et al. Posttraumatic epilepsy - Disease or comorbidity? Epilepsy Behav. 38, 19–24 (2014).
Sirota, M. et al. Discovery and preclinical validation of drug indications using compendia of public gene expression data. Sci. Transl. Med. 3, 96ra77 (2011).
Wacker, S. A., Houghtaling, B. R., Elemento, O. & Kapoor, T. M. Using transcriptome sequencing to identify mechanisms of drug action and resistance. Nat. Chem. Biol. 8, 235–7 (2012).
Das, M. et al. Lateral fluid percussion injury of the brain induces CCL20 inflammatory chemokine expression in rats. J. Neuroinflammation 8, 148 (2011).
Raghavendra Rao, V. L., Dhodda, V. K., Song, G., Bowen, K. K. & Dempsey, R. J. Traumatic brain injury-induced acute gene expression changes in rat cerebral cortex identified by GeneChip analysis. J. Neurosci. Res. 71, 208–19 (2003).
Pitkänen, A. et al. Gender issues in antiepileptogenic treatments. Neurobiol. Dis. 72 Pt B, 224–32 (2014).
Guo, D., Zeng, L., Brody, D. L. & Wong, M. Rapamycin attenuates the development of posttraumatic epilepsy in a mouse model of traumatic brain injury. PLoS One 8, e64078 (2013).
Sandberg, M., Patil, J., D’Angelo, B., Weber, S. G. & Mallard, C. NRF2-regulation in brain health and disease: implication of cerebral inflammation. Neuropharmacology 79, 298–306 (2014).
Hardingham, G. E. & Do, K. Q. Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat. Rev. Neurosci. 17, 125–134 (2016).
Gallorini, M. et al. Activation of the Nrf2-regulated antioxidant cell response inhibits HEMA-induced oxidative stress and supports cell viability. Biomaterials 56, 114–28 (2015).
Satoh, T. et al. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic [correction of electrophillic] phase II inducers. Proc. Natl. Acad. Sci. USA. 103, 768–73 (2006).
Mazzuferi, M. et al. Nrf2 defense pathway: Experimental evidence for its protective role in epilepsy. Ann. Neurol. 74, 560–8 (2013).
De León, M., Van Eldik, L. J. & Shooter, E. M. Differential regulation of S100 beta and mRNAs coding for S100-like proteins (42A and 42C) during development and after lesion of rat sciatic nerve. J. Neurosci. Res. 29, 155–62 (1991).
Dmytriyeva, O. et al. The metastasis-promoting S100A4 protein confers neuroprotection in brain injury. Nat. Commun. 3, 1197 (2012).
Romina, R. & Renato, B. Epilepsy in Multigene Tubulin Family Mutations. J Neurol Neurophysiol (2015).
Cushion, T. D. et al. De novo mutations in the beta-tubulin gene TUBB2A cause simplified gyral patterning and infantile-onset epilepsy. Am. J. Hum. Genet. 94, 634–41 (2014).
Chattopadhyay, M. et al. Comparison between 3-Nitrooxyphenyl acetylsalicylate (NO-ASA) and O2-(acetylsalicyloxymethyl)-1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolate (NONO-ASA) as safe anti-inflammatory, analgesic, antipyretic, antioxidant prodrugs. J Pharmacol Exp Ther 335, 443–450 (2010).
Liu, C. & Hermann, T. E. Characterization of ionomycin as a calcium ionophore. J. Biol. Chem. 253, 5892–5894 (1978).
Lesley, J., Hyman, R. & Kincade, P. W. CD44 and its interaction with extracellular matrix. Adv. Immunol. 54, 271–335 (1993).
Stylli, S. S., Kaye, A. H. & Novak, U. Induction of CD44 expression in stab wounds of the brain: long term persistence of CD44 expression. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 7, 137–140 (2000).
McIntosh, T. K. et al. Traumatic brain injury in the rat: Characterization of a lateral fluid-percussion model. Neuroscience 28, 233–244 (1989).
Kharatishvili, I., Nissinen, . J. P., Intosh, T. K. M. C., McIntosh, T. K. & Pitkänen, a. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience 140, 685–97 (2006).
Andrews, S. FastQC, a quality control tool for high throughput sequence data. at http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (2010).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA. 102, 15545–50 (2005).
Shannon, P. et al. Cytoscape: A software Environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Acknowledgements
This study was supported by the Academy of Finland (A.P.), EpiGENet EUROEPINOMICS (A.P.), FP7-HEALTH project 602102 (EPITARGET), ERA-NET Neuron [TBI Epilepsy]. We thank Mr. Jarmo Hartikainen and Mrs. Merja Lukkari for their excellent technical assistance. We also grateful to Mr. Juha Kekäläinen, for his excellent help in bioinformatics analysis.
Author information
Authors and Affiliations
Contributions
A.L. and A.P. wrote the main manuscript text. A.L. and J.P. analyzed data. N.P. sampled rat brain. A.L. prepared figures. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lipponen, A., Paananen, J., Puhakka, N. et al. Analysis of Post-Traumatic Brain Injury Gene Expression Signature Reveals Tubulins, Nfe2l2, Nfkb, Cd44 and S100a4 as Treatment Targets. Sci Rep 6, 31570 (2016). https://doi.org/10.1038/srep31570
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep31570
This article is cited by
-
Identification of potential crucial genes and therapeutic targets for epilepsy
European Journal of Medical Research (2024)
-
“Omics” in traumatic brain injury: novel approaches to a complex disease
Acta Neurochirurgica (2021)
-
Real-Time Noninvasive Bioluminescence, Ultrasound and Photoacoustic Imaging in NFκB-RE-Luc Transgenic Mice Reveal Glia Maturation Factor-Mediated Immediate and Sustained Spatio-Temporal Activation of NFκB Signaling Post-Traumatic Brain Injury in a Gender-Specific Manner
Cellular and Molecular Neurobiology (2021)
-
Translational Genomics in Neurocritical Care: a Review
Neurotherapeutics (2020)
-
Dynamics of clusterin protein expression in the brain and plasma following experimental traumatic brain injury
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.