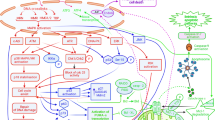
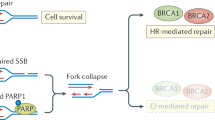
Abstract
Platinum (Pt) compounds entered the clinic as anticancer agents when cisplatin was approved in 1978. More than 40 years later, even in the era of precision medicine and immunotherapy, Pt drugs remain among the most widely used anticancer drugs. As Pt drugs mainly target DNA, it is not surprising that recent insights into alterations of DNA repair mechanisms provide a useful explanation for their success. Many cancers have defective DNA repair, a feature that also sheds new light on the mechanisms of secondary drug resistance, such as the restoration of DNA repair pathways. In addition, genome-wide functional screening approaches have revealed interesting insights into Pt drug uptake. About half of cisplatin and carboplatin but not oxaliplatin may enter cells through the widely expressed volume-regulated anion channel (VRAC). The analysis of this heteromeric channel in tumour biopsies may therefore be a useful biomarker to stratify patients for initial Pt treatments. Moreover, Pt-based approaches may be improved in the future by the optimization of combinations with immunotherapy, management of side effects and use of nanodelivery devices. Hence, Pt drugs may still be part of the standard of care for several cancers in the coming years.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
US Food and Drug Administration. Drugs@FDA: FDA-approved drugs. New drug application (NDA): 018057 (FDA, 2019).
Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 7, 573–584 (2007).
Kauffman, G. B., Pentimalli, R., Doldi, S. & Hall, M. D. Michele Peyrone (1813–1883), discoverer of cisplatin. Platin Met. Rev. 54, 250–256 (2010).
Rosenberg, B., van Camp, L. & Krigas, T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 205, 698–699 (1965).
Wiltshaw, E. Cisplatin in the treatment of cancer. Platin Met. Rev. 23, 90–98 (1979).
US Food and Drug Administration. Drugs@FDA: FDA-approved drugs. Abbreviated new drug application (ANDA): 077139 (FDA, 2012).
Perego, P. & Robert, J. Oxaliplatin in the era of personalized medicine: from mechanistic studies to clinical efficacy. Cancer Chemother. Pharmacol. 77, 5–18 (2016).
Dilruba, S. & Kalayda, G. V. Platinum-based drugs: past, present and future. Cancer Chemother. Pharmacol. 77, 1103–1124 (2016).
Lippard, S. J. New chemistry of an old molecule: cis-[Pt(NH3)2Cl2. Science 218, 1075–1082 (1982).
Wang, D. & Lippard, S. J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 4, 307–320 (2005).
Burger, H. et al. Drug transporters of platinum-based anticancer agents and their clinical significance. Drug Resist. Updat. 14, 22–34 (2011).
Borst, P., Rottenberg, S. & Jonkers, J. How do real tumors become resistant to cisplatin? Cell Cycle 7, 1353–1359 (2008).
Sakai, W. et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature 451, 1116–1120 (2008). This study shows that secondary intragenic BRCA2 mutations restore the wild-type reading frame as a mechanism of resistance to cisplatin in cancer cell lines and clinical specimens.
Zhao, W., Wiese, C., Kwon, Y., Hromas, R. & Sung, P. The BRCA tumor suppressor network in chromosome damage repair by homologous recombination. Annu. Rev. Biochem. 88, 221–245 (2019).
Harris, A. L. DNA repair and resistance to chemotherapy. Cancer Surv. 4, 601–624 (1985).
Lord, C. J. & Ashworth, A. The DNA damage response and cancer therapy. Nature 481, 287–294 (2012).
Nickoloff, J. A., Jones, D., Lee, S. H., Williamson, E. A. & Hromas, R. Drugging the cancers addicted to DNA repair. J. Natl. Cancer Inst. 109, djx059 (2017).
Miki, Y. et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266, 66–71 (1994).
Wooster, R. et al. Identification of the breast cancer susceptibility gene BRCA2. Nature 378, 789–792 (1995).
Nik-Zainal, S. et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature 534, 47–54 (2016).
von Minckwitz, G. et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 15, 747–756 (2014).
Telli, M. L. et al. Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin. Cancer Res. 22, 3764–3773 (2016).
Silver, D. P. et al. Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J. Clin. Oncol. 28, 1145–1153 (2010).
Vollebergh, M. A. et al. An aCGH classifier derived from BRCA1-mutated breast cancer and benefit of high-dose platinum-based chemotherapy in HER2-negative breast cancer patients. Ann. Oncol. 22, 1561–1570 (2011).
Vollebergh, M. A. et al. Genomic patterns resembling BRCA1- and BRCA2-mutated breast cancers predict benefit of intensified carboplatin-based chemotherapy. Breast Cancer Res. 16, R47 (2014).
Bryant, H. E. et al. Specific killing of BRCA2-deficient tumors with inhibitors of poly(ADP-ribose) polymerase. Nature 434, 913–917 (2005).
Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005).
Ledermann, J. et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 15, 852–861 (2014).
Ledermann, J. A. & Pujade-Lauraine, E. Olaparib as maintenance treatment for patients with platinum-sensitive relapsed ovarian cancer. Ther. Adv. Med. Oncol. 11, 1758835919849753 (2019).
Welsh, C. et al. Reduced levels of XPA, ERCC1 and XPF DNA repair proteins in testis tumor cell lines. Int. J. Cancer 110, 352–361 (2004).
Fenske, A. E. et al. Cisplatin resistance induced in germ cell tumor cells is due to reduced susceptibility towards cell death but not to altered DNA damage induction or repair. Cancer Lett. 324, 171–178 (2012).
Bagrodia, A. et al. Genetic determinants of cisplatin resistance in patients with advanced germ cell tumors. J. Clin. Oncol. 34, 4000–4007 (2016).
Luvero, D. et al. Ovarian cancer relapse: from the latest scientific evidence to the best practice. Crit. Rev. Oncol. Hematol. 140, 28–38 (2019).
Lo Russo, G., Imbimbo, M. & Garassino, M. C. Is the chemotherapy era in advanced non-small cell lung cancer really over? Maybe not yet. Tumori 3, 223–225 (2016).
Gately, D. P. & Howell, S. B. Cellular accumulation of the anticancer agent cisplatin: a review. Br. J. Cancer 67, 1171–1176 (1993).
Harrach, S. & Ciarimboli, G. Role of transporters in the distribution of platinum-based drugs. Front. Pharmacol. 6, 85 (2015).
Pan, B. F., Sweet, D. H., Pritchard, J. B., Chen, R. & Nelson, J. A. A transfected cell model for the renal toxin transporter, rOCT2. Toxicol. Sci. 47, 181–186 (1999).
Jong, N. N., Nakanishi, T., Liu, J. J., Tamai, I. & McKeage, M. J. Oxaliplatin transport mediated by organic cation/carnitine transporters OCTN1 and OCTN2 in overexpressing human embryonic kidney 293 cells and rat dorsal root ganglion neurons. J. Pharmacol. Exp. Ther. 338, 537–547 (2011).
Ishida, S., Lee, J., Thiele, D. J. & Herskowitz, I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc. Natl Acad. Sci. USA 99, 14298–14302 (2002).
Wen, X. et al. Transgenic expression of the human MRP2 transporter reduces cisplatin accumulation and nephrotoxicity in Mrp2-null mice. Am. J. Pathol. 184, 1299–1308 (2014).
Myint, K., Li, Y., Paxton, J. & McKeage, M. Multidrug resistance-associated protein 2 (MRP2) mediated transport of oxaliplatin-derived platinum in membrane vesicles. PLoS ONE 10, e0130727 (2015).
Myint, K. et al. Identification of MRP2 as a targetable factor limiting oxaliplatin accumulation and response in gastrointestinal cancer. Sci. Rep. 9, 2245 (2019).
Hall, M. D., Okabe, M., Shen, D. W., Liang, X. J. & Gottesman, M. M. The role of cellular accumulation in determining sensitivity to platinum-based chemotherapy. Annu. Rev. Pharmacol. Toxicol. 48, 495–535 (2008).
De Luca, A. et al. A structure-based mechanism of cisplatin resistance mediated by glutathione transferase P1-1. Proc. Natl Acad. Sci. USA 116, 13943–13951 (2019).
Planells-Cases, R. et al. Subunit composition of VRAC channels determines substrate specificity and cellular resistance to Pt-based anti-cancer drugs. EMBO J. 34, 2993–3008 (2015). This study demonstrates that around 50% of cisplatin uptake is dependent on the LRRC8A and LRRC8D VRAC subunits.
He, Y. J. et al. DYNLL1 binds to MRE11 to limit DNA end resection in BRCA1-deficient cells. Nature 563, 522–526 (2018).
Sorensen, B. H., Dam, C. S., Sturup, S. & Lambert, I. H. Dual role of LRRC8A-containing transporters on cisplatin resistance in human ovarian cancer cells. J. Inorg. Biochem. 160, 287–295 (2016).
Perez, R. P. Cellular and molecular determinants of cisplatin resistance. Eur. J. Cancer 34, 1535–1542 (1998).
Cossa, G., Gatti, L., Zunino, F. & Perego, P. Strategies to improve the efficacy of platinum compounds. Curr. Med. Chem. 16, 2355–2365 (2009).
Perego, P. et al. Association between cisplatin resistance and mutation of p53 gene and reduced bax expression in ovarian carcinoma cell systems. Cancer Res. 56, 556–562 (1996).
Wu, A. Y. et al. Fn14 overcomes cisplatin resistance of high-grade serous ovarian cancer by promoting Mdm2-mediated p53-R248Q ubiquitination and degradation. J. Exp. Clin. Cancer Res. 38, 176 (2019).
Hanna, N. H. & Einhorn, L. H. Testicular cancer — discoveries and updates. N. Engl. J. Med. 371, 2005–2016 (2014).
Swisher, E. M. et al. Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 68, 2581–2586 (2008). This study describes secondary BRCA1 mutations as a mechanism of resistance to cisplatin in ovarian carcinoma clinical specimens.
Rottenberg, S. et al. Selective induction of chemotherapy resistance of mammary tumors in a conditional mouse model for hereditary breast cancer. Proc. Natl Acad. Sci. USA 104, 12117–12122 (2007).
Pajic, M. et al. Selected alkylating agents can overcome drug tolerance of G0-like tumor cells and eradicate BRCA1-deficient mammary tumors in mice. Clin. Cancer Res. 23, 7020–7033 (2017).
Jaspers, J. E. et al. Loss of 53BP1 causes PARP inhibitor resistance in Brca1-mutated mouse mammary tumors. Cancer Discov. 3, 68–81 (2013).
Cavallo, F. et al. Reduced proficiency in homologous recombination underlies the high sensitivity of embryonal carcinoma testicular germ cell tumors to cisplatin and poly(ADP-ribose) polymerase inhibition. PLoS ONE 7, e51563 (2012).
Chaudhuri, A. R. et al. Replication fork stability confers chemoresistance in BRCA-deficient cells. Nature 535, 382–387 (2016). This paper demonstrates the relevance of replication fork stability as a determinant of resistance to cisplatin.
Becker, J. R. et al. The ASCIZ–DYNLL1 axis promotes 53BP1-dependent non-homologous end joining and PARP inhibitor sensitivity. Nat. Commun. 9, 5406 (2018).
Elaimy, A. L. et al. The VEGF receptor neuropilin 2 promotes homologous recombination by stimulating YAP/TAZ-mediated Rad51 expression. Proc. Natl Acad. Sci. USA 116, 14174–14180 (2019).
Liptay, M., Barbosa, J. S. & Rottenberg, S. Replication fork remodeling and therapy escape in DNA damage response-deficient cancers. Front. Oncol. 10, 670 (2020).
Schlacher, K. et al. Double-strand break repair-independent role for BRCA2 in blocking stalled replication fork degradation by MRE11. Cell 145, 529–542 (2011).
Li, Q. et al. ERCC2 helicase domain mutations confer nucleotide excision repair deficiency and drive cisplatin sensitivity in muscle-invasive bladder cancer. Clin. Cancer Res. 25, 977–988 (2019).
Wojtaszek, J. L. et al. A small molecule targeting mutagenic translesion synthesis improves chemotherapy. Cell 178, 152–159.e11 (2019). This study identifies the first compound that sensitizes cells to cisplatin while inhibiting REV1-dependent mutagenic TLS.
Kuczynski, E. A., Sargent, D. J., Grothey, A. & Kerbel, R. S. Drug rechallenge and treatment beyond progression — implications for drug resistance. Nat. Rev. Clin. Oncol. 10, 571–587 (2013).
Glasspool, R. M., Teodoridis, J. M. & Brown, R. Epigenetics as a mechanism driving polygenic clinical drug resistance. Br. J. Cancer 94, 1087–1092 (2006).
Sharma, S. V. et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 141, 69–80 (2010). This paper demonstrates the occurrence of unstable and non-hereditable drug resistance upon cisplatin treatment.
Borst, P. Cancer drug pan-resistance: pumps, cancer stem cells, quiescence, epithelial to mesenchymal transition, blocked cell death pathways, persisters or what? Open Biol. 2, 120066 (2012).
Clevers, H. The cancer stem cell: premises, promises and challenges. Nat. Med. 17, 313–319 (2011).
Sharma, A. et al. Longitudinal single-cell RNA sequencing of patient-derived primary cells reveals drug-induced infidelity in stem cell hierarchy. Nat. Commun. 9, 4931 (2018).
Hou, M. F. et al. The NuRD complex-mediated p21 suppression facilitates chemoresistance in BRCA-proficient breast cancer. Exp. Cell Res. 359, 458–465 (2017).
Guillemette, S. et al. Resistance to therapy in BRCA2 mutant cells due to loss of the nucleosome remodeling factor CHD4. Genes Dev. 29, 489–494 (2015).
Almeida, L. O. et al. NFκB mediates cisplatin resistance through histone modifications in head and neck squamous cell carcinoma (HNSCC). FEBS Open Bio 4, 96–104 (2013).
Hu, S. et al. Overexpression of EZH2 contributes to acquired cisplatin resistance in ovarian cancer cells in vitro and in vivo. Cancer Biol. Ther. 10, 788–795 (2010).
Brown, R., Curry, E., Magnani, L., Wilhelm-Benartzi, C. S. & Borley, J. Poised epigenetic states and acquired drug resistance in cancer. Nat. Rev. Cancer 14, 747–753 (2014).
Rottenberg, S. et al. Impact of intertumoral heterogeneity on predicting chemotherapy response of BRCA1-deficient mammary tumors. Cancer Res. 72, 2350–2361 (2012).
Schouten, P. C. et al. High XIST and low 53BP1 expression predict poor outcome after high-dose alkylating chemotherapy in patients with a BRCA1-like breast cancer. Mol. Cancer Ther. 15, 190–198 (2016).
Sun, W., Zu, Y., Fu, X. & Deng, Y. Knockdown of lncRNA-XIST enhances the chemosensitivity of NSCLC cells via suppression of autophagy. Oncol. Rep. 38, 3347–3354 (2017).
Cassinelli, G. et al. Targeting the Akt kinase to modulate survival, invasiveness and drug resistance of cancer cells. Curr. Med. Chem. 20, 1923–1945 (2013).
Cossa, G. et al. Modulation of sensitivity to antitumor agents by targeting the MAPK survival pathway. Curr. Pharm. Des. 19, 883–894 (2013).
Jin, L. et al. MAST1 drives cisplatin resistance in human cancers by rewiring cRaf-independent MEK activation. Cancer Cell 34, 315–330.e7 (2018). This study strengthens the relevance of pathways inhibiting cisplatin-induced apoptosis in drug resistance, providing new options for treating resistant cancers with activation of survival pathways.
Cossa, G. et al. Differential outcome of MEK1/2 inhibitor–platinum combinations in platinum-sensitive and -resistant ovarian carcinoma cells. Cancer Lett. 347, 212–224 (2014).
Ishibashi, M. et al. Tyrosine kinase receptor TIE-1 mediates platinum resistance by promoting nucleotide excision repair in ovarian cancer. Sci. Rep. 8, 13207 (2018).
Olive, K. P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009).
Coffelt, S. B. & de Visser, K. E. Immune-mediated mechanisms influencing the efficacy of anticancer therapies. Trends Immunol. 36, 198–216 (2015).
Wu, T. & Dai, Y. Tumor microenvironment and therapeutic response. Cancer Lett. 387, 61–68 (2017).
Wang, W. et al. Effector T cells abrogate stroma-mediated chemoresistance in ovarian cancer. Cell 165, 1092–1105 (2016). This study shows that cisplatin resistance can occur by a non-genetic mechanism in the TME in which CAFs regulate thiol metabolism, thereby impairing cisplatin accumulation in ovarian cancer cells.
Diaz-Maroto, N. G. et al. Noncanonical TGFβ pathway relieves the blockade of IL1β/TGFβ-mediated crosstalk between tumor and stroma: TGFBR1 and TAK1 inhibition in colorectal cancer. Clin. Cancer Res. 25, 4466–4479 (2019).
Dijkgraaf, E. M. et al. Chemotherapy alters monocyte differentiation to favor generation of cancer-supporting M2 macrophages in the tumor microenvironment. Cancer Res. 73, 2480–2492 (2013).
Sommariva, M. et al. TLR9 agonists oppositely modulate DNA repair genes in tumor versus immune cells and enhance chemotherapy effects. Cancer Res. 71, 6382–6390 (2011).
Iida, N. et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342, 967–970 (2013). This study shows in mouse models of cancer that an intact microbiota is required for optimal activity of oxaliplatin, which is associated with induction of ROS contributed by tumour-infiltrating myeloid cells.
Bienvenu, P., Caron, L., Gasparutto, D. & Kergonou, J. F. Assessing and counteracting the prooxidant effects of anticancer drugs. EXS 62, 257–265 (1992).
Socinski, M. A. et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Engl. J. Med. 378, 2288–2301 (2018).
West, H. et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 20, 924–937 (2019).
Gandhi, L. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 378, 2078–2092 (2018).
Schmid, P. et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 382, 810–821 (2020).
Le, D. T. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Paz-Ares, L. et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 394, 1929–1939 (2019).
Kroon, P. et al. Radiotherapy and cisplatin increase immunotherapy efficacy by enabling local and systemic intratumoral T-cell activity. Cancer Immunol. Res. 7, 670–682 (2019).
Ramakrishnan, R. et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J. Clin. Invest. 120, 1111–1124 (2010).
Galluzzi, L., Buque, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17, 97–111 (2017).
Ghiringhelli, F. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nat. Med. 15, 1170–1178 (2009).
Obeid, M. et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 13, 54–61 (2007).
Tesniere, A. et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 29, 482–491 (2010).
Munn, D. H. & Bronte, V. Immune suppressive mechanisms in the tumor microenvironment. Curr. Opin. Immunol. 39, 1–6 (2016).
de Biasi, A. R., Villena-Vargas, J. & Adusumilli, P. S. Cisplatin-induced antitumor immunomodulation: a review of preclinical and clinical evidence. Clin. Cancer Res. 20, 5384–5391 (2014).
Galluzzi, L., Buque, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 28, 690–714 (2015).
Lesterhuis, W. J. et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J. Clin. Invest. 121, 3100–3108 (2011). This study highlights the capability of Pt compounds to down-modulate immunosuppressive molecules, particularly PDL2.
Blatter, S. et al. Chemotherapy induces an immunosuppressive gene expression signature in residual BRCA1/p53-deficient mouse mammary tumors. J. Mol. Clin. Med. 1, 7–17 (2018).
Grabosch, S. et al. Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene 38, 2380–2393 (2019).
Khoo, L. T. & Chen, L. Y. Role of the cGAS–STING pathway in cancer development and oncotherapeutic approaches. EMBO Rep. 19, e46935 (2018).
Della Corte, C. M. et al. STING pathway expression identifies NSCLC with an immune-responsive phenotype. J. Thorac. Oncol. 15, 777–791 (2020).
Harabuchi, S. et al. Intratumoral STING activations overcome negative impact of cisplatin on antitumor immunity by inflaming tumor microenvironment in squamous cell carcinoma. Biochem. Biophys. Res. Commun. 522, 408–414 (2020).
Fu, D. et al. T cell recruitment triggered by optimal dose platinum compounds contributes to the therapeutic efficacy of sequential PD-1 blockade in a mouse model of colon cancer. Am. J. Cancer Res. 10, 473–490 (2020).
Lips, E. H. et al. BRCAness digitalMLPA profiling predicts benefit of intensified platinum-based chemotherapy in triple-negative and luminal-type breast cancer. Breast Cancer Res. 22, 79 (2020).
Sarkar, A. Novel platinum compounds and nanoparticles as anticancer agents. Pharm. Pat. Anal. 7, 33–46 (2018).
Johnstone, T. C., Suntharalingam, K. & Lippard, S. J. The next generation of platinum drugs: targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem. Rev. 116, 3436–3486 (2016).
Komeda, S. et al. The phosphate clamp: a small and independent motif for nucleic acid backbone recognition. Nucleic Acids Res. 39, 325–336 (2011).
Rosa, N. M. P., Ferreira, F. H. D. C., Farrell, N. P. & Costa, L. A. S. TriplatinNC and biomolecules: building models based on non-covalent interactions. Front. Chem. 7, 307 (2019).
Gatti, L. et al. Novel bis-platinum complexes endowed with an improved pharmacological profile. Mol. Pharm. 7, 207–216 (2010).
Almaqwashi, A. A. et al. DNA intercalation facilitates efficient DNA-targeted covalent binding of phenanthriplatin. J. Am. Chem. Soc. 141, 1537–1545 (2019).
Zheng, Y. R. et al. Pt(IV) prodrugs designed to bind non-covalently to human serum albumin for drug delivery. J. Am. Chem. Soc. 136, 8790–8798 (2014).
Arosio, D., Manzoni, L., Corno, C. & Perego, P. Integrin-targeted peptide- and peptidomimetic-drug conjugates for the treatment of tumors. Recent Pat. Anticancer Drug Discov. 12, 148–168 (2017).
Stathopoulos, G. P. et al. Comparison of liposomal cisplatin versus cisplatin in non-squamous cell non-small-cell lung cancer. Cancer Chemother. Pharmacol. 68, 945–950 (2011).
Ghaferi, M., Asadollahzadeh, M. J., Akbarzadeh, A., Ebrahimi Shahmabadi, H. & Alavi, S. E. Enhanced efficacy of PEGylated liposomal cisplatin: in vitro and in vivo evaluation. Int. J. Mol. Sci. 21, 559 (2020).
Baumann, P. et al. CD24 expression causes the acquisition of multiple cellular properties associated with tumor growth and metastasis. Cancer Res. 65, 10783–10793 (2005).
Ashihara, K. et al. Pharmacokinetic evaluation and antitumor potency of liposomal nanoparticle encapsulated cisplatin targeted to CD24-positive cells in ovarian cancer. Oncol. Lett. 19, 1872–1880 (2020).
Wolff, J. E., Berrak, S., Koontz Webb, S. E. & Zhang, M. Nitrosourea efficacy in high-grade glioma: a survival gain analysis summarizing 504 cohorts with 24193 patients. J. Neurooncol. 88, 57–63 (2008).
Brock, P. R. et al. Sodium thiosulfate for protection from cisplatin-induced hearing loss. N. Engl. J. Med. 378, 2376–2385 (2018).
Berndtsson, M. et al. Acute apoptosis by cisplatin requires induction of reactive oxygen species but is not associated with damage to nuclear DNA. Int. J. Cancer 120, 175–180 (2007).
Alberti, E., Zampakou, M. & Donghi, D. Covalent and non-covalent binding of metal complexes to RNA. J. Inorg. Biochem. 163, 278–291 (2016).
Russo Krauss, I., Ferraro, G. & Merlino, A. Cisplatin–protein interactions: unexpected drug binding to N-terminal amine and lysine side chains. Inorg. Chem. 55, 7814–7816 (2016).
Takasato, M. et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564–568 (2015).
Freyer, D. R. et al. Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 18, 63–74 (2017).
Oun, R., Moussa, Y. E. & Wheate, N. J. The side effects of platinum-based chemotherapy drugs: a review for chemists. Dalton Trans. 47, 6645–6653 (2018).
Lv, F., Ma, Y., Zhang, Y. & Li, Z. Relationship between GSTP1 rs1695 gene polymorphism and myelosuppression induced by platinum-based drugs: a meta-analysis. Int. J. Biol. Markers 33, 364–371 (2018).
Crona, D. J. et al. A systematic review of strategies to prevent cisplatin-induced nephrotoxicity. Oncologist 22, 609–619 (2017).
Kanat, O., Ertas, H. & Caner, B. Platinum-induced neurotoxicity: a review of possible mechanisms. World J. Clin. Oncol. 8, 329–335 (2017).
Avan, A. et al. Platinum-induced neurotoxicity and preventive strategies: past, present, and future. Oncologist 20, 411–432 (2015).
Yan, F., Liu, J. J., Ip, V., Jamieson, S. M. & McKeage, M. J. Role of platinum DNA damage-induced transcriptional inhibition in chemotherapy-induced neuronal atrophy and peripheral neurotoxicity. J. Neurochem. 135, 1099–1112 (2015).
Karasawa, T. & Steyger, P. S. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol. Lett. 237, 219–227 (2015).
More, S. S. et al. Role of the copper transporter, CTR1, in platinum-induced ototoxicity. J. Neurosci. 30, 9500–9509 (2010).
Ciarimboli, G. et al. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am. J. Pathol. 176, 1169–1180 (2010).
Breglio, A. M. et al. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat. Commun. 8, 1654 (2017).
Sooriyaarachchi, M., Gailer, J., Dolgova, N. V., Pickering, I. J. & George, G. N. Chemical basis for the detoxification of cisplatin-derived hydrolysis products by sodium thiosulfate. J. Inorg. Biochem. 162, 96–101 (2016).
Elferink, F., van der Vijgh, W. J., Klein, I. & Pinedo, H. M. Interaction of cisplatin and carboplatin with sodium thiosulfate: reaction rates and protein binding. Clin. Chem. 32, 641–645 (1986).
Allan, S. G., Smyth, J. F., Hay, F. G., Leonard, R. C. & Wolf, C. R. Protective effect of sodium-2-mercaptoethanesulfonate on the gastrointestinal toxicity and lethality of cis-diamminedichloroplatinum. Cancer Res. 46, 3569–3573 (1986).
Perales-Puchalt, A. et al. Frontline science: microbiota reconstitution restores intestinal integrity after cisplatin therapy. J. Leukoc. Biol. 103, 799–805 (2018).
Acknowledgements
The authors thank P. Borst (The Netherlands Cancer Institute, Amsterdam), P. Francica (University of Bern, Switzerland), M. Mutlu (University of Bern, Switzerland), D. Colombo (University of Milan, Italy), M. Rodolfo (Istituto Nazionale dei Tumori, Milan, Italy) and G. Cossa (University of Würzbug, Germany) for critical reading of the manuscript. The authors’ research projects are supported by the Swiss National Science Foundation (310030_179360 to S.R.), the Swiss Cancer League (KLS-4282-08-2017 to S.R.), the European Union (ERC CoG-681572 to S.R.), the Wilhelm Sander Foundation (no. 2019.069.1 to S.R.) and the Italian Ministry of Health, Fondazione AIRC per la Ricerca sul Cancro and Fondazione Cariplo-Regione Lombardia (grant 2016-1019) to the P.P. laboratory.
Author information
Authors and Affiliations
Contributions
C.D. and P.P. researched data for the article, S.R. and P.P. made substantial contribution to the discussion of content and all authors wrote, reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
The authors dedicate this article to the memory of Lloyd Kelland, who greatly contributed to the field of the pharmacology of platinum agents.
Peer review information
Nature Reviews Cancer thanks W. Lesterhuis, M. McKeage and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Monoadducts
-
Platinum–DNA entities in which the platinum drug has only one of the two leaving groups (that is, the chlorides for cisplatin) displaced when bound to the target DNA.
- DNA crosslinks
-
Crosslinks (either intrastrand or interstrand) that block DNA replication and/or DNA transcription and occur when various exogenous or endogenous agents react with two different positions in the DNA to form covalent adducts with DNA bases.
- Alkylating agents
-
Agents that add alkyl groups to the bases of DNA, which can lead to DNA breaks and crosslinks and interference with DNA replication and transcription, all resulting in cell death.
- Nucleophilic residues
-
Parts of molecules, for instance biological macromolecules, with an electron pair available to generate a covalent bond; electrophilic agents such as platinum drugs tend to react with nucleophilic residues.
- Homologous recombination
-
(HR). High-fidelity repair of DNA lesions, including double-strand breaks, in the S and G2 phases of the cell cycle, using a sister chromatid as a template.
- Triple-negative breast cancers
-
(TNBCs). A highly aggressive subtype of breast cancer defined by the absence of oestrogen receptor, progesterone receptor and ERBB2 gene amplification.
- Nucleotide excision repair
-
(NER). A process that removes large DNA adducts or base modifications that distort the double helix and uses the opposite strand as a template for repair.
- Cisplatin–glutathione conjugate
-
A non-toxic conjugate in which glutathione binds cisplatin to become a substrate for transporters of the ATP binding cassette superfamily, such as multidrug resistance-associated protein 2 (MRP2), and therefore can be extruded from cells.
- RAD51 foci
-
The local accumulation of RAD51 protein at the sites of DNA double-strand breaks visualized through microscopic imaging.
- Hippo pathway
-
An evolutionary conserved signalling pathway involved in vertebrate development, with a key role in angiogenesis; the pathway negatively regulates the activity of the transcriptional co-activators Yes associated protein (YAP) and Transcriptional co-activator with PDZ-binding motif (TAZ).
- Translesion synthesis
-
(TLS). A DNA repair process introducing a nucleotide opposite to the lesion, followed by the elongation of the 3′ DNA terminus through DNA polymerases specialized to bypass the DNA lesion.
- Drug-tolerant cells
-
(DTCs). Populations of tumour cells that survive acute treatment and rapidly adapt to therapy.
- γ-Glutamyl transpeptidase family
-
Enzymes that act to promote extracellular glutathione degradation, allowing the platinum drug — no longer sequestered by glutathione — to reach the target DNA.
- ‘Warm’ tumours
-
Tumours with poor infiltration by T cells.
- ‘Hot’ tumours
-
Tumours with a T cell inflamed phenotype, that is, exhibiting T cell infiltration and tumour cell expression of type I interferons, as well as the presence of interferon-γ (IFNγ) in the tumour microenvironment.
- Polynuclear Pt agents
-
Agents that contain more than one reactive platinum (Pt) centre available to form crosslinks in the DNA.
- Trans-geometry Pt(II) complexes
-
Complexes characterized by leaving groups (that is, chlorides for cisplatin) in a trans configuration, resembling trans-platin, the inactive stereoisomer of cisplatin.
- Monofunctional coordinating agents
-
Analogues of cisplatin containing only one leaving group (for example, chloride).
- Pt(IV) prodrugs
-
Compounds with a +4 oxidation state undergoing intracellular reduction to generate active Pt(II) species; they contain four ligands of a Pt(II) precursor of known anticancer activity with two additional ligands.
- Conventional Pt agents
-
Platinum (Pt) agents, such as cisplatin, carboplatin or oxaliplatin, in which Pt has a +2 oxidation state.
- Ligand substitution
-
A reaction that occurs by the displacement of leaving groups (for example, cisplatin chlorides) by a nucleophile (for example, water, guanine-N7) that is pivotal for the interaction with the DNA target and is slow for compounds that are less prone to substitution.
Rights and permissions
About this article
Cite this article
Rottenberg, S., Disler, C. & Perego, P. The rediscovery of platinum-based cancer therapy. Nat Rev Cancer 21, 37–50 (2021). https://doi.org/10.1038/s41568-020-00308-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-020-00308-y
This article is cited by
-
Reductive prodrug and AIE copolymer nanoparticle for monitoring and chemotherapy
BMC Cancer (2024)
-
N6-methyladenosine-modified circPLPP4 sustains cisplatin resistance in ovarian cancer cells via PIK3R1 upregulation
Molecular Cancer (2024)
-
Deubiquitinase USP7 stabilizes KDM5B and promotes tumor progression and cisplatin resistance in nasopharyngeal carcinoma through the ZBTB16/TOP2A axis
Cell Death & Differentiation (2024)
-
Nanoparticles targeting mutant p53 overcome chemoresistance and tumor recurrence in non-small cell lung cancer
Nature Communications (2024)
-
Targeting ATR in patients with cancer
Nature Reviews Clinical Oncology (2024)