Abstract
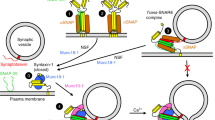
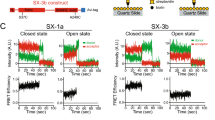
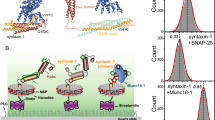
During the priming step that leaves synaptic vesicles ready for neurotransmitter release, the SNARE syntaxin-1 transitions from a closed conformation that binds Munc18-1 tightly to an open conformation within the highly stable SNARE complex. Control of this conformational transition is important for brain function, but the underlying mechanism is unknown. NMR and fluorescence experiments now show that the Munc13-1 MUN domain, which plays a central role in vesicle priming, markedly accelerates the transition from the syntaxin-1–Munc18-1 complex to the SNARE complex. This activity depends on weak interactions of the MUN domain with the syntaxin-1 SNARE motif, and probably with Munc18-1. Together with available physiological data, these results provide a defined molecular basis for synaptic vesicle priming, and they illustrate how weak protein-protein interactions can play crucial biological roles by promoting transitions between high-affinity macromolecular assemblies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sudhof, T.C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 27, 509–547 (2004).
Brunger, A.T. Structure and function of SNARE and SNARE-interacting proteins. Q. Rev. Biophys. 38, 1–47 (2005).
Jahn, R. & Scheller, R.H. SNAREs—engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 7, 631–643 (2006).
Rizo, J. & Rosenmund, C. Synaptic vesicle fusion. Nat. Struct. Mol. Biol. 15, 665–674 (2008).
Südhof, T.C. & Rothman, J.E. Membrane fusion: grappling with SNARE and SM proteins. Science 323, 474–477 (2009).
Poirier, M.A. et al. The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat. Struct. Biol. 5, 765–769 (1998).
Sutton, R.B., Fasshauer, D., Jahn, R. & Brunger, A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395, 347–353 (1998).
Hanson, P.I., Roth, R., Morisaki, H., Jahn, R. & Heuser, J.E. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell 90, 523–535 (1997).
Fernandez, I. et al. Three-dimensional structure of an evolutionarily conserved N-terminal domain of syntaxin 1A. Cell 94, 841–849 (1998).
Dulubova, I. et al. A conformational switch in syntaxin during exocytosis: role of munc18. EMBO J. 18, 4372–4382 (1999).
Misura, K.M., Scheller, R.H. & Weis, W.I. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature 404, 355–362 (2000).
Gerber, S.H. et al. Conformational switch of syntaxin-1 controls synaptic vesicle fusion. Science 321, 1507–1510 (2008).
Dulubova, I. et al. Munc18–1 binds directly to the neuronal SNARE complex. Proc. Natl. Acad. Sci. USA 104, 2697–2702 (2007).
Shen, J., Tareste, D.C., Paumet, F., Rothman, J.E. & Melia, T.J. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell 128, 183–195 (2007).
Khvotchev, M. et al. Dual modes of Munc18–1/SNARE interactions are coupled by functionally critical binding to syntaxin-1 N terminus. J. Neurosci. 27, 12147–12155 (2007).
Deák, F. et al. Munc18–1 binding to the neuronal SNARE complex controls synaptic vesicle priming. J. Cell Biol. 184, 751–764 (2009).
Rizo, J., Chen, X. & Arac, D. Unraveling the mechanisms of synaptotagmin and SNARE function in neurotransmitter release. Trends Cell Biol. 16, 339–350 (2006).
Diao, J. et al. Single-vesicle fusion assay reveals Munc18–1 binding to the SNARE core is sufficient for stimulating membrane fusion. ACS Chem. Neurosci. 1, 168–174 (2010).
Carr, C.M. & Rizo, J. At the junction of SNARE and SM protein function. Curr. Opin. Cell Biol. 22, 488–495 (2010).
Walter, A.M., Wiederhold, K., Bruns, D., Fasshauer, D. & Sorensen, J.B. Synaptobrevin N-terminally bound to syntaxin–SNAP-25 defines the primed vesicle state in regulated exocytosis. J. Cell Biol. 188, 401–413 (2010).
Richmond, J.E., Davis, W.S. & Jorgensen, E.M. UNC-13 is required for synaptic vesicle fusion in C. elegans. Nat. Neurosci. 2, 959–964 (1999).
Augustin, I., Rosenmund, C., Sudhof, T.C. & Brose, N. Munc13–1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature 400, 457–461 (1999).
Varoqueaux, F. et al. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc. Natl. Acad. Sci. USA 99, 9037–9042 (2002).
Aravamudan, B., Fergestad, T., Davis, W.S., Rodesch, C.K. & Broadie, K. Drosophila UNC-13 is essential for synaptic transmission. Nat. Neurosci. 2, 965–971 (1999).
Koushika, S.P. et al. A post-docking role for active zone protein Rim. Nat. Neurosci. 4, 997–1005 (2001).
Schoch, S. et al. RIM1α forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature 415, 321–326 (2002).
Schoch, S. et al. Redundant functions of RIM1α and RIM2α in Ca2+-triggered neurotransmitter release. EMBO J. 25, 5852–5863 (2006).
Richmond, J.E., Weimer, R.M. & Jorgensen, E.M. An open form of syntaxin bypasses the requirement for UNC-13 in vesicle priming. Nature 412, 338–341 (2001).
Betz, A., Okamoto, M., Benseler, F. & Brose, N. Direct interaction of the rat unc-13 homologue Munc13–1 with the N terminus of syntaxin. J. Biol. Chem. 272, 2520–2526 (1997).
Stevens, D.R. et al. Identification of the minimal protein domain required for priming activity of Munc13–1. Curr. Biol. 15, 2243–2248 (2005).
Madison, J.M., Nurrish, S. & Kaplan, J.M. UNC-13 interaction with syntaxin is required for synaptic transmission. Curr. Biol. 15, 2236–2242 (2005).
Basu, J. et al. A minimal domain responsible for Munc13 activity. Nat. Struct. Mol. Biol. 12, 1017–1018 (2005).
Betz, A. et al. Functional interaction of the active zone proteins Munc13–1 and RIM1 in synaptic vesicle priming. Neuron 30, 183–196 (2001).
Dulubova, I. et al. A Munc13/RIM/Rab3 tripartite complex: from priming to plasticity? EMBO J. 24, 2839–2850 (2005).
Lu, J. et al. Structural Basis for a Munc13–1 homodimer to Munc13–1/RIM heterodimer switch. PLoS Biol. 4, e192 (2006).
Wang, X. et al. A protein interaction node at the neurotransmitter release site: domains of Aczonin/Piccolo, Bassoon, CAST, and rim converge on the N-terminal domain of Munc13–1. J. Neurosci. 29, 12584–12596 (2009).
Guan, R., Dai, H. & Rizo, J. Binding of the Munc13–1 MUN domain to membrane-anchored SNARE complexes. Biochemistry 47, 1474–1481 (2008).
Weninger, K., Bowen, M.E., Choi, U.B., Chu, S. & Brunger, A.T. Accessory proteins stabilize the acceptor complex for synaptobrevin, the 1:1 syntaxin/SNAP-25 complex. Structure 16, 308–320 (2008).
Burkhardt, P., Hattendorf, D.A., Weis, W.I. & Fasshauer, D. Munc18a controls SNARE assembly through its interaction with the syntaxin N-peptide. EMBO J. 27, 923–933 (2008).
Fasshauer, D., Antonin, W., Subramaniam, V. & Jahn, R. SNARE assembly and disassembly exhibit a pronounced hysteresis. Nat. Struct. Biol. 9, 144–151 (2002).
Araç, D., Murphy, T. & Rizo, J. Facile detection of protein-protein interactions by one-dimensional NMR spectroscopy. Biochemistry 42, 2774–2780 (2003).
Ruschak, A.M. & Kay, L.E. Methyl groups as probes of supra-molecular structure, dynamics and function. J. Biomol. NMR 46, 75–87 (2010).
Brose, N., Hofmann, K., Hata, Y. & Sudhof, T.C. Mammalian homologues of Caenorhabditis elegans unc-13 gene define novel family of C2-domain proteins. J. Biol. Chem. 270, 25273–25280 (1995).
Xu, Y., Su, L. & Rizo, J. Binding of Munc18–1 to synaptobrevin and to the SNARE four-helix bundle. Biochemistry 49, 1568–1576 (2010).
Xue, M., Ma, C., Craig, T.K., Rosenmund, C. & Rizo, J. The Janus-faced nature of the C2B domain is fundamental for synaptotagmin-1 function. Nat. Struct. Mol. Biol. 15, 1160–1168 (2008).
Chen, X., Lu, J., Dulubova, I. & Rizo, J. NMR analysis of the closed conformation of syntaxin-1. J. Biomol. NMR 41, 43–54 (2008).
Dai, H., Shen, N., Arac, D. & Rizo, J.A. Quaternary SNARE-synaptotagmin-Ca2+-phospholipid complex in neurotransmitter release. J. Mol. Biol. 367, 848–863 (2007).
Xue, M. et al. Binding of the complexin N terminus to the SNARE complex potentiates synaptic-vesicle fusogenicity. Nat. Struct. Mol. Biol. 17, 568–575 (2010).
Verhage, M. et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science 287, 864–869 (2000).
Medine, C.N., Rickman, C., Chamberlain, L.H. & Duncan, R.R. Munc18-1 prevents the formation of ectopic SNARE complexes in living cells. J. Cell Sci. 120, 4407–4415 (2007).
Shen, J., Rathore, S.S., Khandan, L. & Rothman, J.E. SNARE bundle and syntaxin N-peptide constitute a minimal complement for Munc18-1 activation of membrane fusion. J. Cell Biol. 190, 55–63 (2010).
Chen, X. et al. Three-dimensional structure of the complexin/SNARE complex. Neuron 33, 397–409 (2002).
James, D.J., Kowalchyk, J., Daily, N., Petrie, M. & Martin, T.F. CAPS drives trans-SNARE complex formation and membrane fusion through syntaxin interactions. Proc. Natl. Acad. Sci. USA 106, 17308–17313 (2009).
Liu, Y. et al. Two distinct secretory vesicle-priming steps in adrenal chromaffin cells. J. Cell Biol. 190, 1067–1077 (2010).
Pei, J., Ma, C., Rizo, J. & Grishin, N.V. Remote homology between Munc13 MUN domain and vesicle tethering complexes. J. Mol. Biol. 391, 509–517 (2009).
Ren, Y. et al. A structure-based mechanism for vesicle capture by the multisubunit tethering complex Dsl1. Cell 139, 1119–1129 (2009).
Basu, J., Betz, A., Brose, N. & Rosenmund, C. Munc13-1 C1 domain activation lowers the energy barrier for synaptic vesicle fusion. J. Neurosci. 27, 1200–1210 (2007).
Tugarinov, V., Sprangers, R. & Kay, L.E. Line narrowing in methyl-TROSY using zero-quantum 1H-13C NMR spectroscopy. J. Am. Chem. Soc. 126, 4921–4925 (2004).
Delaglio, F. et al. NMRPipe:a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 (1995).
Johnson, B.A. & Blevins, R.A. NMR View: a computer-program for the visualization and analysis of NMR data. J. Biomol. NMR 4, 603–614 (1994).
Acknowledgements
We thank Y. Sun of the University of Texas Southwestern Medical Center (UT Southwestern) for expert technical assistance, Y. Xu and L. Su (UT Southwestern) for fruitful discussions, M. Rosen (UT Southwestern) and L. Kay (University of Toronto) for advice on NMR experiments and W. Wickner (Dartmouth University) for insightful comments on the manuscript. This work was supported by a postdoctoral fellowship from the American Heart Association (to Y.X.), by Welch foundation grant I-1304 and by US National Institutes of Health grant NS37200 (both to J.R.).
Author information
Authors and Affiliations
Contributions
C.M. did the kinetic studies of SNARE complex formation. C.M., W.L. and J.R. conducted the NMR experiments. Y.X. did initial biochemical studies of the MUN domain. J.R. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9, Supplementary Notes 1 and 2, and Supplementary Methods (PDF 2304 kb)
Rights and permissions
About this article
Cite this article
Ma, C., Li, W., Xu, Y. et al. Munc13 mediates the transition from the closed syntaxin–Munc18 complex to the SNARE complex. Nat Struct Mol Biol 18, 542–549 (2011). https://doi.org/10.1038/nsmb.2047
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2047
This article is cited by
-
The activation of mGluR4 rescues parallel fiber synaptic transmission and LTP, motor learning and social behavior in a mouse model of Fragile X Syndrome
Molecular Autism (2023)
-
Identification of residues critical for the extension of Munc18-1 domain 3a
BMC Biology (2023)
-
Vesicle trafficking and vesicle fusion: mechanisms, biological functions, and their implications for potential disease therapy
Molecular Biomedicine (2022)
-
Inhibition of calcium-triggered secretion by hydrocarbon-stapled peptides
Nature (2022)
-
Molecular encoding and synaptic decoding of context during salt chemotaxis in C. elegans
Nature Communications (2022)