Abstract
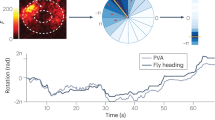
Neural circuits construct distributed representations of key variables—external stimuli or internal constructs of quantities relevant for survival, such as an estimate of one’s location in the world—as vectors of population activity. Although population activity vectors may have thousands of entries (dimensions), we consider that they trace out a low-dimensional manifold whose dimension and topology match the represented variable. This manifold perspective enables blind discovery and decoding of the represented variable using only neural population activity (without knowledge of the input, output, behavior or topography). We characterize and directly visualize manifold structure in the mammalian head direction circuit, revealing that the states form a topologically nontrivial one-dimensional ring. The ring exhibits isometry and is invariant across waking and rapid eye movement sleep. This result directly demonstrates that there are continuous attractor dynamics and enables powerful inference about mechanism. Finally, external rather than internal noise limits memory fidelity, and the manifold approach reveals new dynamical trajectories during sleep.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data have been previously reported21 and are available on the CRCNS website at http://crcns.org/data-sets/thalamus/th-1.
Code availability
The code is available at https://fietelab.mit.edu/code/.
References
Amari, S.-I. Dynamics of pattern formation in lateral-inhibition type neural fields. Biol. Cybern. 27, 77–87 (1977).
Hopfield, J. J. Neural networks and physical systems with emergent collective computational abilities. Proc. Natl Acad. Sci. USA 79, 2554–2558 (1982).
Seung, H. S. How the brain keeps the eyes still. Proc. Natl Acad. Sci. USA 93, 13339–13344 (1996).
Zhang, K. Representation of spatial orientation by the intrinsic dynamics of the head-direction cell ensemble: a theory. J. Neurosci. 15, 2112–2126 (1996).
Burak, Y. & Fiete, I. R. Accurate path integration in continuous attractor network models of grid cells. PLoS Comput. Biol. 5, e1000291 (2009).
Mazor, O. & Laurent, G. Transient dynamics versus fixed points in odor representations by locust antennal lobe projection neurons. Neuron 48, 661–673 (2005).
Mante, V., Sussillo, D., Shenoy, K. V. & Newsome, W. T. Context-dependent computation by recurrent dynamics in prefrontal cortex. Nature 503, 78–84 (2013).
Gallego, J. A., Perich, M. G., Miller, L. E. & Solla, S. A. Neural manifolds for the control of movement. Neuron 94, 978–984 (2017).
Ranck, J. B. in Electrical Activity of Archicortex (eds Buzsaki, G. & Vanderwolf, C.) 217–220 (Akademiai Kiado, 1985).
Taube, J. S., Muller, R. U. & Ranck, J. B. Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J. Neurosci. 10, 420–435 (1990).
Taube, J. S., Muller, R. U. & Ranck, J. B. Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J. Neurosci. 10, 436–447 (1990).
Finkelstein, A. et al. Three-dimensional head-direction coding in the bat brain. Nature 517, 159–164 (2015).
Seelig, J. D. & Jayaraman, V. Neural dynamics for landmark orientation and angular path integration. Nature 521, 186–191 (2015).
Green, J. et al. A neural circuit architecture for angular integration in Drosophila. Nature 546, 101–106 (2017).
Kim, S. S., Rouault, H., Druckmann, S. & Jayaraman, V. Ring attractor dynamics in the Drosophila central brain. Science 356, 849–853 (2017).
Skaggs, W. E., Knierim, J. J., Kudrimoti, H. S. & McNaughton, B. L. A model of the neural basis of the rat’s sense of direction. Adv. Neural Inf. Process. Syst. 7, 173–180 (1995).
Sharp, P. E., Blair, H. T. & Brown, M. Neural network modeling of the hippocampal formation spatial signals and their possible role in navigation: a modular approach. Hippocampus 6, 720–734 (1996).
Aronov, D., Nevers, R. & Tank, D. W. Mapping of a non-spatial dimension by the hippocampal–entorhinal circuit. Nature 543, 719–722 (2017).
Mizumori, S. & Williams, J. Directionally selective mnemonic properties of neurons in the lateral dorsal nucleus of the thalamus of rats. J. Neurosci. 13, 4015–4028 (1993).
Knierim, J. J., Kudrimoti, H. S. & McNaughton, B. L. Interactions between idiothetic cues and external landmarks in the control of place cells and head direction cells. J. Neurophysiol. 80, 425–446 (1998).
Peyrache, A., Lacroix, M. M., Petersen, P. C. & Buzsáki, G. Internally organized mechanisms of the head direction sense. Nat. Neurosci. 18, 569–575 (2015).
Chaudhuri, R., Gercek, B., Pandey, B. & Fiete, I. Unsupervised latent variable extraction from neural data to characterize processing across states. In Computational and Systems Neuroscience (CoSyNe) I-56 http://cosyne.org/cosyne17/Cosyne2017_program_book.pdf (2017).
Ghrist, R. Barcodes: the persistent topology of data. Bull. Am. Math. Soc. 45, 61–75 (2008).
Grassberger, P. & Procaccia, I. Measuring the strangeness of strange attractors. Phys. D. 9, 189–208 (1983).
Chen, Z. & Wilson, M. A. Deciphering neural codes of memory during sleep. Trends Neurosci. 40, 260–275 (2017).
Burak, Y. & Fiete, I. R. Fundamental limits on persistent activity in networks of noisy neurons. Proc. Natl Acad. Sci. USA 109, 17645–17650 (2012).
Moreno-Bote, R. et al. Information-limiting correlations. Nat. Neurosci. 17, 1410–1417 (2014).
Bialek, W. Physical limits to sensation and perception. Annu. Rev. Biophys. Biophys. Chem. 16, 455–478 (1987).
Osborne, L. C., Lisberger, S. G. & Bialek, W. A sensory source for motor variation. Nature 437, 412–416 (2005).
Pavlides, C. & Winson, J. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J. Neurosci. 9, 2907–2918 (1989).
Lee, A. K. & Wilson, M. A. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36, 1183–1194 (2002).
Diekelmann, S. & Born, J. The memory function of sleep. Nat. Rev. Neurosci. 11, 114–126 (2010).
Brandon, M. P., Bogaard, A. R., Andrews, C. M. & Hasselmo, M. E. Head direction cells in the postsubiculum do not show replay of prior waking sequences during sleep. Hippocampus 22, 604–618 (2012).
Massimini, M. et al. Breakdown of cortical effective connectivity during sleep. Science 309, 2228–2232 (2005).
Steriade, M., McCormick, D. A. & Sejnowski, T. J. Thalamocortical oscillations in the sleeping and aroused brain. Science 262, 679–685 (1993).
Gardner, R. J., Lu, L., Wernle, T., Moser, M.-B. & Moser, E. I. Correlation structure of grid cells is preserved during sleep. Nat. Neurosci. 22, 598–608 (2019).
Trettel, S. G., Trimper, J. B., Hwaun, E., Fiete, I. R. & Colgin, L. L. Grid cell co-activity patterns during sleep reflect spatial overlap of grid fields during active behaviors. Nat. Neurosci. 22, 609–617 (2019).
Siapas, A. G. & Wilson, M. A. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron 21, 1123–1128 (1998).
Wimmer, K., Nykamp, D. Q., Constantinidis, C. & Compte, A. Bump attractor dynamics in prefrontal cortex explains behavioral precision in spatial working memory. Nat. Neurosci. 17, 431–439 (2014).
Yoon, K. et al. Specific evidence of low-dimensional continuous attractor dynamics in grid cells. Nat. Neurosci. 16, 1077–1084 (2013).
Low, R. J., Lewallen, S., Aronov, D., Nevers, R. & Tank, D. W. Probing variability in a cognitive map using manifold inference from neural dynamics. Preprint at biorXiv https://www.biorxiv.org/content/10.1101/418939v2 (2018).
Bassett, J. P., Wills, T. J. & Cacucci, F. Self-organised attractor dynamics in the developing head direction circuit. Curr. Biol. 28, 609–615 (2018).
Tenenbaum, J. B., De Silva, V. & Langford, J. C. A global geometric framework for nonlinear dimensionality reduction. Science 290, 2319–2323 (2000).
Curto, C. & Itskov, V. Cell groups reveal structure of stimulus space. PLoS Comput. Biol. 4, e1000205 (2008).
Dabaghian, Y., Mémoli, F., Frank, L. & Carlsson, G. A topological paradigm for hippocampal spatial map formation using persistent homology. PLoS Comput. Biol. 8, e1002581 (2012).
Singh, G. et al. Topological analysis of population activity in visual cortex. J. Vis. 8, 1–18 (2008).
Spreemann, G., Dunn, B., Botnan, M. B. & Baas, N. A. Using persistent homology to reveal hidden information in neural data. Preprint at arXiv https://arxiv.org/abs/1510.06629 (2015).
Rybakken, E., Baas, N. & Dunn, B. Decoding of neural data using cohomological feature extraction. Neural Comput. 31, 68–93 (2019).
Park, M. et al. Bayesian manifold learning: the locally linear latent variable model. Adv. Neural Inf. Process. Syst. 28, 154–162 (2015).
Rubin, A. et al. Revealing neural correlates of behavior without behavioral measurements. Preprint at biorXiv https://www.biorxiv.org/content/10.1101/540195v1 (2019).
Bauer, U., Tralie, C. & Saul, N. Ripser. Github https://github.com/ctralie/ripser (2017).
Knierim, J. J., Kudrimoti, H. S. & McNaughton, B. L. Place cells, head direction cells, and the learning of landmark stability. J. Neurosci. 15, 1648–1659 (1995).
Acknowledgements
The authors thank D. Tank, S. Lewallen and R. Low for insightful discussions, and F. Caccuci, T. Wills, S. Deneve, Y. Burak, J. Murray, J. Pillow, L. Paninski and M. Sahani for comments on the work or manuscript. I.F. is grateful to G. Prasad for pointing her to the field of topological data analysis several years ago, and to W. Bialek for raising the possibility of unsupervised discovery of encoded variables from neural data, also several years ago. This work was supported in part by grants from the NIH (U01-NS094330-03), the Simons Foundation (SCGB and the International Brain Laboratory) and the Howard Hughes Medical Institute through the Faculty Scholars Program to I.F., and by the Canadian Research Chair in Systems Neuroscience (245716), a CIHR Project Grant (155957), a NSERC Discovery Grant (RGPIN-2018-04600) and the IRDC (108877-001) to A.P. Part of this work was performed by R.C. and I.F. in residence at the Simons Institute for the Theory of Computing at UC Berkeley, where R.C. was a Google Research Fellow.
Author information
Authors and Affiliations
Contributions
R.C. and I.F. conceived the decoding method and analyses. R.C., B.G. and B.P. implemented the analyses. A.P. collected the original data and advised on the data and analyses. R.C. and I.F. wrote the paper with input from the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information: Nature Neuroscience thanks Vivek Jayaraman, Kate Jeffery, Sung Soo Kim, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1
Low-dimensional structure in the head direction circuit.
Supplementary Figure 2
Distortion of manifold by linear methods and by inhomogeneous sampling.
Supplementary Figure 3
Toroidal manifold in simulated grid cell activity.
Supplementary Figure 4
Decoding performance does not strongly depend on embedding dimension or number of spline knots and performance is good with ~20 neurons and several minutes of data.
Supplementary Figure 5
Wake dynamics for other animals.
Supplementary Figure 6
Spiking variance and tuning curves explained by decoding.
Supplementary Figure 7
Ring manifold preserved during REM across animals.
Supplementary Figure 8
REM occupancy and flows.
Supplementary Figure 9
REM decoding across animals.
Supplementary Figure 10
Waking and REM states and dynamics replicated in a continuous attractor model.
Supplementary Figure 11
Loss of ring and higher-dimensional nREM manifold across animals.
Supplementary Figure 12
nREM decoding and dynamics controls.
Supplementary Figure 13
Decoding, dynamics, and trajectory statistics during nREM for Mouse 25 are similar to those observed for Mouse 28.
Supplementary Figure 14
Qualitative reproduction of nREM trajectory, dynamics, and statistics in continuous attractor ring network model.
Supplementary information
Supplementary Information
Supplementary Figs. 1–14 and Supplementary Notes 1–4.
Supplementary Video 1
Wake manifold. Three-dimensional Isomap embedding of the waking manifold from Mouse 28, session 140313.
Supplementary Video 2
REM manifold. Three-dimensional Isomap embedding of the REM manifold from mouse 28, session 140313.
Supplementary Video 3
Waking dynamics. Dynamics during 200 s of waking (projection generated using Isomap). The moving trace shows 1 s (most recent point is darkest). The blue points in the background show the waking manifold.
Supplementary Video 4
REM dynamics. Dynamics during 200 s of REM sleep (projection generated using Isomap). The moving trace shows 1 s (most recent point is darkest). The green scatter plot in the background shows the REM manifold. Note that REM sleep intervals are typically quite short, so the 200 s comprises multiple concatenated intervals.
Supplementary Video 5
nREM manifold. Three-dimensional Isomap embedding of the nREM manifold from mouse 28, session 140313, shown in mustard yellow, along with the waking manifold for comparison, shown in blue.
Supplementary Video 6
nREM dynamics. Dynamics during 200 s of nREM sleep (projection generated using Isomap). The moving trace shows 1 s (most recent point is darkest). The mustard yellow scatter plot in the background shows the nREM manifold, and the blue points show the waking manifold.
Rights and permissions
About this article
Cite this article
Chaudhuri, R., Gerçek, B., Pandey, B. et al. The intrinsic attractor manifold and population dynamics of a canonical cognitive circuit across waking and sleep. Nat Neurosci 22, 1512–1520 (2019). https://doi.org/10.1038/s41593-019-0460-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-019-0460-x
This article is cited by
-
Environment geometry alters subiculum boundary vector cell receptive fields in adulthood and early development
Nature Communications (2024)
-
Local origin of excitatory–inhibitory tuning equivalence in a cortical network
Nature Neuroscience (2024)
-
Preparatory activity and the expansive null-space
Nature Reviews Neuroscience (2024)
-
Minute-scale oscillatory sequences in medial entorhinal cortex
Nature (2024)
-
Mysterious ultraslow and ordered activity observed in the cortex
Nature (2024)