Key Points
-
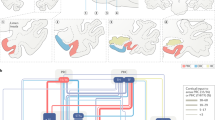
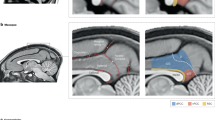
The perirhinal cortex (PRC), parahippocampal cortex (PHC) and retrosplenial cortex (RSC) are crucial for memory in humans, so it is essential to understand the functions of these areas.
-
The PHC and RSC exhibit patterns of anatomical and functional connectivity that are highly similar to one another and strikingly different from the PRC.
-
The PRC is important for familiarity-based item recognition, associating features of objects and fine-grained perceptual or semantic discriminations.
-
The PHC and RSC are important for recollection-based memories, memory for spatial and episodic context, scene perception, simulation of hypothetical events and certain aspects of spatial navigation and social cognition.
-
Integrating this evidence, we propose a new framework in which the PRC, PHC and RSC are embedded in two extended networks that support different forms of memory-guided behaviour.
-
The PRC is situated in an anterior temporal (AT) system that also includes the amygdala, ventral temporopolar cortex and lateral orbitofrontal cortex. This system may collectively support the assessment of the significance of specific entities.
-
The PHC and RSC are situated in a posterior medial (PM) system that also includes the mammillary bodies, anterior thalamic nuclei, pre- and parasubiculum, posterior cingulate, precuneus and angular gyrus. This system may support the construction of situation models in support of memory, spatial navigation and social cognition.
-
The AT and PM systems are differentially targeted by neurological disorders. For instance, semantic dementia is associated with disproportionate atrophy and dysfunction within the AT system relative to Alzheimer's disease, which disproportionately affects the PM system.
Abstract
Although the perirhinal cortex (PRC), parahippocampal cortex (PHC) and retrosplenial cortex (RSC) have an essential role in memory, the precise functions of these areas are poorly understood. Here, we review the anatomical and functional characteristics of these areas based on studies in humans, monkeys and rats. Our Review suggests that the PRC and PHC–RSC are core components of two separate large-scale cortical networks that are dissociable by neuroanatomy, susceptibility to disease and function. These networks not only support different types of memory but also appear to support different aspects of cognition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Squire, L. R. & Zola-Morgan, S. The medial temporal lobe memory system. Science 253, 1380–1386 (1991).
Brown, M. W. & Aggleton, J. P. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nature Rev. Neurosci. 2, 51–61 (2001).
Eichenbaum, H., Yonelinas, A. P. & Ranganath, C. The medial temporal lobe and recognition memory. Annu. Rev. Neurosci. 30, 123–152 (2007). This article reviews research on the roles of MTL subregions in recognition memory in rats, monkeys and humans. The authors propose that the PRC represents specific items, the PHC represents context information, and the hippocampus is crucial for associating item and context information (also see references 29, 30, 45, 83, 143 and 171).
Mishkin, M., Suzuki, W. A., Gadian, D. G. & Vargha-Khadem, F. Hierarchical organization of cognitive memory. Phil. Trans. R. Soc. Lond. B 352, 1461–1467 (1997).
Vann, S. D., Aggleton, J. P. & Maguire, E. A. What does the retrosplenial cortex do? Nature Rev. Neurosci. 10, 792–802 (2009). This article provides a thorough synthesis of evidence concerning the anatomy and function of the RSC, including its essential role in episodic memory and spatial cognition.
Valenstein, E. et al. Retrosplenial amnesia. Brain 110, 1631–1646 (1987).
Witter, M. P. et al. Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus 10, 398–410 (2000).
Aggleton, J. P. Multiple anatomical systems embedded within the primate medial temporal lobe: implications for hippocampal function. Neurosci. Biobehav. Rev. 36, 1579–1596 (2012).
Furtak, S. C., Wei, S. M., Agster, K. L. & Burwell, R. D. Functional neuroanatomy of the parahippocampal region in the rat: the perirhinal and postrhinal cortices. Hippocampus 17, 709–722 (2007).
Aggleton, J. P., Wright, N. F., Vann, S. D. & Saunders, R. C. Medial temporal lobe projections to the retrosplenial cortex of the macaque monkey. Hippocampus 22, 1883–1900 (2012).
Stefanacci, L., Suzuki, W. A. & Amaral, D. G. Organization of connections between the amygdaloid complex and the perirhinal and parahippocampal cortices in macaque monkeys. J. Comp. Neurol. 375, 552–582 (1996).
Kahn, I., Andrews-Hanna, J. R., Vincent, J. L., Snyder, A. Z. & Buckner, R. L. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J. Neurophysiol. 100, 129–139 (2008). This is the first study in which functional connectivity analysis of resting-state fMRI data was used to carefully characterize the connectivity of different MTL subregions in humans. The study demonstrated that the PHC and PRC show strikingly different functional connectivity profiles.
Libby, L. A., Ekstrom, A. D., Ragland, J. D. & Ranganath, C. Differential connectivity of perirhinal and parahippocampal cortices within human hippocampal subregions revealed by high-resolution functional imaging. J. Neurosci. 32, 6550–6560 (2012).
Kobayashi, Y. & Amaral, D. G. Macaque monkey retrosplenial cortex: II. Cortical afferents. J. Comp. Neurol. 466, 48–79 (2003).
Kobayashi, Y. & Amaral, D. G. Macaque monkey retrosplenial cortex: III. Cortical efferents. J. Comp. Neurol. 502, 810–833 (2007).
Suzuki, W. A. & Amaral, D. G. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J. Comp. Neurol. 350, 497–533 (1994).
Lavenex, P., Suzuki, W. A. & Amaral, D. G. Perirhinal and parahippocampal cortices of the macaque monkey: intrinsic projections and interconnections. J. Comp. Neurol. 472, 371–394 (2004).
Lavenex, P., Suzuki, W. A. & Amaral, D. G. Perirhinal and parahippocampal cortices of the macaque monkey: projections to the neocortex. J. Comp. Neurol. 447, 394–420 (2002).
Kondo, H., Saleem, K. S. & Price, J. L. Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. J. Comp. Neurol. 493, 479–509 (2005). This paper is an innovative synthesis of primate neuroanatomy data. The authors propose that the lateral orbitofrontal cortex is a component of an extended cortical network that also includes the PRC, whereas the medial prefrontal cortex is a component of a distributed network that also includes the PHC.
Kravitz, D. J., Saleem, K. S., Baker, C. I. & Mishkin, M. A new neural framework for visuospatial processing. Nature Rev. Neurosci. 12, 217–230 (2011).
Mufson, E. J. & Pandya, D. N. Some observations on the course and composition of the cingulum bundle in the rhesus monkey. J. Comp. Neurol. 225, 31–43 (1984).
Raichle, M. E. et al. A default mode of brain function. Proc. Natl Acad. Sci. USA 98, 676–682 (2001).
Andrews-Hanna, J. R., Reidler, J. S., Sepulcre, J., Poulin, R. & Buckner, R. L. Functional-anatomic fractionation of the brain's default network. Neuron 65, 550–562 (2010).
Hoistad, M. & Barbas, H. Sequence of information processing for emotions through pathways linking temporal and insular cortices with the amygdala. Neuroimage 40, 1016–1033 (2008).
Baxter, M. G. & Murray, E. A. Opposite relationship of hippocampal and rhinal cortex damage to delayed nonmatching-to-sample deficits in monkeys. Hippocampus 11, 61–71 (2001).
Nemanic, S., Alvarado, M. C. & Bachevalier, J. The hippocampal/parahippocampal regions and recognition memory: insights from visual paired comparison versus object-delayed nonmatching in monkeys. J. Neurosci. 24, 2013–2026 (2004).
Aggleton, J. P., Brown, M. W. & Albasser, M. M. Contrasting brain activity patterns for item recognition memory and associative recognition memory: insights from immediate-early gene functional imaging. Neuropsychologia 23 May 2012 (doi:10.1016/j.neuropsychologia.2012.05.018).
Davachi, L. & Goldman-Rakic, P. S. Primate rhinal cortex participates in both visual recognition and working memory tasks: functional mapping with 2-DG. J. Neurophysiol. 2590–2601 (2001).
Ranganath, C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus 20, 1263–1290 (2010).
Montaldi, D. & Mayes, A. R. The role of recollection and familiarity in the functional differentiation of the medial temporal lobes. Hippocampus 20, 1291–1314 (2010).
Bowles, B. et al. Impaired familiarity with preserved recollection after anterior temporal-lobe resection that spares the hippocampus. Proc. Natl Acad. Sci. USA 104, 16382–16387 (2007).
Xiang, J. Z. & Brown, M. W. Differential neuronal encoding of novelty, familiarity and recency in regions of the anterior temporal lobe. Neuropharmacology 37, 657–676 (1998). Building on earlier work from Brown's laboratory, this study presents a detailed characterization of correlates of object recognition memory through a single-unit recording in area TE, the PRC and the entorhinal cortex.
Henson, R. N., Cansino, S., Herron, J. E., Robb, W. G. & Rugg, M. D. A familiarity signal in human anterior medial temporal cortex? Hippocampus 13, 259–262 (2003).
Montaldi, D., Spencer, T. J., Roberts, N. & Mayes, A. R. The neural system that mediates familiarity memory. Hippocampus 16, 504–520 (2006).
Gonsalves, B. D., Kahn, I., Curran, T., Norman, K. A. & Wagner, A. D. Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron 47, 751–761 (2005).
Weis, S. et al. Process dissociation between contextual retrieval and item recognition. Neuroreport 15, 2729–2733 (2004).
Ranganath, C. et al. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia 42, 2–13 (2003).
Davachi, L., Mitchell, J. P. & Wagner, A. D. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc. Natl Acad. Sci. USA 100, 2157–2162 (2003).
Nakamura, K. & Kubota, K. Mnemonic firing of neurons in the monkey temporal pole during a visual recognition memory task. J. Neurophysiol. 74, 162–178 (1995).
Miyashita, Y. Neuronal correlate of visual associative long-term memory in the primate temporal cortex. Nature 335, 817–820 (1988).
Erickson, C. A. & Desimone, R. Responses of macaque perirhinal neurons during and after visual stimulus association learning. J. Neurosci. 19, 10404–10416 (1999).
Fujimichi, R. et al. Unitized representation of paired objects in area 35 of the macaque perirhinal cortex. Eur. J. Neurosci. 32, 659–667 (2010).
Murray, E. A., Gaffan, D. & Mishkin, M. Neural substrates of visual stimulus-stimulus association in rhesus monkeys. J. Neurosci. 13, 4549–4561 (1993).
Haskins, A. L., Yonelinas, A. P., Quamme, J. R. & Ranganath, C. Perirhinal cortex supports encoding and familiarity-based recognition of novel associations. Neuron 59, 554–560 (2008).
Diana, R. A., Yonelinas, A. P. & Ranganath, C. Medial temporal lobe activity during source retrieval reflects information type, not memory strength. J. Cogn. Neurosci. 22, 1808–1818 (2010).
Staresina, B. P. & Davachi, L. Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. J. Cogn. Neurosci. 20, 1478–1489 (2008).
Liu, Z. & Richmond, B. J. Response differences in monkey TE and perirhinal cortex: stimulus association related to reward schedules. J. Neurophysiol. 83, 1677–1692 (2000).
Liu, Z., Murray, E. A. & Richmond, B. J. Learning motivational significance of visual cues for reward schedules requires rhinal cortex. Nature Neurosci. 3, 1307–1315 (2000).
Liu, Z. et al. DNA targeting of rhinal cortex D2 receptor protein reversibly blocks learning of cues that predict reward. Proc. Natl Acad. Sci. USA 101, 12336–12341 (2004).
Lindquist, D. H., Jarrard, L. E. & Brown, T. H. Perirhinal cortex supports delay fear conditioning to rat ultrasonic social signals. J. Neurosci. 24, 3610–3617 (2004).
Kholodar-Smith, D. B., Allen, T. A. & Brown, T. H. Fear conditioning to discontinuous auditory cues requires perirhinal cortical function. Behav. Neurosci. 122, 1178–1185 (2008).
Kholodar-Smith, D. B., Boguszewski, P. & Brown, T. H. Auditory trace fear conditioning requires perirhinal cortex. Neurobiol. Learn. Mem. 90, 537–543 (2008).
Otto, T., Cousens, G. & Herzog, C. Behavioral and neuropsychological foundations of olfactory fear conditioning. Behav. Brain Res. 110, 119–128 (2000).
Furtak, S. C., Allen, T. A. & Brown, T. H. Single-unit firing in rat perirhinal cortex caused by fear conditioning to arbitrary and ecological stimuli. J. Neurosci. 27, 12277–12291 (2007).
Boxer, A. L. et al. Cinguloparietal atrophy distinguishes Alzheimer disease from semantic dementia. Arch. Neurol. 60, 949–956 (2003).
Davies, R. R., Graham, K. S., Xuereb, J. H., Williams, G. B. & Hodges, J. R. The human perirhinal cortex and semantic memory. Eur. J. Neurosci. 20, 2441–2446 (2004).
Patterson, K., Nestor, P. J. & Rogers, T. T. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Rev. Neurosci. 8, 976–987 (2007).
Moss, H. E., Rodd, J. M., Stamatakis, E. A., Bright, P. & Tyler, L. K. Anteromedial temporal cortex supports fine-grained differentiation among objects. Cereb. Cortex 15, 616–627 (2005).
Noppeney, U. et al. Temporal lobe lesions and semantic impairment: a comparison of herpes simplex virus encephalitis and semantic dementia. Brain 130, 1138–1147 (2007).
Stefanacci, L., Buffalo, E. A., Schmolck, H. & Squire, L. R. Profound amnesia after damage to the medial temporal lobe: a neuroanatomical and neuropsychological profile of patient E. P. J. Neurosci. 20, 7024–7036 (2000).
Taylor, K. I., Moss, H. E., Stamatakis, E. A. & Tyler, L. K. Binding crossmodal object features in perirhinal cortex. Proc. Natl Acad. Sci. USA 103, 8239–8244 (2006).
Lambon Ralph, M. A., Ehsan, S., Baker, G. A. & Rogers, T. T. Semantic memory is impaired in patients with unilateral anterior temporal lobe resection for temporal lobe epilepsy. Brain 135, 242–258 (2012).
Nobre, A. C. & McCarthy, G. Language-related field potentials in the anterior-medial temporal lobe: II. Effects of word type and semantic priming. J. Neurosci. 15, 1090–1098 (1995). Along with its companion paper, this study provided the first detailed characterization of the AMTL N400, demonstrating that field potentials recorded directly from the PRC are sensitive to semantic processing.
Chan, A. M. et al. First-pass selectivity for semantic categories in human anteroventral temporal lobe. J. Neurosci. 31, 18119–18129 (2011).
Tyler, L. K. et al. Processing objects at different levels of specificity. J. Cogn. Neurosci. 16, 351–362 (2004).
Visser, M., Jefferies, E. & Lambon Ralph, M. A. Semantic processing in the anterior temporal lobes: a meta-analysis of the functional neuroimaging literature. J. Cogn. Neurosci. 22, 1083–1094 (2010).
Wang, W. C., Lazzara, M. M., Ranganath, C., Knight, R. T. & Yonelinas, A. P. The medial temporal lobe supports conceptual implicit memory. Neuron 68, 835–842 (2010). This study presents results showing that patients with damage to the left PRC are impaired at conceptual priming, and converging evidence showing that left perirhinal activation in healthy individuals is predictive of successful conceptual priming.
Voss, J. L., Hauner, K. K. & Paller, K. A. Establishing a relationship between activity reduction in human perirhinal cortex and priming. Hippocampus 19, 773–778 (2009).
Marinkovic, K. et al. Spatiotemporal dynamics of modality-specific and supramodal word processing. Neuron 38, 487–497 (2003).
Graham, K. S., Barense, M. D. & Lee, A. C. Going beyond LTM in the MTL: a synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia 48, 831–853 (2010).
Bussey, T. J., Saksida, L. M. & Murray, E. A. The perceptual-mnemonic/feature conjunction model of perirhinal cortex function. Q. J. Exp. Psychol. B 58, 269–282 (2005).
Naya, Y., Yoshida, M. & Miyashita, Y. Forward processing of long-term associative memory in monkey inferotemporal cortex. J. Neurosci. 23, 2861–2871 (2003).
Buckley, M. J., Booth, M. C., Rolls, E. T. & Gaffan, D. Selective perceptual impairments after perirhinal cortex ablation. J. Neurosci. 21, 9824–9836 (2001).
Bussey, T. J., Saksida, L. M. & Murray, E. A. Perirhinal cortex resolves feature ambiguity in complex visual discriminations. Eur. J. Neurosci. 15, 365–374 (2002).
Lee, A. C. et al. Perceptual deficits in amnesia: challenging the medial temporal lobe 'mnemonic' view. Neuropsychologia 43, 1–11 (2005). A groundbreaking study suggesting that human patients with damage to the PRC show subtle deficits in object perception.
Lee, A. C. et al. Differentiating the roles of the hippocampus and perirhinal cortex in processes beyond long-term declarative memory: a double dissociation in dementia. J. Neurosci. 26, 5198–5203 (2006).
Barense, M. D. et al. Functional specialization in the human medial temporal lobe. J. Neurosci. 25, 10239–10246 (2005).
Shrager, Y., Gold, J. J., Hopkins, R. O. & Squire, L. R. Intact visual perception in memory-impaired patients with medial temporal lobe lesions. J. Neurosci. 26, 2235–2240 (2006).
Lee, A. C., Bandelow, S., Schwarzbauer, C., Henson, R. N. & Graham, K. S. Perirhinal cortex activity during visual object discrimination: an event-related fMRI study. Neuroimage 33, 362–373 (2006).
O'Neil, E. B., Cate, A. D. & Kohler, S. Perirhinal cortex contributes to accuracy in recognition memory and perceptual discriminations. J. Neurosci. 29, 8329–8334 (2009).
Holdstock, J. S., Hocking, J., Notley, P., Devlin, J. T. & Price, C. J. Integrating visual and tactile information in the perirhinal cortex. Cereb. Cortex 19, 2993–3000 (2009).
Diana, R. A., Yonelinas, A. P. & Ranganath, C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn. Sci. 11, 379–386 (2007).
Davachi, L. Item, context and relational episodic encoding in humans. Curr. Opin. Neurobiol. 16, 693–700 (2006).
Spaniol, J. et al. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia 47, 1765–1779 (2009).
Vilberg, K. L. & Rugg, M. D. Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia 46, 1787–1799 (2008).
Tubridy, S. & Davachi, L. Medial temporal lobe contributions to episodic sequence encoding. Cereb. Cortex 21, 272–280 (2011).
Jenkins, L. J. & Ranganath, C. Prefrontal and medial temporal lobe activity at encoding predicts temporal context memory. J. Neurosci. 30, 15558–15565 (2010).
Yonelinas, A. P., Otten, L. J., Shaw, K. N. & Rugg, M. D. Separating the brain regions involved in recollection and familiarity in recognition memory. J. Neurosci. 25, 3002–3008 (2005).
Johnson, J. D., McDuff, S. G., Rugg, M. D. & Norman, K. A. Recollection, familiarity, and cortical reinstatement: a multivoxel pattern analysis. Neuron 63, 697–708 (2009).
Daselaar, S. M., Fleck, M. S. & Cabeza, R. Triple dissociation in the medial temporal lobes: recollection, familiarity, and novelty. J. Neurophysiol. 96, 1902–1911 (2006).
Daselaar, S. M. et al. Posterior midline and ventral parietal activity is associated with retrieval success and encoding failure. Front. Hum. Neurosci. 3, 13 (2009).
Leshikar, E. D. & Duarte, A. Medial prefrontal cortex supports source memory accuracy for self-referenced items. Soc. Neurosci. 7, 126–145 (2011).
Martin, V. C., Schacter, D. L., Corballis, M. C. & Addis, D. R. A role for the hippocampus in encoding simulations of future events. Proc. Natl Acad. Sci. USA 108, 13858–13863 (2011).
Ritchey, M., LaBar, K. S. & Cabeza, R. Level of processing modulates the neural correlates of emotional memory formation. J. Cogn. Neurosci. 23, 757–771 (2011).
Buckner, R. L., Andrews-Hanna, J. R. & Schacter, D. L. The brain's default network: anatomy, function, and relevance to disease. Ann. NY Acad. Sci. 1124, 1–38 (2008).
Maguire, E. A. The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings. Scand. J. Psychol. 42, 225–238 (2001).
Svoboda, E., McKinnon, M. C. & Levine, B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia 44, 2189–2208 (2006).
St Jacques, P. L., Conway, M. A., Lowder, M. W. & Cabeza, R. Watching my mind unfold versus yours: an fMRI study using a novel camera technology to examine neural differences in self-projection of self versus other perspectives. J. Cogn. Neurosci. 23, 1275–1284 (2010).
Spreng, R. N., Mar, R. A. & Kim, A. S. N. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci. 21, 489–510 (2009). This article is a meta-analysis of fMRI studies showing that the PHC, RSC, posterior hippocampus and default network are reliably recruited across autobiographical memory, virtual spatial navigation, theory of mind and episodic simulation tasks.
Szpunar, K. K., Watson, J. M. & McDermott, K. B. Neural substrates of envisioning the future. Proc. Natl Acad. Sci. USA 104, 642–647 (2007).
Szpunar, K. K., Chan, J. C. K. & McDermott, K. B. Contextual processing in episodic future thought. Cereb. Cortex 19, 1539–1548 (2009).
Addis, D. R., Pan, L., Vu, M.-A., Laiser, N. & Schacter, D. L. Constructive episodic simulation of the future and the past: distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia 47, 2222–2238 (2009).
Hassabis, D. & Maguire, E. A. Deconstructing episodic memory with construction. Trends Cogn. Sci. 11, 299–306 (2007).
Buckner, R. L. & Carroll, D. C. Self-projection and the brain. Trends Cogn. Sci. 11, 49–57 (2007).
Norman, G. & Eacott, M. J. Dissociable effects of lesions to the perirhinal cortex and the postrhinal cortex on memory for context and objects in rats. Behav. Neurosci. 119, 557–566 (2005). This article reports a double dissociation between the effects of PRC and postrhinal cortex lesions, such that the former impair object recognition and the latter impair context recognition.
Wan, H., Aggleton, J. P. & Brown, M. W. Different contributions of the hippocampus and perirhinal cortex to recognition memory. J. Neurosci. 19, 1142–1148 (1999).
Bucci, D. J., Saddoris, M. P. & Burwell, R. D. Contextual fear discrimination is impaired by damage to the postrhinal or perirhinal cortex. Behav. Neurosci. 116, 479–488 (2002).
Burwell, R. D., Saddoris, M. P., Bucci, D. J. & Wiig, K. A. Corticohippocampal contributions to spatial and contextual learning. J. Neurosci. 24, 3826–3836 (2004).
Vann, S. D. & Aggleton, J. P. Extensive cytotoxic lesions of the rat retrosplenial cortex reveal consistent deficits on tasks that tax allocentric spatial memory. Behav. Neurosci. 116, 85–94 (2002).
Keene, C. S. & Bucci, D. J. Neurotoxic lesions of retrosplenial cortex disrupt signaled and unsignaled contextual fear conditioning. Behav. Neurosci. 122, 1070–1077 (2008).
Alvarado, M. C. & Bachevalier, J. Comparison of the effects of damage to the perirhinal and parahippocampal cortex on transverse patterning and location memory in rhesus macaques. J. Neurosci. 25, 1599–1609 (2005).
Bachevalier, J. & Nemanic, S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus 18, 64–80 (2008).
Malkova, L. & Mishkin, M. One-trial memory for object-place associations after separate lesions of hippocampus and posterior parahippocampal region in the monkey. J. Neurosci. 23, 1956–1965 (2003).
Bohbot, V. D., Allen, J. J. & Nadel, L. Memory deficits characterized by patterns of lesions to the hippocampus and parahippocampal cortex. Ann. NY Acad. Sci. 911, 355–368 (2000).
Uncapher, M. R., Otten, L. J. & Rugg, M. D. Episodic encoding is more than the sum of its parts: an fMRI investigation of multifeatural contextual encoding. Neuron 52, 547–556 (2006).
Sommer, T., Rose, M., Weiller, C. & Buchel, C. Contributions of occipital, parietal and parahippocampal cortex to encoding of object-location associations. Neuropsychologia 43, 732–743 (2005).
Ross, R. S. & Slotnick, S. D. The hippocampus is preferentially associated with memory for spatial context. J. Cogn. Neurosci. 20, 432–446 (2008).
Cansino, S., Maquet, P., Dolan, R. J. & Rugg, M. D. Brain activity underlying encoding and retrieval of source memory. Cereb. Cortex 12, 1048–1056 (2002).
Baumann, O., Chan, E. & Mattingley, J. B. Dissociable neural circuits for encoding and retrieval of object locations during active navigation in humans. Neuroimage 49, 2816–2825 (2010).
Janzen, G. & van Turennout, M. Selective neural representation of objects relevant for navigation. Nature Neurosci. 7, 673–677 (2004).
Schinazi, V. R. & Epstein, R. A. Neural correlates of real-world route learning. Neuroimage 53, 725–735 (2010).
Ekstrom, A. D., Copara, M. S., Isham, E. A., Wang, W.-C. & Yonelinas, A. P. Dissociable networks involved in spatial and temporal order source retrieval. Neuroimage 56, 1803–1813 (2011).
Epstein, R. & Kanwisher, N. A cortical representation of the local visual environment. Nature 392, 598–601 (1998).
Epstein, R., Harris, A., Stanley, D. & Kanwisher, N. The parahippocampal place area: recognition, navigation, or encoding? Neuron 23, 115–125 (1999).
Mullally, S. L. & Maguire, E. A. A. New role for the parahippocampal cortex in representing space. J. Neurosci. 31, 7441–7449 (2011).
Bar, M. & Aminoff, E. Cortical analysis of visual context. Neuron 38, 347–358 (2003).
Takahashi, N. & Kawamura, M. Pure topographical disorientation — the anatomical basis of landmark agnosia. Cortex 38, 717–725 (2002).
Landis, T., Cummings, J. L., Benson, D. F. & Palmer, E. P. Loss of topographic familiarity. An environmental agnosia. Arch. Neurol. 43, 132–136 (1986).
Ekstrom, A. D. et al. Cellular networks underlying human spatial navigation. Nature 425, 184–188 (2003).
Burwell, R. D. & Hafeman, D. M. Positional firing properties of postrhinal cortex neurons. Neuroscience 119, 577–588 (2003).
Hassabis, D. et al. Decoding neuronal ensembles in the human hippocampus. Curr. Biol. 19, 546–554 (2009).
O'Craven, K. M. & Kanwisher, N. Mental imagery of faces and places activates corresponding stiimulus-specific brain regions. J. Cogn. Neurosci. 12, 1013–1023 (2000).
Park, S., Intraub, H., Yi, D.-J., Widders, D. & Chun, M. M. Beyond the edges of a view: boundary extension in human scene-selective visual cortex. Neuron 54, 335–342 (2007).
Ekstrom, A. D. et al. Human hippocampal theta activity during virtual navigation. Hippocampus 15, 881–889 (2005).
Epstein, R. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn. Sci. 12, 388–396 (2008). A succinct but thorough review of evidence regarding the roles of PHC and RSC in spatial cognition.
Aguirre, G. K. & D'Esposito, M. Topographical disorientation: a synthesis and taxonomy. Brain 122, 1613–1628 (1999).
Kelley, W. M. et al. Finding the self? An event-related fMRI study. J. Cogn. Neurosci. 14, 785–794 (2002).
D'Argembeau, A. et al. The neural basis of personal goal processing when envisioning future events. J. Cogn. Neurosci. 22, 1701–1713 (2010).
Moran, J. M., Macrae, C. N., Heatherton, T. F., Wyland, C. L. & Kelley, W. M. Neuroanatomical evidence for distinct cognitive and affective components of self. J. Cogn. Neurosci. 18, 1586–1594 (2006).
Sajonz, B. et al. Delineating self-referential processing from episodic memory retrieval: common and dissociable networks. NeuroImage 50, 1606–1617 (2010).
Greene, J. & Haidt, J. How (and where) does moral judgment work? Trends Cogn. Sci. 6, 517–523 (2002).
Aggleton, J. P. & Pearce, J. M. Neural systems underlying episodic memory: insights from animal research. Phil. Trans. R. Soc. Lond. B 356, 1467–1482 (2001).
Bird, C. M. & Burgess, N. The hippocampus and memory: insights from spatial processing. Nature Rev. Neurosci. 9, 182–194 (2008).
Murray, E. A. The amygdala, reward and emotion. Trends Cogn. Sci. 11, 489–497 (2007).
Horel, J. A., Voytko, M. L. & Salsbury, K. G. Visual learning suppressed by cooling the temporal pole. Behav. Neurosci. 98, 310–324 (1984).
Farovik, A., Place, R. J., Miller, D. R. & Eichenbaum, H. Amygdala lesions selectively impair familiarity in recognition memory. Nature Neurosci. 14, 1416–1417 (2011).
Meunier, M., Bachevalier, J. & Mishkin, M. Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia 35, 999–1015 (1997).
Xiang, J. Z. & Brown, M. W. Neuronal responses related to long-term recognition memory processes in prefrontal cortex. Neuron 42, 817–829 (2004).
Olson, I. R., Plotzker, A. & Ezzyat, Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain 130, 1718–1731 (2007).
LaBar, K. S. & Cabeza, R. Cognitive neuroscience of emotional memory. Nature Rev. Neurosci. 7, 54–64 (2006).
Kluver, H. & Bucy, P.C. “Psychic blindness” and other symptoms following bilateral temporal lobectomy in Rhesus monkeys. Am. J. Physiol. 119, 352–353 (1937).
Adolphs, R. What does the amygdala contribute to social cognition? Ann. NY Acad. Sci. 1191, 42–61 (2010).
Phelps, E. A. & LeDoux, J. E. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48, 175–187 (2005).
Murty, V. Ritchey, M., Adcock, R. A. & LaBar, K. S. fMRI studies of successful emotional memory encoding: a quantitative meta-analysis. Neuropsychologia 48, 3459–3469 (2010).
Rushworth, M. F. S., Noonan, M. P., Boorman, E. D., Walton, M. E. & Behrens, T. E. Frontal cortex and reward-guided learning and decision-making. Neuron 70, 1054–1069 (2011).
Tranel, D., Damasio, H. & Damasio, A. R. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia 35, 1319–1327 (1997).
Mayes, A. R., Meudell, R., Mann, D. & Pickering, A. Location of lesions in Korsakoff's Syndrome: neuropsychological and neuropathological data on two patients. Cortex 24, 367–388 (1987).
Aggleton, J. P. et al. Hippocampal-anterior thalamic pathways for memory: uncovering a network of direct and indirect actions. Eur. J. Neurosci. 31, 2292–2307 (2010).
Garden, D. L. et al. Anterior thalamic lesions stop synaptic plasticity in retrosplenial cortex slices: expanding the pathology of diencephalic amnesia. Brainy 132, 1847–1857 (2009).
Cabeza, R., Ciaramelli, E., Olson, I. R. & Moscovitch, M. The parietal cortex and episodic memory: an attentional account. Nature Rev. Neurosci. 9, 613–625 (2008).
Berryhill, M. E., Phuong, L., Picasso, L., Cabeza, R. & Olson, I. R. Parietal lobe and episodic memory: bilateral damage causes impaired free recall of autobiographical memory. J. Neurosci. 27, 14415–14423 (2007).
Simons, J. S., Peers, P. V., Mazuz, Y. S., Berryhill, M. E. & Olson, I. R. Dissociation between memory accuracy and memory confidence following bilateral parietal lesions. Cereb. Cortex 20, 479–485 (2010).
Yoder, R. M., Clark, B. J. & Taube, J. S. Origins of landmark encoding in the brain. Trends Neurosci. 34, 561–571 (2011).
Bar, M. Visual objects in context. Nature Rev. Neurosci. 5, 617–629 (2004). This article presents the view that the PHC and RSC extract information about context on the basis of global visual scene information, thereby facilitating object recognition.
Zwaan, R. A. & Radvansky, G. A. Situation models in language comprehension and memory. Psychol. Bull. 123, 162–185 (1998). A well-written review and theoretical synthesis of behavioural research on situation models in memory, language and spatial cognition.
Kintsch, W. The role of knowledge in discourse comprehension: a construction-integration model. Psychol. Rev. 95, 163–182 (1988).
Tse, D. et al. Schema-dependent gene activation and memory encoding in neocortex. Science 333, 891–895 (2011).
Oliva, A. & Torralba, A. The role of context in object recognition. Trends Cogn. Sci. 11, 520–527 (2007).
Ranganath, C. & Rainer, G. Neural mechanisms for detecting and remembering novel events. Nature Rev. Neurosci. 4, 193–202 (2003).
de Curtis, M. & Pare, D. The rhinal cortices: a wall of inhibition between the neocortex and the hippocampus. Prog. Neurobiol. 74, 101–110 (2004). A review of physiological research suggesting that excitatory inputs from the PRC to the hippocampal formation are subject to intense, long-range inhibition, thus limiting the extent to which the two regions can functionally interact.
Eacott, M. J. & Gaffan, E. A. The roles of perirhinal cortex, postrhinal cortex, and the fornix in memory for objects, contexts, and events in the rat. Q. J. Exp. Psychol. B 58, 202–217 (2005).
Ranganath, C. & Blumenfeld, R. S. in Learning and Memory: A Comprehensive Reference (ed. Byrne, J. H.) 261–279 (Academic Press, 2008).
Suzuki, W. A. & Amaral, D. G. Where are the perirhinal and parahippocampal cortices? A historical overview of the nomenclature and boundaries applied to the primate medial temporal lobe. Neuroscience 120, 893–906 (2003).
Seeley, W. W., Crawford, R. K., Zhou, J., Miller, B. L. & Greicius, M. D. Neurodegenerative diseases target large-scale human brain networks. Neuron 62, 42–52 (2009). This study demonstrates that functional connectivity analysis of resting-state fMRI data and analysis of covariance in cortical thickness provide converging evidence regarding networks in the brain, and that these networks are differentially targeted by neurodegenerative diseases. Semantic dementia was associated with degeneration of an anterior temporal network that included the ventral temporopolar cortex, whereas Alzheimer's disease was associated with degeneration of a posterior network that included the medial and ventrolateral parietal cortex and posterior cingulate.
Nestor, P. J., Fryer, T. D. & Hodges, J. R. Declarative memory impairments in Alzheimer's disease and semantic dementia. Neuroimage 30, 1010–1020 (2006).
Staresina, B. P., Duncan, K. D. & Davachi, L. Perirhinal and parahippocampal cortices differentially contribute to later recollection of object- and scene-related event details. J. Neurosci. 31, 8739–8747 (2011).
Bernasconi, N. et al. Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain 126, 462–469 (2003).
Bonilha, L., Kobayashi, E., Rorden, C., Cendes, F. & Li, L. M. Medial temporal lobe atrophy in patients with refractory temporal lobe epilepsy. J. Neurol. Neurosurg. Psychiatry 74, 1627–1630 (2003).
Galton, C. J. et al. Differing patterns of temporal atrophy in Alzheimer's disease and semantic dementia. Neurology 57, 216–225 (2001).
Yarkoni, T., Poldrack, R. A., Nichols, T. E., Van Essen, D. C. & Wager. T. D. Large-scale automated synthesis of human functional neuroimaging data. Nature Methods 8, 665–670 (2011).
Acknowledgements
We thank the many colleagues (including three anonymous reviewers) who gave helpful suggestions and comments on previous drafts and apologize to those whose work could not be cited owing to citation limits. This paper was supported by US National Institute of Mental Health grant 1R01MH083734.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Hippocampal formation
-
A term used to collectively describe the entorhinal cortex, dentate gyrus, subfields CA1, CA2 and CA3, and the subiculum.
- Immediate early gene
-
A gene that encodes a transcription factor that is induced within minutes of raised neuronal activity without requiring a protein signal. Immediate early gene activation is therefore used as an indirect marker of neuronal activation.
- Semantic priming
-
A quickening in reaction time for responding to words that are preceded by a semantically related “priming stimulus”.
- Autobiographical memories
-
Memories of personal events from an individual's life.
- Retrograde amnesia
-
Memory loss of events that occurred before the onset of a memory disorder. Typically, following the onset of medial temporal lobe damage, patients show a reduced ability to recollect episodes from the time period before the brain damage occurred.
- Delayed non-matching to place task
-
A spatial recognition memory task in which animals have to distinguish a non-visited arm of a maze from a previously visited arm and enter the non-visited arm in order to receive a reward.
- Theta oscillations
-
Large, rhythmic changes in the amplitude of local field potentials that are seen in the 5–12 Hz frequency in rodents and in the 4–8 Hz range in humans. Theta oscillations are evident during active exploration of novel environments and have been functionally associated with spatial navigation and memory for temporal sequences.
- Theory of mind
-
The ability to understand the mental states — such as beliefs, desires and intentions — of others.
- Connectional fingerprints
-
The patterns of cortico–cortical connections exhibited by cytoarchitectonic areas.
- Systems consolidation
-
A hypothesized process by which the brain regions that support memory of a particular experience are thought to change over time. Systems consolidation theories are typically invoked to explain differential effects of brain lesions on memories of recent and remote events.
Rights and permissions
About this article
Cite this article
Ranganath, C., Ritchey, M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci 13, 713–726 (2012). https://doi.org/10.1038/nrn3338
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3338
This article is cited by
-
A retinotopic code structures the interaction between perception and memory systems
Nature Neuroscience (2024)
-
A generative model of memory construction and consolidation
Nature Human Behaviour (2024)
-
Spatiotemporal dynamics of hippocampal-cortical networks underlying the unique phenomenological properties of trauma-related intrusive memories
Molecular Psychiatry (2024)
-
Targeting vulnerable microcircuits in the ventral hippocampus of male transgenic mice to rescue Alzheimer-like social memory loss
Military Medical Research (2024)
-
Multi-ancestry phenome-wide association of complement component 4 variation with psychiatric and brain phenotypes in youth
Genome Biology (2023)