Abstract
For many of our senses, the role of the cerebral cortex in detecting stimuli is controversial1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17. Here we examine the effects of both acute and chronic inactivation of the primary somatosensory cortex in mice trained to move their large facial whiskers to detect an object by touch and respond with a lever to obtain a water reward. Using transgenic mice, we expressed inhibitory opsins in excitatory cortical neurons. Transient optogenetic inactivation of the primary somatosensory cortex, as well as permanent lesions, initially produced both movement and sensory deficits that impaired detection behaviour, demonstrating the link between sensory and motor systems during active sensing. Unexpectedly, lesioned mice had recovered full behavioural capabilities by the subsequent session. This rapid recovery was experience-dependent, and early re-exposure to the task after lesioning facilitated recovery. Furthermore, ablation of the primary somatosensory cortex before learning did not affect task acquisition. This combined optogenetic and lesion approach suggests that manipulations of the sensory cortex may be only temporarily disruptive to other brain structures that are themselves capable of coordinating multiple, arbitrary movements with sensation. Thus, the somatosensory cortex may be dispensable for active detection of objects in the environment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available from the corresponding author upon reasonable request.
References
Glickfeld, L. L., Histed, M. H. & Maunsell, J. H. R. Mouse primary visual cortex is used to detect both orientation and contrast changes. J. Neurosci. 33, 19416–19422 (2013).
Petruno, S. K., Clark, R. E. & Reinagel, P. Evidence that primary visual cortex is required for image, orientation, and motion discrimination by rats. PLoS ONE 8, e56543 (2013).
Lashley, K. S. The mechanism of vision IV. The cerebral areas necessary for pattern vision in the rat. J. Comp. Neurol. 53, 419–478 (1931).
Kato, H. K., Gillet, S. N. & Isaacson, J. S. Flexible sensory representations in auditory cortex driven by behavioral relevance. Neuron 88, 1027–1039 (2015).
Kelly, J. B. & Glazier, S. J. Auditory cortex lesions and discrimination of spatial location by the rat. Brain Res. 145, 315–321 (1978).
Talwar, S. K., Musial, P. G. & Gerstein, G. L. Role of mammalian auditory cortex in the perception of elementary sound properties. J. Neurophysiol. 85, 2350–2358 (2001).
Oliveira-Maia, A. J. et al. The insular cortex controls food preferences independently of taste receptor signaling. Front. Syst. Neurosci. 6, 5 (2012).
Peng, Y. et al. Sweet and bitter taste in the brain of awake behaving animals. Nature 527, 512–515 (2015).
Waiblinger, C., Brugger, D. & Schwarz, C. Vibrotactile discrimination in the rat whisker system is based on neuronal coding of instantaneous kinematic cues. Cereb. Cortex 25, 1093–1106 (2015).
Hutson, K. A. & Masterton, R. B. The sensory contribution of a single vibrissa’s cortical barrel. J. Neurophysiol. 56, 1196–1223 (1986).
Morita, T., Kang, H., Wolfe, J., Jadhav, S. P. & Feldman, D. E. Psychometric curve and behavioral strategies for whisker-based texture discrimination in rats. PLoS ONE 6, e20437 (2011).
Guo, Z. V. et al. Flow of cortical activity underlying a tactile decision in mice. Neuron 81, 179–194 (2014).
O’Connor, D. H., Peron, S. P., Huber, D. & Svoboda, K. Neural activity in barrel cortex underlying vibrissa-based object localization in mice. Neuron 67, 1048–1061 (2010).
Kwon, S. E., Yang, H., Minamisawa, G. & O’Connor, D. H. Sensory and decision-related activity propagate in a cortical feedback loop during touch perception. Nat. Neurosci. 19, 1243–1249 (2016).
Miyashita, T. & Feldman, D. E. Behavioral detection of passive whisker stimuli requires somatosensory cortex. Cereb. Cortex 23, 1655–1662 (2013).
Sachidhanandam, S., Sreenivasan, V., Kyriakatos, A., Kremer, Y. & Petersen, C. C. H. Membrane potential correlates of sensory perception in mouse barrel cortex. Nat. Neurosci. 16, 1671–1677 (2013).
Stüttgen, M. C. & Schwarz, C. Barrel cortex: what is it good for? Neuroscience 368, 3–16 (2018).
Otchy, T. M. et al. Acute off-target effects of neural circuit manipulations. Nature 528, 358–363 (2015).
Deutsch, D., Pietr, M., Knutsen, P. M., Ahissar, E. & Schneidman, E. Fast feedback in active sensing: touch-induced changes to whisker-object interaction. PLoS ONE 7, e44272 (2012).
Grant, R. A., Mitchinson, B., Fox, C. W. & Prescott, T. J. Active touch sensing in the rat: anticipatory and regulatory control of whisker movements during surface exploration. J. Neurophysiol. 101, 862–874 (2009).
Mitchinson, B. et al. Active vibrissal sensing in rodents and marsupials. Phil. Trans. R. Soc. Lond. B 366, 3037–3048 (2011).
Matyas, F. et al. Motor control by sensory cortex. Science 330, 1240–1243 (2010).
Harvey, M. A., Sachdev, R. N. & Zeigler, H. P. Cortical barrel field ablation and unconditioned whisking kinematics. Somatosens. Mot. Res. 18, 223–227 (2001).
Pammer, L. et al. The mechanical variables underlying object localization along the axis of the whisker. J. Neurosci. 33, 6726–6741 (2013).
Stüttgen, M. C. & Schwarz, C. Psychophysical and neurometric detection performance under stimulus uncertainty. Nat. Neurosci. 11, 1091–1099 (2008).
Keck, T. et al. Synaptic scaling and homeostatic plasticity in the mouse visual cortex in vivo. Neuron 80, 327–334 (2013).
Kawai, R. et al. Motor cortex is required for learning but not for executing a motor skill. Neuron 86, 800–812 (2015).
Brecht, M. The body model theory of somatosensory cortex. Neuron 94, 985–992 (2017).
Stüttgen, M. C., Schwarz, C. & Jäkel, F. Mapping spikes to sensations. Front. Neurosci. 5, 125 (2011).
Cohen, J. D. & Castro-Alamancos, M. A. Detection of low salience whisker stimuli requires synergy of tectal and thalamic sensory relays. J. Neurosci. 30, 2245–2256 (2010).
Huerta, M. F., Frankfurter, A. & Harting, J. K. Studies of the principal sensory and spinal trigeminal nuclei of the rat: projections to the superior colliculus, inferior olive, and cerebellum. J. Comp. Neurol. 220, 147–167 (1983).
Smith, J. B., Mowery, T. M. & Alloway, K. D. Thalamic POm projections to the dorsolateral striatum of rats: potential pathway for mediating stimulus-response associations for sensorimotor habits. J. Neurophysiol. 108, 160–174 (2012).
Gorski, J. A. et al. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci. 22, 6309–6314 (2002).
Scholl, B., Pattadkal, J. J., Dilly, G. A., Priebe, N. J. & Zemelman, B. V. Local integration accounts for weak selectivity of mouse neocortical parvalbumin interneurons. Neuron 87, 424–436 (2015).
O’Connor, D. H. et al. Vibrissa-based object localization in head-fixed mice. J. Neurosci. 30, 1947–1967 (2010).
Clack, N. G. et al. Automated tracking of whiskers in videos of head fixed rodents. PLoS Comput. Biol. 8, e1002591 (2012).
Hill, D. N., Curtis, J. C., Moore, J. D. & Kleinfeld, D. Primary motor cortex reports efferent control of vibrissa motion on multiple timescales. Neuron 72, 344–356 (2011).
Kleinfeld, D. & Deschênes, M. Neuronal basis for object location in the vibrissa scanning sensorimotor system. Neuron 72, 455–468 (2011).
Bruno, R. M. & Simons, D. J. Feedforward mechanisms of excitatory and inhibitory cortical receptive fields. J. Neurosci. 22, 10966–10975 (2002).
Pachitariu, M., Steinmetz, N., Kadir, S., Carandini, M. & Harris, K. D. Kilosort: realtime spike-sorting for extracellular electrophysiology with hundreds of channels. Preprint at https://www.biorxiv.org/content/early/2016/06/30/061481 (2016).
Rossant, C. et al. Spike sorting for large, dense electrode arrays. Nat. Neurosci. 19, 634–641 (2016).
Paxinos, G. & Franklin, K. B. J. The Mouse Brain in Stereotaxic Coordinates. 2nd ed. (Academic, New York, 2001).
Acknowledgements
We thank B. C. Pil and A. Kase for assistance with mouse training, H. Zeng and M. Kheirbek for Ai39 mice, A. Losonczy for PV–Cre mice, J. Huang for Rosa–H2B–GFP mice, N. Clack for suggestions on video analysis of whisker motion, B. Grewe for advice on cortical aspiration, and J. C. Tapia, M. Goldberg, N. Sawtell, T. Jessell, A. Kinnischtzke, D. Kato and G. Pierce for comments on the manuscript. Funding was provided by NIH R01 NS094659, R01 NS069679, the Klingenstein Fund, the Rita Allen Foundation, the Dana Foundation and the Ludwig Schaefer Scholars Program (R.M.B.); NIH F32 NS084768 (Y.K.H.), T32 MH015174 (Y.K.H., C.O.L.) and F32 NS096819 (C.C.R.).
Reviewer information
Nature thanks C. Schwarz and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Y.K.H. and R.M.B. conceived the experiments, analysed data and wrote the manuscript. Y.K.H. performed the experiments. C.O.L. and Y.K.H. developed the behavioural assay. C.C.R. assembled videography setup, C.C.R. and Y.K.H. performed array recordings, and C.C.R. analysed array data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
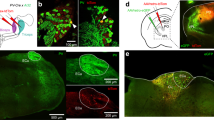
Extended Data Fig. 1 Optogenetic photoinhibition of cortical neurons in Emx1–Halo mice is highly efficacious.
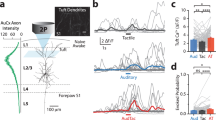
a, Juxtasomal recordings in awake, head-fixed mice were made below an optic fibre placed above a thinned, transparent skull. b, Raster plot for example neuron for randomly interleaved laser off (top) or on (bottom) trials, with air puff schematized below. c, Population peri-stimulus time histograms of 35 cells with regular-spiking (RS) waveforms (cortical depth: 280–1,120 µm, n = 4 mice) for laser-off and -on trials. Both spontaneous and whisker stimulus-evoked spikes are silenced. Shaded area: laser on. d, e, Efficiency of RS cell inactivation as a function of laser power (d) and cortical depth (e), where per cent inactivation is relative to a cell’s spike rate during laser-off trials. f, Lateral extent of inactivation. Illumination of 20–40 mW reliably inactivated an area within a 1-mm radius. g, Photoinhibition at 40 mW fully blocked spontaneous and sensory-evoked spikes (100% inactivation relative to laser-off trials) in 83% of RS cells and >96% of spikes in 94% of RS cells (same cells as in c); P = 3.1 × 10−11, Wilcoxon rank-sum test. h, Fast-spiking (FS) neurons (n = 8 cells) were similarly silenced; P = 3.1 × 10−8, Wilcoxon rank-sum test. i, Estimated area of photoinhibition with 40-mW relative to barrel cortex (1-mm radius around C2 barrel, red circle) depicted with a tangential section through barrel cortex of an Nr5a1–eYFP mouse with layer 4 labelled to visualize barrels. j, Emx1–Halo-mediated cortical inactivation was also assessed during detection behaviour with array recordings (n = 8 session, 3 mice). k, Data from Fig. 1e replotted on a logarithmic scale to show low spike rates during laser-on trials. l, Behavioural performance during laser-off and -on trials did not correlate with spiking activity for each trial type (four sessions from three mice with per cent correct for laser-off trials: 82, 87, 80, and 78%). m, Laser-on data in l, replotted with larger scale to visualize data during laser-on trials. Data shown as median ± interquartile range (d–h); mean ± s.e.m. (l, m).
Extended Data Fig. 2 Optogenetic manipulations of barrel cortex using PV–ChR and Emx1–Halo mice result in similar behavioural, motor, and sensory deficits.
a, Photoinhibition of excitatory neurons in Emx1–Halo mice (orange) and photoactivation of inhibitory neurons in PV–ChR mice (blue) yielded similar behavioural deficits. Negative control mice (Cre-negative, stop-Halo mice; black) were unaffected by 593-nm laser illumination. P values for Emx1–Halo: hit, 1.6 × 10−5; false alarm (FA), 1.7 × 10−3; per cent correct, 3.6 × 10−4; d-prime, 1.8 × 10−3. For PV–ChR: hit, 3.5 × 10−3; per cent correct, 3.9 × 10−3; d-prime, 0.0498. b, Optogenetic inactivation of S1 with either Emx1–Halo or PV–ChR mice decreases whisking kinematics. P values for Emx1–Halo: NOGO peak velocity, 6.6 × 10−3; max angle, 1.4 × 10−3; peak amplitude, 2.6 × 10−2; GO peak velocity, 4.3 × 10−4; max change in curvature (ΔΚ), 1.9 × 10−2; per cent trials with no contacts, 1.2 × 10−2. PV–ChR: NOGO peak velocity, 8.1 × 10−3; max angle, 8.7 × 10−2; peak amplitude, 8.4 × 10−2; GO peak velocity, 5.7 × 10−3; ΔΚ, 8.7 × 10−3; per cent trials with no contacts, 7.5 × 10−3. c, Sensory thresholds increase with optogenetic inactivation. P values for Emx1–Halo: curvature threshold, 9.7 × 10−4; contact threshold, 3.4 × 10−3. PV–ChR: curvature threshold, 1.1 × 10−1; contact threshold, 1.2 × 10−2. Data for negative control and Emx1–Halo mice are the same as in Figs. 2, 3 but repeated here for comparison with PV–ChR mice.
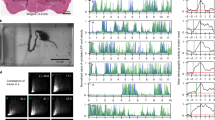
Extended Data Fig. 3 Defining contacts based on whisker angle and change in curvature.
a, Example curvature versus whisker position for a single session. Each circle represents the paired values for curvature and whisker angle for each frame during the session. Values for NOGO trials define whisker parameters during free whisking in air, when no contacts can be made (black); GO trials are shown in red. Linear regression was used to define the line of best fit (blue, solid line) for NOGO parameters, and upper and lower contact thresholds were set by finding the offsets that encompassed the no-contact parameter space (1–5 s.d. from the line of best fit, blue dashed lines). b, Putative contacts were defined as points at which the local maxima or minima of the change in curvature were above (forward contact with whisker) or below (reverse contact with whisker) the defined thresholds (tick marks). Whisking analysis was restricted to the 200-ms time window (yellow shaded area) during sampling, before the average response time (green).
Extended Data Fig. 4 Behavioural performance after lesions did not correlate with lesion size.
a, Mice performed the task with a single C2 whisker. b, The location of the C2 barrel in a coronal section. The C2 barrel was functionally mapped with intrinsic imaging and Alexa-conjugated cholera toxin subunit B (CTB, red) was injected into the centre of the C2 barrel. Blue, DAPI. Mappings in b and c are used to inform the locations of lesions made relative to the C2 barrel column. c, Equivalent location in section from an Nr5a1–eYFP mouse with barrels fluorescently labelled in L4 (white) overlaid on bright-field image to show extent of barrel cortex relative to section (black lines). C2 was located about 1.2–1.5 mm posterior to bregma, varying slightly between mice. Lesions were centred around C2. d, Size and locations of contralateral barrel cortex lesions for the 11 mice with 1-day rest shown in Fig. 3 (arranged from largest to smallest by lesion volume). For each mouse, three locations along the anterior–posterior axis are shown overlaid on atlas images, reproduced with permission from ref. 42. In a few mice (for example, mice 1, 3 and 8), lesions extended into the secondary somatosensory area (S2). Numbers along anterior–posterior axis indicate approximate location relative to bregma. e, Lesion size did not correlate with the degree of impairment on the first (grey) or second post-lesion session when behavioural performance had recovered (red). Performance was normalized to the pre-lesion performance for each mouse. f, Lesion sizes were similar between groups with 1 or 3 days of rest after lesioning (P = 0.91).
Extended Data Fig. 5 Ipsilateral S1 does not compensate for loss of contralateral S1.
a, Behavioural performance of mice recovered rapidly after contralateral S1 lesions, as shown in Fig. 3c (red). Subsequent ipsilateral lesion (n = 6) had effects similar to sham and ipsilateral-only manipulations shown in Fig. 3c, indicating that ipsilateral S1 was not compensating for loss of contralateral S1 activity. b, C2 whisker-trim control. Performance of bilaterally lesioned mice dropped to chance when the C2 whisker was removed (P = 2.7 × 10−3). c, Sizes of bilateral lesions.
Extended Data Fig. 6 Example histology from lesioned mice depicting lesion of contralateral S1 only or additional damage to striatum.
a, Unlesioned example. b, c, Examples of S1-only lesions from two mice. d–f, Three examples of damage to striatum in addition to S1. Even minimal damage (arrows) to the dorsolateral striatum resulted in permanent behaviour deficits. Scale bar, 1 mm.
Extended Data Fig. 7 Learning curves for unlesioned and lesioned mice in learning experiment.
a, Individual learning curves for unlesioned mice (n = 11, blue lines) and mice with lesions of contralateral barrel cortex (n = 9, orange lines) that learned the detection task to criterion (74% correct performance for two consecutive sessions). Mice were first introduced to the pole with 90% GO trials (red lines). This intermediate step ensured that mice maintained a stable weight before moving on to the last step of training. Mice were moved onto 60% GO trials for the rest of the learning assessment. Mice were given 48 sessions to learn the task. b, Unlesioned and lesioned groups spent similar times on 90% GO sessions (3.8 ± 1.8 versus 3.5 ± 1.1 sessions for unlesioned and lesioned mice, respectively; P = 0.64). c, By the end of the 90% GO sessions, performance was still at chance levels (P = 0.34 and P = 0.37 for unlesioned and lesioned mice, respectively; one-sample t-test).
Supplementary information
Rights and permissions
About this article
Cite this article
Hong, Y.K., Lacefield, C.O., Rodgers, C.C. et al. Sensation, movement and learning in the absence of barrel cortex. Nature 561, 542–546 (2018). https://doi.org/10.1038/s41586-018-0527-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0527-y
Keywords
This article is cited by
-
The influence of cortical activity on perception depends on behavioral state and sensory context
Nature Communications (2024)
-
Direct contribution of the sensory cortex to the judgment of stimulus duration
Nature Communications (2024)
-
Nigrostriatal dopamine modulates the striatal-amygdala pathway in auditory fear conditioning
Nature Communications (2023)
-
How deep is the brain? The shallow brain hypothesis
Nature Reviews Neuroscience (2023)
-
Behaviorally relevant decision coding in primary somatosensory cortex neurons
Nature Neuroscience (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.