Abstract
The biguanide metformin is used for its antidiabetic effect for many years but how metformin acts remains poorly understood and controversial. AMP-activated protein kinase (AMPK), a protein kinase that plays a key role in maintaining energy homeostasis, is assumed to be one of the prime targets of metformin. However, since our demonstration that AMPK is not required for the beneficial effects of metformin on the control of glycemia, the list of AMPK-independent actions of metformin is rapidly increasing. Given the conflicting results on the effects of metformin we sought, using our genetic mouse models deficient in the catalytic subunits of AMPK, to determine whether this kinase is involved in the effects of metformin on the expression of the iron-regulatory hormone hepcidin, as recently proposed. Here we demonstrate, using different approaches, either isolated hepatocytes that lack AMPK, or direct AMPK activators, that, AMPK activation is not necessary for metformin to inhibit BMP6-induced hepcidin gene expression. These results may shed new lights on the increasingly recognized AMPK-independent metformin’s molecular action, an important area of current research.
Similar content being viewed by others
Introduction
In a recent study, Kim et al. reported an interesting finding of transcriptional regulation of the iron-regulatory hormone hepcidin expression by the antidiabetic biguanide drug metformin1. Hepcidin plays a critical role in the control of systemic iron balance by coordinating the major fluxes of iron into blood plasma through the regulation of intestinal iron absorption, delivery of recycled iron from macrophages, and release of stored iron from hepatocytes2. It is known that dietary iron regulates hepcidin expression through BMP6-mediated activation of SMAD1/5/8 signaling3. Kim et al. showed that metformin treatment blunted the BMP6-controlled hepcidin response and attenuated the BMP6-related changes of iron metabolism in mice. While the detailed mechanisms underlying the effects of metformin have not been completely elucidated, there is prior evidence that its primary effect is in mitochondria, where the drug interferes with respiratory complex I and reduces ATP production4,5, leading to the activation of AMP-activated protein kinase (AMPK)6, a protein kinase that has a key role in maintaining energy homeostasis7.
In view of their previous observation that expression of the small heterodimer partner (SHP; NR0B2), an atypical orphan member of the nuclear receptor superfamily8, was strongly induced by metformin through AMPK activation9, the authors further tested the hypothesis that AMPK activation and subsequent SHP expression could affect BMP6 signaling-induced hepcidin expression. In line with this hypothesis, the authors indeed showed that metformin treatment suppresses BMP6-mediated hepcidin response both in hepatoma cells and primary hepatocytes, a suppressive effect that was attenuated by either SHP knockdown, treatment with a non specific inhibitor of AMPK (Compound C, also known as dorsomorphin) or a dominant negative form of AMPK. They therefore concluded that AMPK signaling is connected to the regulation of iron metabolism through induction of SHP and control of hepcidin gene expression.
Since the precise mechanism of action of metformin remains controversial, with activation of both AMPK-dependent and AMPK-independent pathways10, we were interested to further investigate the putative involvement of AMPK in suppression of BMP6-mediated hepcidin up-regulation by metformin.
Methods
All methods were carried out in accordance with relevant guidelines and regulations.
Animals
All procedures were performed in accordance with the principles and guidelines established in the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (Council of Europe, ETS no. 123, 1991). Animal studies described herein were reviewed and approved (agreement no. 75–886) by the Directeur Départemental des Services Vétérinaires of the Préfecture de Police de Paris. Mice lacking both AMPKα1 and AMPKα2 catalytic subunits in the liver were previously described11,12,13. Unless challenged in conditions inducing changes in the ATP/AMP ratio (such as pharmacological-induced energy stress11,12 or nutrient deprivation13), these mice presented no metabolic defect in basal conditions.
Littermates from the same breeding pair were used in these experiments. Hepatocytes were isolated and cultured as described11. Briefly, hepatocytes were isolated from fed adult male mice of 8–10 week-old by the collagenase method. The cells were plated in M199 medium supplemented with 10% FCS. After attachment (3–4 hours), hepatocytes were maintained in M199 medium for additional 24 h in presence of 0.1% FCS. Medium was changed and AMPK activators (0.25 to 1 mM metformin, 3 μM 991 or 3 μM C13) were added for 1 h prior supplemental 24 h of culture in presence of 10 nM BMP6.
Reverse transcription and real-time quantitative PCR
RNA extraction, reverse transcription and quantitative PCR were performed as previously described14. All samples were normalized to the threshold cycle value for cyclophilinA.
Western blot
Total lysate extracts from hepatocytes were prepared as described11. Briefly, hepatocytes were lysed in ice-cold lysis buffer and sonicated on ice. The homogenate was centrifuged for 10 minutes at 10000 g at 4 °C and the supernatants were removed for determination of total protein content. Twenty micrograms of proteins from the supernatant was separated on 10% SDS-PAGE gels and transferred onto nitrocellulose membranes. Immunoblotting was performed following standard procedures, and the signals were detected by chemiluminescence reagents. Primary antibodies were directed against total AMPKα, AMPKα phosphorylated at Thr172, total ACC and ACC phosphorylated at Ser79 (from Cell Signaling Technology).
Statistical analysis
Statistical analysis was performed using the Newmans-Keuls test. P values less than .05 were considered statistically significant.
Results and Discussion
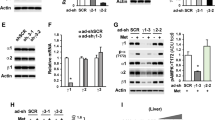
To determine the role of AMPK on the effect of metformin on hepcidin activation by BMP6, we isolated and treated hepatocytes from WT and AMPK livKO. Importantly, at 2 mM metformin, we noticed a strong toxic effect of the drug leading to a high mortality rate of the hepatocytes after 24 h of culture. We thus decided to work with metformin concentrations ranging from 0.25 to 1 mM. Here, we confirmed in the WT hepatocytes that metformin acts in a dose- dependent manner to inhibit BMP6-mediated hepcidin gene expression. However, strikingly, an even stronger repression was observed in AMPK- deficient hepatocytes (Fig. 1A), clearly demonstrating that metformin-induced repression of hepcidin gene expression is not mediated through AMPK activation. As expected, metformin efficiently stimulated AMPK activation as illustrated by increased phosphorylation of AMPK at Thr172 and of its well-established substrate, acetyl-CoA carboxylase (ACC) in WT hepatocytes only (Fig. 1B).
Effect of metformin on BMP6-induced hepcidin gene expression in WT and AMPKLiv KO mice.(A) Q-PCR analysis showing hepcidin mRNA levels in mouse primary hepatocytes treated with BMP6 (10 nM) or/and metformin (from 0.25 to 1 mM) for 24 h. RNA extraction and real-time quantification of transcripts were performed as described14. mRNA expression was calculated using the ΔΔCt method and normalized to the expression of cyclophilin A. Hepcidin gene expression in BMP6 treated hepatocytes, with or without metformin, is expressed as-fold hepcidin expression of that in non-treated hepatocytes. Error bars represent SD for n = 3 samples in each group. Statistical significance is indicated by * (*p < 0.05, **p < 0.005, ***p < 0.0005 as compared to the BMP6 condition). A typical representative experiment is shown. Similar results were confirmed in three separate experiments. (B) Immunoblots showing pAMPK/AMPK and pACC/ACC in hepatocyte extracts prepared as previously described, treated either with 10 nM BMP6, 1 mM metformin or both.
To further demonstrate the lack of AMPK-mediated regulation of hepcidin gene expression, we tested the effect of two direct allosteric AMPK activators, compound 991, a small-molecule benzimidazole derivative15 and compound C1316, whose activities were shown to be largely ineffective in AMPKLiv KO hepatocytes16,17. As previously reported, at 3 µM, 991 and C13 were ineffective in increasing AMPK phosphorylation in WT primary hepatocytes16,18, but a robust increase in ACC phosphorylation was readily observed, indicating AMPK activation (Fig. 2A). However, while AMPK is well activated by these small molecule activators, they were inefficient in inhibiting hepcidin gene expression in response to BMP6 treatment, whatever the genotype of the mice (Fig. 2B). Noteworthy, none of the AMPK activators tested (metformin, 991 and C13) were found to alter hepcidin gene expression in absence of BMP6 (Supp. Fig. 1).
Effect of 991 and C13 on BMP6-induced hepcidin gene expression in WT and AMPKLiv KO mice. (A) Immunoblots showing pAMPK/AMPK and pACC/ACC in hepatocyte extracts prepared as previously described, treated with 10 nM BMP6, 3 μM 991 or 3 μM C13. (B) Q-PCR analysis showing hepcidin mRNA levels in mouse primary hepatocytes treated with BMP6 (10 nM), 991 (3μM) or C13 (3μM) for 24 h. RNA extraction and real-time quantification of transcripts were performed as described. mRNA expression was calculated using the ΔΔCt method and normalized to the expression of cyclophilin A. Hepcidin gene expression is expressed in % of BMP6 treated hepatocytes. Error bars represent SD for n = 3 samples in each group.
In view of this AMPK- independent effect of metformin, we questioned the role of SHP in the repression of hepcidin, SHP being supposedly induced by AMPK activation. Surprisingly and hardly reconcilable with such a role for SHP, we found a drastic dose- dependent reduction of SHP mRNA levels after treatment with metformin, regardless of the presence of BMP6 (Fig. 3 and Supp. Fig. 2), in contrast to the data reported by Kim et al.1. Noteworthy, our results are consistent with previous observations showing a repression of SHP mRNA levels by metformin in mouse hepatocytes19. In addition, Krausova et al. did not detect any increase in SHP mRNA levels in response to metformin in human primary hepatocytes20 and observed a significant down regulation in the MZ-Hep1 hepatoma cell line incubated for 4 h with 2 mM metformin.
Effect of metformin on BMP6-mediated SHP gene expression in WT and AMPKLiv KO mice. (A) Q-PCR analysis showing SHP mRNA levels in mouse primary hepatocytes treated with BMP6 (10 nM) or/and metformin (from 0.25 to 1 mM) for 24 h. RNA extraction and real-time quantification of transcripts were performed as described. mRNA expression was calculated using the ΔΔCt method and normalized to the expression of cyclophilin A. SHP gene expression in BMP6 treated hepatocytes, with or without metformin, is expressed as-fold SHP expression of that in non-treated hepatocytes. Error bars represent SD for n = 3 samples in each group. Statistical significance is indicated by * (*p < 0.05, **p < 0.005, ***p < 0.0005 as compared to the BMP6 condition).
There is today an abundant literature pointing out AMPK- independent effects of metformin. Metformin is currently the drug of first choice for the treatment of type 2 diabetes for its glucose-lowering effect10. By using AMPKLiv KO mice and hepatocytes, we established for the first time that AMPK is not required for metformin-mediated inhibition of hepatic glucose production and gluconeogenic gene expression11. We proposed that a decreased of cellular energy charge (concomitant decrease in ATP and increase in AMP intracellular levels) resulting from metformin’s inhibition of mitochondrial respiratory-chain complex I4,5 was a plausible explanation of its AMPK-independent action11,21. Hence, metformin-dependent deficit in ATP levels reduces the energy-demanding process of gluconeogenesis. In addition, the concomitant increase in AMP levels functions as a key signalling mediator to allosterically inhibit the gluconeogenic enzyme fructose-1,6-bisphosphatase and also cAMP–PKA signalling through suppression of adenylate cyclase10. More recently, Madiraju et al. proposed that the reduction of gluconeogenesis evoked by metformin may be a result of increased cytosolic redox state and decreased mitochondrial redox state elicited by direct inhibition of mitochondrial glycerophosphate dehydrogenase22.
Apart from these metabolic effects of metformin, it is now well recognized that AMPK is also dispensable for some of the other pleiotropic effects of the drug. To name a few, we can mention the antineoplastic potential of metformin shown to be mediated through REDD1, a negative regulator of mTOR23. Along with these studies, metformin was also reported to down-regulate mTORC1 through the RAG family of GTPases24. Recently, Cameron et al. demonstrated that metformin treatment suppressed TNFα-induced degradation of the NF-κB negative regulator IκB and that AMPK was not required for these effects in the liver25.
In conclusion, our data on the effect of metformin on hepcidin cannot reliably support the conclusion that metformin-mediated repression of BMP6-induced hepcidin gene expression is mediated through an AMPK/SHP axis. Further analyses are now necessary to determine the precise role of SHP and whether identified alternative pathways, such as the ones discussed here, or novel ones, are required for the effect of metformin.
References
Kim, D. K. et al. Orphan nuclear receptor SHP regulates iron metabolism through inhibition of BMP6-mediated hepcidin expression. Sci Rep 6, 34630, https://doi.org/10.1038/srep34630 (2016).
Ganz, T. Systemic iron homeostasis. Physiol Rev 93, 1721–1741, https://doi.org/10.1152/physrev.00008.2013 (2013).
Kautz, L. et al. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood 112, 1503–1509, https://doi.org/10.1182/blood-2008-03-143354 (2008).
El-Mir, M. Y. et al. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem 275, 223–228 (2000).
Owen, M. R., Doran, E. & Halestrap, A. P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 348(Pt 3), 607–614 (2000).
Zhou, G. et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108, 1167–1174, https://doi.org/10.1172/JCI13505 (2001).
Carling, D. & Viollet, B. Beyond energy homeostasis: the expanding role of AMP-activated protein kinase in regulating metabolism. Cell Metab 21, 799–804, https://doi.org/10.1016/j.cmet.2015.05.005 (2015).
Lee, Y. S., Chanda, D., Sim, J., Park, Y. Y. & Choi, H. S. Structure and function of the atypical orphan nuclear receptor small heterodimer partner. Int Rev Cytol 261, 117–158, https://doi.org/10.1016/S0074-7696(07)61003-1 (2007).
Kim, Y. D. et al. Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes 57, 306–314, https://doi.org/10.2337/db07-0381 (2008).
Foretz, M., Guigas, B., Bertrand, L., Pollak, M. & Viollet, B. Metformin: from mechanisms of action to therapies. Cell Metab 20, 953–966, https://doi.org/10.1016/j.cmet.2014.09.018 (2014).
Foretz, M. et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 120, 2355–2369, https://doi.org/10.1172/JCI40671 (2010).
Hasenour, C. M. et al. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside (AICAR) effect on glucose production, but not energy metabolism, is independent of hepatic AMPK in vivo. J Biol Chem 289, 5950–5959, https://doi.org/10.1074/jbc.M113.528232 (2014).
Hasenour, C. M. et al. Liver AMP-Activated Protein Kinase Is Unnecessary for Gluconeogenesis but Protects Energy State during Nutrient Deprivation. PLoS One 12, e0170382, https://doi.org/10.1371/journal.pone.0170382 (2017).
Deschemin, J. C. & Vaulont, S. Role of hepcidin in the setting of hypoferremia during acute inflammation. PLoS One 8, e61050, https://doi.org/10.1371/journal.pone.0061050 (2013).
Lai, Y. C. et al. A small-molecule benzimidazole derivative that potently activates AMPK to increase glucose transport in skeletal muscle: comparison with effects of contraction and other AMPK activators. Biochem J 460, 363–375, https://doi.org/10.1042/BJ20131673 (2014).
Hunter, R. W. et al. Mechanism of action of compound-13: an alpha1-selective small molecule activator of AMPK. Chem Biol 21, 866–879, https://doi.org/10.1016/j.chembiol.2014.05.014 (2014).
Johanns, M. et al. AMPK antagonizes hepatic glucagon-stimulated cyclic AMP signalling via phosphorylation-induced activation of cyclic nucleotide phosphodiesterase 4B. Nat Commun 7, 10856, https://doi.org/10.1038/ncomms10856 (2016).
Woods, A. et al. Liver-Specific Activation of AMPK Prevents Steatosis on a High-Fructose Diet. Cell Rep 18, 3043–3051, https://doi.org/10.1016/j.celrep.2017.03.011 (2017).
Aatsinki, S. M. et al. Metformin induces PGC-1alpha expression and selectively affects hepatic PGC-1alpha functions. Br J Pharmacol 171, 2351–2363, https://doi.org/10.1111/bph.12585 (2014).
Krausova, L. et al. Metformin suppresses pregnane X receptor (PXR)-regulated transactivation of CYP3A4 gene. Biochem Pharmacol 82, 1771–1780, https://doi.org/10.1016/j.bcp.2011.08.023 (2011).
Stephenne, X. et al. Metformin activates AMP-activated protein kinase in primary human hepatocytes by decreasing cellular energy status. Diabetologia 54, 3101–3110, https://doi.org/10.1007/s00125-011-2311-5 (2011).
Madiraju, A. K. et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510, 542–546, https://doi.org/10.1038/nature13270 (2014).
Ben Sahra, I. et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res 71, 4366–4372, https://doi.org/10.1158/0008-5472.CAN-10-1769 (2011).
Kalender, A. et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab 11, 390–401, https://doi.org/10.1016/j.cmet.2010.03.014 (2010).
Cameron, A. R. et al. Anti-Inflammatory Effects of Metformin Irrespective of Diabetes Status. Circ Res 119, 652–665, https://doi.org/10.1161/CIRCRESAHA.116.308445 (2016).
Author information
Authors and Affiliations
Contributions
S.V. conceived the study. S.V. and B.V. wrote the manuscript. J.C.D. and M.F. performed experiments. J.C.D., M.F., B.V. and S.V. analysed and interpreted the data. All authors critically revised the manuscript and approved its final version.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deschemin, JC., Foretz, M., Viollet, B. et al. AMPK is not required for the effect of metformin on the inhibition of BMP6-induced hepcidin gene expression in hepatocytes. Sci Rep 7, 12679 (2017). https://doi.org/10.1038/s41598-017-12976-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-12976-2
This article is cited by
-
The gastrointestinal tract is a major source of the acute metformin-stimulated rise in GDF15
Scientific Reports (2024)
-
Exercise mimetics: harnessing the therapeutic effects of physical activity
Nature Reviews Drug Discovery (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.