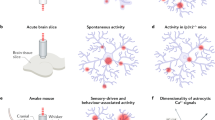
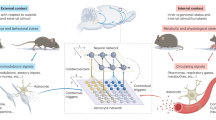
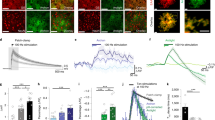
Abstract
Astrocyte Ca2+ signalling has been proposed to link neuronal information in different spatial–temporal dimensions to achieve a higher level of brain integration. However, some discrepancies in the results of recent studies challenge this view and highlight key insufficiencies in our current understanding. In parallel, new experimental approaches that enable the study of astrocyte physiology at higher spatial–temporal resolution in intact brain preparations are beginning to reveal an unexpected level of compartmentalization and sophistication in astrocytic Ca2+ dynamics. This newly revealed complexity needs to be attentively considered in order to understand how astrocytes may contribute to brain information processing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Dani, J. W., Chernjavsky, A. & Smith, S. J. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron 8, 429–440 (1992).
Porter, J. T. & McCarthy, K. D. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J. Neurosci. 16, 5073–5081 (1996).
Pasti, L., Volterra, A., Pozzan, T. & Carmignoto, G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J. Neurosci. 17, 7817–7830 (1997).
Wang, X. et al. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nature Neurosci. 9, 816–823 (2006).
Petzold, G. C., Albeanu, D. F., Sato, T. F. & Murthy, V. N. Coupling of neural activity to blood flow in olfactory glomeruli is mediated by astrocytic pathways. Neuron 58, 897–910 (2008).
Schummers, J., Yu, H. & Sur, M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science 320, 1638–1643 (2008).
Nimmerjahn, A., Mukamel, E. A. & Schnitzer, M. J. Motor behavior activates Bergmann glial networks. Neuron 62, 400–412 (2009).
Gourine, A. V. et al. Astrocytes control breathing through pH-dependent release of ATP. Science 329, 571–575 (2010).
Volterra, A. & Meldolesi, J. Astrocytes, from brain glue to communication elements: the revolution continues. Nature Rev. Neurosci. 6, 626–640 (2005).
Perea, G., Navarrete, M. & Araque, A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 32, 421–431 (2009).
Halassa, M. M. & Haydon, P. G. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu. Rev. Physiol. 72, 335–355 (2010).
Volterra, A. in Neuroglia (eds Kettenmann, H. & Ransom, B. R.) 816–823 (Oxford Univ. Press, 2013).
Waldo, G. L. & Harden, T. K. Agonist binding and Gq-stimulating activities of the purified human P2Y1 receptor. Mol. Pharmacol. 65, 426–436 (2004).
Pinto, J. C. et al. Cannabinoid receptor binding and agonist activity of amides and esters of arachidonic acid. Mol. Pharmacol. 46, 516–522 (1994).
Bowery, N. G., Hill, D. R. & Hudson, A. L. Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes. Br. J. Pharmacol. 78, 191–206 (1983).
Nash, M. S. et al. Determinants of metabotropic glutamate receptor-5-mediated Ca2+ and inositol 1,4,5-trisphosphate oscillation frequency. Receptor density versus agonist concentration. J. Biol. Chem. 277, 35947–35960 (2002).
Berridge, M. J., Bootman, M. D. & Roderick, H. L. Calcium signalling: dynamics, homeostasis and remodelling. Nature Rev. Mol. Cell Biol. 4, 517–529 (2003).
Rizzuto, R. & Pozzan, T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 86, 369–408 (2006).
Verkhratsky, A. & Kirchhoff, F. NMDA receptors in glia. Neuroscientist 13, 28–37 (2007).
Matsui, K. & Jahr, C. E. Exocytosis unbound. Curr. Opin. Neurobiol. 16, 305–311 (2006).
Kirischuk, S., Kettenmann, H. & Verkhratsky, A. Na+/Ca2+ exchanger modulates kainate-triggered Ca2+ signaling in Bergmann glial cells in situ. FASEB J. 11, 566–572 (1997).
Doengi, M. et al. GABA uptake-dependent Ca2+ signaling in developing olfactory bulb astrocytes. Proc. Natl Acad. Sci. USA 106, 17570–17575 (2009).
Parri, H. R., Gould, T. M. & Crunelli, V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nature Neurosci. 4, 803–812 (2001).
Nett, W. J., Oloff, S. H. & McCarthy, K. D. Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J. Neurophysiol. 87, 528–537 (2002).
Aguado, F., Espinosa-Parrilla, J. F., Carmona, M. A. & Soriano, E. Neuronal activity regulates correlated network properties of spontaneous calcium transients in astrocytes in situ. J. Neurosci. 22, 9430–9444 (2002).
Honsek, S. D., Walz, C., Kafitz, K. W. & Rose, C. R. Astrocyte calcium signals at Schaffer collateral to CA1 pyramidal cell synapses correlate with the number of activated synapses but not with synaptic strength. Hippocampus 22, 29–42 (2012).
Shigetomi, E., Tong, X., Kwan, K. Y., Corey, D. P. & Khakh, B. S. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nature Neurosci. 15, 70–80 (2012).
Shigetomi, E., Jackson-Weaver, O., Huckstepp, R. T., O'Dell, T. J. & Khakh, B. S. TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J. Neurosci. 33, 10143–10153 (2013).
Pasti, L., Zonta, M., Pozzan, T., Vicini, S. & Carmignoto, G. Cytosolic calcium oscillations in astrocytes may regulate exocytotic release of glutamate. J. Neurosci. 21, 477–484 (2001).
Bezzi, P. et al. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nature Neurosci. 7, 613–620 (2004).
Marchaland, J. et al. Fast subplasma membrane Ca2+ transients control exo-endocytosis of synaptic-like microvesicles in astrocytes. J. Neurosci. 28, 9122–9132 (2008).
Woo, D. H. et al. TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell 151, 25–40 (2012).
Parpura, V. et al. Glutamate-mediated astrocyte-neuron signalling. Nature 369, 744–747 (1994).
Bezzi, P. et al. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 391, 281–285 (1998).
Bezzi, P. & Volterra, A. A neuron–glia signalling network in the active brain. Curr. Opin. Neurobiol. 11, 387–394 (2001).
Shigetomi, E., Bowser, D. N., Sofroniew, M. V. & Khakh, B. S. Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J. Neurosci. 28, 6659–6663 (2008).
Montana, V., Ni, Y., Sunjara, V., Hua, X. & Parpura, V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J. Neurosci. 24, 2633–2642 (2004).
Mothet, J. P. et al. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc. Natl Acad. Sci. USA 102, 5606–5611 (2005).
Santello, M., Bezzi, P. & Volterra, A. TNFα controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron 69, 988–1001 (2011).
Fiacco, T. A. & McCarthy, K. D. Intracellular astrocyte calcium waves in situ increase the frequency of spontaneous AMPA receptor currents in CA1 pyramidal neurons. J. Neurosci. 24, 722–732 (2004).
Fellin, T. et al. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43, 729–743 (2004).
Pascual, O. et al. Astrocytic purinergic signaling coordinates synaptic networks. Science 310, 113–116 (2005).
Panatier, A. et al. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 125, 775–784 (2006).
Jourdain, P. et al. Glutamate exocytosis from astrocytes controls synaptic strength. Nature Neurosci. 10, 331–339 (2007).
Perea, G. & Araque, A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science 317, 1083–1086 (2007).
Panatier, A. et al. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146, 785–798 (2011).
Liu, Q. S., Xu, Q., Arcuino, G., Kang, J. & Nedergaard, M. Astrocyte-mediated activation of neuronal kainate receptors. Proc. Natl Acad. Sci. USA 101, 3172–3177 (2004).
Agulhon, C., Fiacco, T. A. & McCarthy, K. D. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 327, 1250–1254 (2010).
Fiacco, T. A. et al. Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron 54, 611–626 (2007).
Henneberger, C., Papouin, T., Oliet, S. H. & Rusakov, D. A. Long-term potentiation depends on release of D-serine from astrocytes. Nature 463, 232–236 (2010).
Navarrete, M. et al. Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Biol. 10, e1001259 (2012).
Petravicz, J., Fiacco, T. A. & McCarthy, K. D. Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J. Neurosci. 28, 4967–4973 (2008).
Serrano, A., Haddjeri, N., Lacaille, J. C. & Robitaille, R. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J. Neurosci. 26, 5370–5382 (2006).
Navarrete, M. & Araque, A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68, 113–126 (2010).
Di Castro, M. A. et al. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nature Neurosci. 14, 1276–1284 (2011).
Poskanzer, K. E. & Yuste, R. Astrocytic regulation of cortical UP states. Proc. Natl Acad. Sci. USA 108, 18453–18458 (2011).
Min, R. & Nevian, T. Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nature Neurosci. 15, 746–753 (2012).
Takata, N. et al. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J. Neurosci. 31, 18155–18165 (2011).
Han, J. et al. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 148, 1039–1050 (2012).
Chen, N. et al. Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. Proc. Natl Acad. Sci. USA 109, E2832–E2841 (2012).
Grosche, J. et al. Microdomains for neuron–glia interaction: parallel fiber signaling to Bergmann glial cells. Nature Neurosci. 2, 139–143 (1999).
Matyash, V., Filippov, V., Mohrhagen, K. & Kettenmann, H. Nitric oxide signals parallel fiber activity to Bergmann glial cells in the mouse cerebellar slice. Mol. Cell Neurosci. 18, 664–670 (2001).
Perea, G. & Araque, A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J. Neurosci. 25, 2192–2203 (2005).
De Pitta, M. et al. Computational quest for understanding the role of astrocyte signaling in synaptic transmission and plasticity. Front. Comput. Neurosci. 6, 98 (2012).
Shigetomi, E. et al. Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J. Gen. Physiol. 141, 633–647 (2013).
Smith, I. F. & Parker, I. Imaging the quantal substructure of single IP3R channel activity during Ca2+ puffs in intact mammalian cells. Proc. Natl Acad. Sci. USA 106, 6404–6409 (2009).
Clarke, L. E. & Barres, B. A. Emerging roles of astrocytes in neural circuit development. Nature Rev. Neurosci. 14, 311–321 (2013).
Tanaka, M. et al. Astrocytic Ca2+ signals are required for the functional integrity of tripartite synapses. Mol. Brain 6, 6 (2013).
Gordon, G. R. et al. Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron 64, 391–403 (2009).
Schulz, K. et al. Simultaneous BOLD fMRI and fiber-optic calcium recording in rat neocortex. Nature Methods 9, 597–602 (2012).
Wang, F. et al. Astrocytes modulate neural network activity by Ca2+-dependent uptake of extracellular K+. Sci. Signal. 5, ra26 (2012).
Wang, F., Xu, Q., Wang, W., Takano, T. & Nedergaard, M. Bergmann glia modulate cerebellar Purkinje cell bistability via Ca2+-dependent K+ uptake. Proc. Natl Acad. Sci. USA 109, 7911–7916 (2012).
Li, W., Llopis, J., Whitney, M., Zlokarnik, G. & Tsien, R. Y. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature 392, 936–941 (1998).
Dolmetsch, R. E., Xu, K. & Lewis, R. S. Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392, 933–936 (1998).
De Koninck, P. & Schulman, H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science 279, 227–230 (1998).
Hirase, H., Qian, L., Bartho, P. & Buzsaki, G. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol. 2, E96 (2004).
Nimmerjahn, A., Kirchhoff, F., Kerr, J. N. & Helmchen, F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nature Methods 1, 31–37 (2004).
Kuchibhotla, K. V., Lattarulo, C. R., Hyman, B. T. & Bacskai, B. J. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science 323, 1211–1215 (2009).
Sun, W. et al. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science 339, 197–200 (2013).
Grienberger, C. & Konnerth, A. Imaging calcium in neurons. Neuron 73, 862–885 (2012).
Neher, E. & Augustine, G. J. Calcium gradients and buffers in bovine chromaffin cells. J. Physiol. 450, 273–301 (1992).
Helmchen, F., Imoto, K. & Sakmann, B. Ca2+ buffering and action potential-evoked Ca2+ signaling in dendrites of pyramidal neurons. Biophys. J. 70, 1069–1081 (1996).
Tian, L., Hires, S. A. & Looger, L. L. Imaging neuronal activity with genetically encoded calcium indicators. Cold Spring Harb. Protoc. 2012, 647–656 (2012).
Akerboom, J. et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 32, 13819–13840 (2012).
Thomas, D. et al. A comparison of fluorescent Ca2+ indicator properties and their use in measuring elementary and global Ca2+ signals. Cell Calcium 28, 213–223 (2000).
Miyawaki, A. et al. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 388, 882–887 (1997).
Yamada, Y. & Mikoshiba, K. Quantitative comparison of novel GCaMP-type genetically encoded Ca2+ indicators in mammalian neurons. Front. Cell Neurosci. 6, 41 (2012).
Margolis, D. J. et al. Reorganization of cortical population activity imaged throughout long-term sensory deprivation. Nature Neurosci. 15, 1539–1546 (2012).
Lutcke, H., Margolis, D. J. & Helmchen, F. Steady or changing? Long-term monitoring of neuronal population activity. Trends Neurosci. 36, 375–384 (2013).
Tian, L. et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nature Methods 6, 875–881 (2009).
Chen, Q. et al. Imaging neural activity using Thy1-GCaMP transgenic mice. Neuron 76, 297–308 (2012).
Hasan, M. T. et al. Functional fluorescent Ca2+ indicator proteins in transgenic mice under TET control. PLoS Biol. 2, e163 (2004).
Pfrieger, F. W. & Slezak, M. Genetic approaches to study glial cells in the rodent brain. Glia 60, 681–701 (2012).
Ohkura, M. et al. Genetically encoded green fluorescent Ca2+ indicators with improved detectability for neuronal Ca2+ signals. PLoS ONE 7, e51286 (2012).
Sun, X. R. et al. Fast GCaMPs for improved tracking of neuronal activity. Nature Commun. 4, 2170 (2013).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Smith, K. Neuroscience: settling the great glia debate. Nature 468, 160–162 (2010).
Duffy, S. & MacVicar, B. A. Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. J. Neurosci. 15, 5535–5550 (1995).
Arizono, M. et al. Receptor-selective diffusion barrier enhances sensitivity of astrocytic processes to metabotropic glutamate receptor stimulation. Sci. Signal. 5, ra27 (2012).
Lavialle, M. et al. Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc. Natl Acad. Sci. USA 108, 12915–12919 (2011).
Tonnesen, J. & Nagerl, U. V. Two-color STED imaging of synapses in living brain slices. Methods Mol. Biol. 950, 65–80 (2013).
Wilms, C. D., Schmidt, H. & Eilers, J. Quantitative two-photon Ca2+ imaging via fluorescence lifetime analysis. Cell Calcium 40, 73–79 (2006).
Akerboom, J. et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front. Mol. Neurosci. 6, 2 (2013).
Pastrana, E. Calcium sensors reach new heights. Nature Methods 10, 824 (2013).
Rusakov, D. A., Zheng, K. & Henneberger, C. Astrocytes as regulators of synaptic function: a quest for the Ca2+ master key. Neuroscientist 17, 513–523 (2011).
Katona, G. et al. Fast two-photon in vivo imaging with three-dimensional random-access scanning in large tissue volumes. Nature Methods 9, 201–208 (2012).
Bushong, E. A., Martone, M. E., Jones, Y. Z. & Ellisman, M. H. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci. 22, 183–192 (2002).
Halassa, M. M., Fellin, T., Takano, H., Dong, J. H. & Haydon, P. G. Synaptic islands defined by the territory of a single astrocyte. J. Neurosci. 27, 6473–6477 (2007).
Gordon, G. R., Choi, H. B., Rungta, R. L., Ellis-Davies, G. C. & MacVicar, B. A. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 456, 745–749 (2008).
Yasuda, R. et al. Imaging calcium concentration dynamics in small neuronal compartments. Sci. STKE 2004, pl5 (2004).
Nevian, T. & Helmchen, F. Calcium indicator loading of neurons using single-cell electroporation. Pflugers Arch. 454, 675–688 (2007).
Darabid, H., Arbour, D. & Robitaille, R. Glial cells decipher synaptic competition at the mammalian neuromuscular junction. J. Neurosci. 33, 1297–1313 (2013).
Nolte, C. et al. GFAP promoter-controlled EGFP-expressing transgenic mice: a tool to visualize astrocytes and astrogliosis in living brain tissue. Glia 33, 72–86 (2001).
Tokuyasu, K. T. A technique for ultracryotomy of cell suspensions and tissues. J. Cell Biol. 57, 551–565 (1973).
Chao, T. I., Rickmann, M. & Wolff, J. R. in The Tripartite Synapse: Glia in Synaptic Transmission (eds Volterra, A., Magistretti, P. J. & Haydon, P. G.) 3–23 (Oxford Univ. Press, 2002).
Acknowledgements
Research in the Volterra laboratory is supported by the ERC Advanced grant 340368 “Astromnesis” and by Swiss National Science Foundation grants 31003A 140999, NCCR “Synapsy” and NCCR “Transcure”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Volterra, A., Liaudet, N. & Savtchouk, I. Astrocyte Ca2+ signalling: an unexpected complexity. Nat Rev Neurosci 15, 327–335 (2014). https://doi.org/10.1038/nrn3725
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3725
This article is cited by
-
Adenosine-independent regulation of the sleep–wake cycle by astrocyte activity
Cell Discovery (2023)
-
Dysfunctional serotonergic neuron-astrocyte signaling in depressive-like states
Molecular Psychiatry (2023)
-
Revisiting the critical roles of reactive astrocytes in neurodegeneration
Molecular Psychiatry (2023)
-
Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration
Nature Reviews Neurology (2023)
-
Analysis of Network Models with Neuron-Astrocyte Interactions
Neuroinformatics (2023)