Key Points
-
The expectant brain undergoes many changes to maximize the likelihood of a successful outcome of the pregnancy. These adaptations are driven by pregnancy hormones and ensure adequate nutrient supply to the fetus, protection from maternal stress hormones, appropriate organization of parturition and the delivery of maternal care.
-
Exposure to stress or glucocorticoids during pregnancy can adversely programme the fetuses, making them more susceptible to disease in adulthood. One protective mechanism against this effect involves endogenous-opioid inhibition of the mother's responses to stress in pregnancy, which reduces the exposure of the fetus to maternal glucocorticoids.
-
Increased food intake in pregnancy is permitted by the resetting of central appetite control mechanisms, for example, the emergence of central leptin resistance. This resetting ensures sufficient nutrients for the fetus(es), extra energy for the mother, and a surplus of energy for storage as fat in preparation for lactation.
-
Inhibitory-opioid mechanisms prevent the premature activation of oxytocin neurons (and hence preterm birth) and aid the accumulation of neurohypophysial oxytocin stores. Allopregnanolone, a neuroactive metabolite of progesterone, restrains oxytocin neurons by enhancing the effectiveness of GABA synapses, but also induces opioid inhibition.
-
Dopamine neurons in the hypothalamus inhibit prolactin secretion. Before term, the stimulatory action of prolactin on these neurons is switched off, permitting increased prolactin secretion for the stimulation of lactation and maternal behaviour.
-
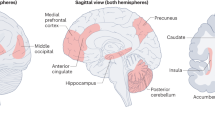
Maternal behaviour emerges rapidly after birth. This depends on 'priming' of the neural circuitry that organizes the components of maternal behaviour and the motivation to perform it. Priming involves the action of oestrogen, progesterone and lactogens, particularly in the medial preoptic area.
-
The offspring are protected from harm by a marked increase in maternal aggressiveness soon after birth. This element of maternal behaviour involves multiple neurochemical changes, in particular, increased oxytocin release and decreased activity of serotonin neurons.
-
In humans, withdrawal of the hormones of pregnancy might predispose women to the 'blues' soon after birth, and in vulnerable women might later trigger major puerperal depression.
Abstract
A successful pregnancy requires multiple adaptations of the mother's physiology to optimize fetal growth and development, to protect the fetus from adverse programming, to provide impetus for timely parturition and to ensure that adequate maternal care is provided after parturition. Many of these adaptations are organized by the mother's brain, predominantly through changes in neuroendocrine systems, and these changes are primarily driven by the hormones of pregnancy. By contrast, adaptations in the mother's brain during lactation are maintained by external stimuli from the young. The changes in pregnancy are not necessarily innocuous: they may predispose the mother to post-partum mood disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Russell, J. A., Douglas, A. J. & Ingram, C. D. Brain preparations for maternity: adaptive changes in behavioral and neuroendocrine systems during pregnancy and lactation. An overview. Prog. Brain Res. 133, 1–38 (2001).
Barker, D. J. Fetal programming of coronary heart disease. Trends Endocrinol. Metab. 13, 364–368 (2002).
Levitt, N. S. et al. Impaired glucose tolerance and elevated blood pressure in low birth weight, nonobese, young South African adults: early programming of cortisol axis. J. Clin. Endocrinol. Metab. 85, 4611–4618 (2000).
Welberg, L. A. M. & Seckl, J. R. Prenatal stress, glucocorticoids and the programming of the brain. J. Neuroendocrinol. 13, 113–128 (2001). This review considers the role of prenatal exposure to excess glucocorticoid in the adverse programming of stress responses. It discusses animal models and human disorders.
Welberg, L. A. M., Seckl, J. R. & Holmes, M. C. Inhibition of 11β-hydroxysteroid dehydrogenase, the foetoplacental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety-like behaviour in the offspring. Eur. J. Neurosci. 12, 1047–1054 (2000).
Vickers, M. H., Breier, B. H., Cutfield, W. S., Hofman, P. L. & Gluckman, P. D. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am. J. Physiol. Endocrinol. Metab. 279, E83–E87 (2000).
Lesage, J. et al. Perinatal maternal undernutrition programs the offspring hypothalamo-pituitary-adrenal (HPA) axis. Stress 9, 183–198 (2006).
Ozanne, S. E. & Constancia, M. Mechanisms of disease: the developmental origins of disease and the role of the epigenotype. Nature Clin. Pract. Endocrinol. Metab. 3, 539–546 (2007).
Gorski, J. N., Dunn-Meynell, A. A. & Levin, B. E. Maternal obesity increases hypothalamic leptin receptor expression and sensitivity in juvenile obesity-prone rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1782–R1791 (2007).
Johnstone, H. A. et al. Attenuation of hypothalamic-pituitary-adrenal axis stress responses in late pregnancy: changes in feedforward and feedback mechanisms. J. Neuroendocrinol. 12, 811–822 (2000).
Atkinson, H. C. & Waddell, B. J. The hypothalamic-pituitary-adrenal axis in rat pregnancy and lactation: circadian variation and interrelationship of plasma adrenocorticotropin and corticosterone. Endocrinology 136, 512–520 (1995).
Carr, B. R., Parker, C. R., Madden, J. D., MacDonald, P. C. & Porter, J. C. Maternal plasma ACTH and cortisol relationships throughout human pregnancy. Am. J. Obstet. Gynecol. 139, 416–421 (1981).
Waddell, B. J. & Atkinson, H. C. Production rate, metabolic clearance rate and uterine extraction of corticosterone during rat pregnancy. J. Endocrinol. 143, 183–190 (1994).
Douglas, A. J., Brunton, P. J., Bosch, O. J., Russell, J. A. & Neumann, I. D. Neuroendocrine responses to stress in mice: hyporesponsiveness in pregnancy and parturition. Endocrinology 144, 5268–5276 (2003).
Schulte, H. M., Weisner, D. & Allolio, B. The corticotrophin releasing hormone test in late pregnancy: lack of adrenocorticotrophin and cortisol response. Clin. Endocrinol. 33, 99–106 (1990).
Hartikainen-Sorri, A. L., Kirkinen, P., Sorri, M., Anttonen, H. & Tuimala, R. No effect of experimental noise exposure on human pregnancy. Obstet. Gynecol. 77, 611–615 (1991).
Neumann, I. D. et al. Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. J. Physiol. 508, 289–300 (1998). This study provided the first detailed analysis of reduced HPA-axis responses to stress in pregnant rats.
Wigger, A. et al. Nonresponsiveness of the rat hypothalamo-pituitary-adrenocortical axis to parturition-related events: inhibitory action of endogenous opioids. Endocrinology 140, 2843–2849 (1999).
Windle, R. J. et al. Endocrine and behavioural responses to noise stress: comparison of virgin and lactating female rats during non-disrupted maternal activity. J. Neuroendocrinol. 9, 407–414 (1997).
da Costa, A. P. C., Wood, S., Ingram, C. D. & Lightman, S. L. Region-specific reduction in stress-induced c-fos mRNA expression during pregnancy and lactation. Brain Res. 742, 177–184 (1996).
Day, H. E. W., Campeau, S., Watson, S. J. Jr & Akil, H. Expression of α1b adrenoceptor mRNA in corticotropin-releasing hormone-containing cells of the rat hypothalamus and its regulation by corticosterone. J. Neurosci. 19, 10098–10106 (1999).
Cole, R. L. & Sawchenko, P. E. Neurotransmitter regulation of cellular activation and neuropeptide gene expression in the paraventricular nucleus of the hypothalamus. J. Neurosci. 22, 959–969 (2002).
Itoi, K. et al. Microinjection of norepinephrine into the paraventricular nucleus of the hypothalamus stimulates corticotropin-releasing factor gene expression in conscious rats. Endocrinology 135, 2177–2182 (1994).
Kiss, A., Palkovits, M. & Aguilera, G. Neural regulation of corticotropin releasing hormone (CRH) and CRH receptor mRNA in the hypothalamic paraventricular nucleus in the rat. J. Neuroendocrinol. 8, 103–112 (1996).
Brunton, P. J. et al. Endogenous opioids and attenuated hypothalamic-pituitary-adrenal axis responses to immune challenge in pregnant rats. J. Neurosci. 25, 5117–5126 (2005). This study used an immune challenge to demonstrate that suppressed HPA-axis responses in late pregnancy result from endogenous-opioid inhibition of noradrenergic input to the CRH neurons.
Douglas, A. J., Meddle, S. L., Toschi, N., Bosch, O. J. & Neumann, I. D. Reduced activity of the noradrenergic system in the paraventricular nucleus at the end of pregnancy: implications for stress hyporesponsiveness. J. Neuroendocrinol. 17, 40–48 (2005).
Douglas, A. J., Johnstone, H. A., Wigger, A., Landgraf, R. & Neumann, I. D. The role of endogenous opioids in neurohypophysial and hypothalamo-pituitary-adrenal axis hormone secretory responses to stress in pregnant rats. J. Endocrinol. 158, 285–293 (1998).
Sawchenko, P. E., Swanson, L. W. & Joseph, S. A. The distribution and cells of origin of ACTH (1–39)-stained varicosities in the paraventricular and supraoptic nuclei. Brain Res. 232, 365–374 (1982).
Douglas, A. J., Bicknell, R. J., Leng, G., Russell, J. A. & Meddle, S. L. β-endorphin cells in the arcuate nucleus: projections to the supraoptic nucleus and changes in expression during pregnancy and parturition. J. Neuroendocrinol. 14, 768–777 (2002).
Brady, L. S., Lynn, A. B., Herkenham, M. & Gottesfeld, Z. Systemic interleukin-1 induces early and late patterns of c-fos mRNA expression in brain. J. Neurosci. 14, 4951–4964 (1994).
Ceccatelli, S., Seroogy, K. B., Millhorn, D. E. & Terenius, L. Presence of a dynorphin-like peptide in a restricted subpopulation of catecholaminergic neurons in rat nucleus tractus solitarii. Brain Res. 589, 225–230 (1992).
Bronstein, D. M., Schafer, M. K. H., Watson, S. J. & Akil, H. Evidence that β-endorphin is synthesized in cells in the nucleus tractus solitarius: detection of POMC mRNA. Brain Res. 587, 269–275 (1992).
Windle, R. J., Shanks, N., Lightman, S. L. & Ingram, C. D. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology 138, 2829–2834 (1997).
Neumann, I. D., Torner, L. & Wigger, A. Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience 95, 567–575 (2000).
Shaikh, A. A. Estrone and estradiol levels in the ovarian venous blood from rats during the estrous cycle and pregnancy. Biol. Reprod. 5, 297–307 (1971).
Concas, A. et al. Role of brain allopregnanolone in the plasticity of γ-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc. Natl Acad. Sci. USA 95, 13284–13289 (1998).
Douglas, A. J., Johnstone, H., Brunton, P. & Russell, J. A. Sex-steroid induction of endogenous opioid inhibition on oxytocin secretory responses to stress. J. Neuroendocrinol. 12, 343–350 (2000).
Gao, C. Q., Dhooge, W. S., Kaufman, J. M., Weyne, J. J. & Eechaute, W. P. Hypothalamic 5α-reductase and 3α-oxidoreductase activity in the male rat. J. Steroid Biochem. Mol. Biol. 80, 91–98 (2002).
Russell, D. W. & Wilson, J. D. Steroid 5α-reductase: two genes/two enzymes. Annu. Rev. Biochem. 63, 25–61 (1994).
Penning, T. M. et al. Structure and function of 3α-hydroxysteroid dehydrogenase. Steroids 62, 101–111 (1997).
Brussaard, A. B., Devay, P., Leyting-Vermeulen, J. L. & Kits, K. S. Changes in properties and neurosteroid regulation of GABAergic synapses in the supraoptic nucleus during the mammalian female reproductive cycle. J. Physiol. 516, 513–524 (1999).
Patchev, V. K., Hassan, A. H. S., Holsboer, F. & Almeida, O. F. X. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology 15, 533–540 (1996).
Brunton, P. J., Harrison, C. E. L. & Russell, J. A. Allopregnanolone is involved in reduced HPA axis responses to immune challenge in late pregnancy. Endocrine Abstracts 7, OC1 (2004).
Blyth, B. J., Hauger, R. L., Purdy, R. H. & Amico, J. A. The neurosteroid allopregnanolone modulates oxytocin expression in the hypothalamic paraventricular nucleus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R684–R691 (2000).
Bali, B. & Kovacs, K. J. GABAergic control of neuropeptide gene expression in parvocellular neurons of the hypothalamic paraventricular nucleus. Eur. J. Neurosci. 18, 1518–1526 (2003).
Russell, J. A. & Brunton, P. J. Oxytocin: peripheral/central actions and their regulation. In The New Encyclopedia of Neuroscience (eds Squire, L. et al.) (in the press).
Russell, J. A. & Leng, G. Sex, parturition and motherhood without oxytocin? J. Endocrinol. 157, 342–359 (1998).
Russell, J. A., Leng, G. & Douglas, A. J. The magnocellular oxytocin system, the fount of maternity: adaptations in pregnancy. Front. Neuroendocrinol. 24, 27–61 (2003).
Lang, R. E. et al. Oxytocin unlike vasopressin is a stress hormone in the rat. Neuroendocrinology 37, 314–316 (1983).
Brunton, P. J., Sabatier, N., Leng, G. & Russell, J. A. Suppressed oxytocin neuron responses to immune challenge in late pregnant rats: a role for endogenous opioids. Eur. J. Neurosci. 23, 1241–1247 (2006).
Douglas, A. J. et al. Central endogenous opioid inhibition of supraoptic oxytocin neurons in pregnant rats. J. Neurosci. 15, 5049–5057 (1995).
Onaka, T., Luckman, S. M., Antonijevic, I., Palmer, R. & Leng, G. Involvement of the noradrenergic afferents from the nucleus tractus solitarii to the supraoptic nucleus in oxytocin release after peripheral cholecystokinin octapeptide in the rat. Neuroscience 66, 403–412 (1995).
Buller, K. M., Xu, Y., Dayas, C. V. & Day, T. A. Dorsal and ventral medullary catecholamine cell groups contribute differentially to systemic interleukin-1β-induced HPA axis responses. Neuroendocrinology 73, 129–138 (2001).
Meddle, S. L., Leng, G., Selvarajah, J. R., Bicknell, R. J. & Russell, J. A. Direct pathways to the supraoptic nucleus from the brainstem and the main olfactory bulb are activated at parturition in the rat. Neuroscience 101, 1013–1021 (2000).
Douglas, A. J., Dye, S., Leng, G., Russell, J. A. & Bicknell, R. J. Endogenous opioid regulation of oxytocin secretion through pregnancy in the rat. J. Neuroendocrinol. 5, 307–314 (1993).
Leng, G., Dye, S. & Bicknell, R. J. κ-opioid restraint of oxytocin secretion: plasticity through pregnancy. Neuroendocrinology 66, 378–383 (1997).
Russell, J. A. & Brunton, P. J. Neuroactive steroids attenuate oxytocin stress responses in late pregnancy. Neuroscience 138, 879–889 (2006).
Leng, G. et al. Endogenous opioid actions and effects of environmental disturbance on parturition and oxytocin secretion in rats. J. Reprod. Fertil. 84, 345–356 (1988).
Ericsson, A., Kovacs, K. J. & Sawchenko, P. E. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J. Neurosci. 14, 897–913 (1994).
Francis, K., Meddle, S. L., Bishop, V. R. & Russell, J. A. Progesterone receptor expression in the pregnant and parturient rat hypothalamus and brainstem. Brain Res. 927, 18–26 (2002).
Brussaard, A. B. & Herbison, A. E. Long-term plasticity of postsynaptic GABAA-receptor function in the adult brain: insights from the oxytocin neurone. Trends Neurosci. 23, 190–195 (2000).
Koksma, J. J., Fritschy, J. M., Mack, V., Van Kesteren, R. E. & Brussaard, A. B. Differential GABAA receptor clustering determines GABA synapse plasticity in rat oxytocin neurons around parturition and the onset of lactation. Mol. Cell. Neurosci. 28, 128–140 (2005).
Theodosis, D. T. et al. Oxytocin and estrogen promote rapid formation of functional GABA synapses in the adult supraoptic nucleus. Mol. Cell. Neurosci. 31, 785–794 (2006).
Koksma, J. J. et al. Oxytocin regulates neurosteroid modulation of GABAA receptors in supraoptic nucleus around parturition. J. Neurosci. 23, 788–797 (2003). This intriguing study shows how oxytocin acts on oxytocin neurons to desensitize GABA A receptors to allopregnanolone at the end of pregnancy.
Way, S. A. et al. Endogenous opioid regulation of oxytocin release during parturition is reduced in ovariectomized rats. J. Endocrinol. 138, 13–22 (1993).
Jiang, Q. B. & Wakerley, J. B. Analysis of bursting responses of oxytocin neurones in the rat in late pregnancy, lactation and after weaning. J. Physiol. 486, 237–248 (1995). This paper describes the emergence, in late pregnancy, of the burst-firing responses of oxytocin neurons to suckling. These responses are classically associated with the milk-ejection reflex in lactation.
Wang, Y. F. & Hatton, G. I. Burst firing of oxytocin neurons in male rat hypothalamic slices. Brain Res. 1032, 36–43 (2005).
Teruyama, R. & Armstrong, W. E. Changes in the active membrane properties of rat supraoptic neurones during pregnancy and lactation. J. Neuroendocrinol. 14, 933–944 (2002).
Herbison, A. E., Voisin, D. L., Douglas, A. J. & Chapman, C. Profile of monoamine and excitatory amino acid release in rat supraoptic nucleus over parturition. Endocrinology 138, 33–40 (1997).
Fenelon, V. S. & Herbison, A. E. Progesterone regulation of GABAA receptor plasticity in adult rat supraoptic nucleus. Eur. J. Neurosci. 12, 1617–1623 (2000).
Neumann, I., Douglas, A. J., Pittman, Q. J., Russell, J. A. & Landgraf, R. Oxytocin released within the supraoptic nucleus of the rat brain by positive feedback action is involved in parturition-related events. J. Neuroendocrinol. 8, 227–233 (1996).
Richard, P., Moos, F. & Freund-Mercier, M. J. Central effects of oxytocin. Physiol. Rev. 71, 331–370 (1991).
Hirasawa, M. et al. Dendritically released transmitters cooperate via autocrine and retrograde actions to inhibit afferent excitation in rat brain. J. Physiol. 559, 611–624 (2004).
Leng, G. & Brown, D. The origins and significance of pulsatility in hormone secretion from the pituitary. J. Neuroendocrinol. 9, 493–513 (1997).
Wang, Y. F. & Hatton, G. I. Dominant role of βγ subunits of G-proteins in oxytocin-evoked burst firing. J. Neurosci. 27, 1902–1912 (2007).
Theodosis, D. T. Oxytocin-secreting neurons: a physiological model of morphological neuronal and glial plasticity in the adult hypothalamus. Front. Neuroendocrinol. 23, 101–135 (2002).
Catheline, G., Touquet, B., Lombard, M. C., Poulain, D. A. & Theodosis, D. T. A study of the role of neuro-glial remodeling in the oxytocin system at lactation. Neuroscience 137, 309–316 (2006).
Gaynes, B. N. et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid. Rep. Technol. Assess. (Summ.) 119, 1–8 (2005).
Mann, P. E. & Bridges, R. S. Lactogenic hormone regulation of maternal behavior. Prog. Brain Res. 133, 251–262 (2001). This chapter reviews research that has revealed the importance of prolactin and placental lactogens in the priming of medial preoptic neurons for their key role in the display of maternal behaviour after birth.
Torner, L., Toschi, N., Nava, G., Clapp, C. & Neumann, I. D. Increased hypothalamic expression of prolactin in lactation: involvement in behavioural and neuroendocrine stress responses. Eur. J. Neurosci. 15, 1381–1389 (2002).
Grattan, D. R. Behavioural significance of prolactin signalling in the central nervous system during pregnancy and lactation. Reproduction 123, 497–506 (2002).
Andrews, Z. B. Neuroendocrine regulation of prolactin secretion during late pregnancy: easing the transition into lactation. J. Neuroendocrinol. 17, 466–473 (2005).
Augustine, R. A., Kokay, I. C., Andrews, Z. B., Ladyman, S. R. & Grattan, D. R. Quantitation of prolactin receptor mRNA in the maternal rat brain during pregnancy and lactation. J. Mol. Endocrinol. 31, 221–232 (2003).
Pi, X., Zhang, B., Li, J. & Voogt, J. L. Promoter usage and estrogen regulation of prolactin receptor gene in the brain of the female rat. Neuroendocrinology 77, 187–197 (2003).
Ma, F. Y. et al. Prolactin specifically activates signal transducer and activator of transcription 5b in neuroendocrine dopaminergic neurons. Endocrinology 146, 5112–5119 (2005).
Lee, Y. & Voogt, J. L. Feedback effects of placental lactogens on prolactin levels and Fos-related antigen immunoreactivity of tuberoinfundibular dopaminergic neurons in the arcuate nucleus during pregnancy in the rat. Endocrinology 140, 2159–2166 (1999).
Anderson, G. M. et al. Suppression of prolactin-induced signal transducer and activator of transcription 5b signaling and induction of suppressors of cytokine signaling messenger ribonucleic acid in the hypothalamic arcuate nucleus of the rat during late pregnancy and lactation. Endocrinology 147, 4996–5005 (2006).
Andrews, Z. B. & Grattan, D. R. Opioid control of prolactin secretion in late pregnant rats is mediated by tuberoinfundibular dopamine neurons. Neurosci. Lett. 328, 60–64 (2002).
Andrews, Z. B. & Grattan, D. R. Opioid receptor subtypes involved in the regulation of prolactin secretion during pregnancy and lactation. J. Neuroendocrinol. 15, 227–236 (2003).
Merchenthaler, I., Lennard, D. E., Cianchetta, P., Merchenthaler, A. & Bronstein, D. Induction of proenkephalin in tuberoinfundibular dopaminergic neurons by hyperprolactinemia: the role of sex steroids. Endocrinology 136, 2442–2450 (1995).
Soaje, M., Valdez, S., Bregonzio, C., Penissi, A. & Deis, R. P. Dopaminergic mechanisms involved in prolactin release after mifepristone and naloxone treatment during late pregnancy in the rat. Neuroendocrinology 84, 58–67 (2006).
Egli, M. et al. Prolactin secretory rhythm of mated rats induced by a single injection of oxytocin. Am. J. Physiol. Endocrinol. Metab. 290, E566–E572 (2006).
Bridges, R. S. & Hays, L. E. Steroid-induced alterations in mRNA expression of the long form of the prolactin receptor in the medial preoptic area of female rats: effects of exposure to a pregnancy-like regimen of progesterone and estradiol. Brain Res. Mol. Brain Res. 140, 10–16 (2005).
Fleming, A. S., Vaccarino, F. & Luebke, C. Amygdaloid inhibition of maternal behavior in the nulliparous female rat. Physiol. Behav. 25, 731–743 (1980).
Bridges, R. S., Mann, P. E. & Coppeta, J. S. Hypothalamic involvement in the regulation of maternal behaviour in the rat: inhibitory roles for the ventromedial hypothalamus and the dorsal/anterior hypothalamic areas. J. Neuroendocrinol. 11, 259–266 (1999).
Sukikara, M. H., Mota-Ortiz, S. R., Baldo, M. V., Felicio, L. F. & Canteras, N. S. A role for the periaqueductal gray in switching adaptive behavioral responses. J. Neurosci. 26, 2583–2589 (2006).
Kinsley, C. H. & Bridges, R. S. Morphine treatment and reproductive condition alter olfactory preferences for pup and adult male odors in female rats. Dev. Psychobiol. 23, 331–347 (1990).
Numan, M. Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behav. Cogn. Neurosci. Rev. 5, 163–190 (2006). This review synthesizes a large body of research on the details of the neural circuitry that is involved in different components of maternal behaviour, including motivation and reward.
Numan, M. et al. The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav. Neurosci. 119, 1588–1604 (2005).
Byrnes, E. M., Rigero, B. A. & Bridges, R. S. Opioid receptor antagonism during early lactation results in the increased duration of nursing bouts. Physiol. Behav. 70, 211–216 (2000).
Byrnes, E. M. & Bridges, R. S. Endogenous opioid facilitation of maternal memory in rats. Behav. Neurosci. 114, 797–804 (2000).
Sheehan, T. & Numan, M. Estrogen, progesterone, and pregnancy termination alter neural activity in brain regions that control maternal behavior in rats. Neuroendocrinology 75, 12–23 (2002).
Mattson, B. J. & Morrell, J. I. Preference for cocaine- versus pup-associated cues differentially activates neurons expressing either Fos or cocaine- and amphetamine-regulated transcript in lactating, maternal rodents. Neuroscience 135, 315–328 (2005).
Lonstein, J. S. & De Vries, G. J. Maternal behaviour in lactating rats stimulates c-fos in glutamate decarboxylase-synthesizing neurons of the medial preoptic area, ventral bed nucleus of the stria terminalis, and ventrocaudal periaqueductal gray. Neuroscience 100, 557–568 (2000).
Francis, D. D., Champagne, F. C. & Meaney, M. J. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J. Neuroendocrinol. 12, 1145–1148 (2000).
Jin, D. et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 446, 41–45 (2007).
Young, L. J., Muns, S., Wang, Z. & Insel, T. R. Changes in oxytocin receptor mRNA in rat brain during pregnancy and the effects of estrogen and interleukin-6. J. Neuroendocrinol. 9, 859–865 (1997).
Meddle, S. L., Bishop, V. R., Gkoumassi, E., van Leeuwen, F. W. & Douglas, A. J. Dynamic changes in oxytocin receptor expression and activation at parturition in the rat brain. Endocrinology 148, 5095–5104 (2007).
Insel, T. R. Postpartum increases in brain oxytocin binding. Neuroendocrinology 44, 515–518 (1986).
Windle, R. J. et al. Gonadal steroid modulation of stress-induced hypothalamo-pituitary-adrenal activity and anxiety behavior: role of central oxytocin. Endocrinology 147, 2423–2431 (2006).
Champagne, F., Diorio, J., Sharma, S. & Meaney, M. J. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc. Natl Acad. Sci. USA 98, 12736–12741 (2001).
Lucas, B. K., Ormandy, C. J., Binart, N., Bridges, R. S. & Kelly, P. A. Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology 139, 4102–4107 (1998).
Grattan, D. R. et al. Prolactin receptors in the brain during pregnancy and lactation: implications for behavior. Horm. Behav. 40, 115–124 (2001).
Kendrick, K. M. Oxytocin, motherhood and bonding. Exp. Physiol. 85, 111S–124S (2000).
Rosenblatt, J. S. Psychobiology of maternal behavior: contribution to the clinical understanding of maternal behavior among humans. Acta Paediatr. Suppl. 397, 3–8 (1994).
Lonstein, J. S. & Gammie, S. C. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci. Biobehav. Rev. 26, 869–888 (2002). This paper reviews the remarkable emergence of aggressive behaviour in female rodents after birth and its mechanisms; it touches upon the relationship between aggressive behaviour and anxiety.
Gammie, S. C. et al. Altered gene expression in mice selected for high maternal aggression. Genes Brain Behav. 6, 432–443 (2007).
Gammie, S. C., Bethea, E. D. & Stevenson, S. A. Altered maternal profiles in corticotropin-releasing factor receptor 1 deficient mice. BMC Neurosci. 8, 17 (2007).
Ferreira, A., Picazo, O., Uriarte, N., Pereira, M. & Fernandez-Guasti, A. Inhibitory effect of buspirone and diazepam, but not of 8-OH-DPAT, on maternal behavior and aggression. Pharmacol. Biochem. Behav. 66, 389–396 (2000).
Lee, G. & Gammie, S. C. GABA enhancement of maternal defense in mice: possible neural correlates. Pharmacol. Biochem. Behav. 86, 176–187 (2007).
Klink, R., Robichaud, M. & Debonnel, G. Gender and gonadal status modulation of dorsal raphe nucleus serotonergic neurons. Part I: effects of gender and pregnancy. Neuropharmacology 43, 1119–1128 (2002).
Klink, R., Robichaud, M. & Debonnel, G. Gender and gonadal status modulation of dorsal raphe nucleus serotonergic neurons. Part II. Regulatory mechanisms. Neuropharmacology 43, 1129–1138 (2002).
Robichaud, M. & Debonnel, G. Modulation of the firing activity of female dorsal raphe nucleus serotonergic neurons by neuroactive steroids. J. Endocrinol. 182, 11–21 (2004).
Kaura, V., Ingram, C. D., Gartside, S. E., Young, A. H. & Judge, S. J. The progesterone metabolite allopregnanolone potentiates GABAA receptor-mediated inhibition of 5-HT neuronal activity. Eur. Neuropsychopharmacol. 17, 108–115 (2007).
Bosch, O. J., Meddle, S. L., Beiderbeck, D. I., Douglas, A. J. & Neumann, I. D. Brain oxytocin correlates with maternal aggression: link to anxiety. J. Neurosci. 25, 6807–6815 (2005).
Bosch, O. J., Musch, W., Bredewold, R., Slattery, D. A. & Neumann, I. D. Prenatal stress increases HPA axis activity and impairs maternal care in lactating female offspring: implications for postpartum mood disorder. Psychoneuroendocrinology 32, 267–278 (2007).
Neumann, I. D. Alterations in behavioral and neuroendocrine stress coping strategies in pregnant, parturient and lactating rats. Prog. Brain Res. 133, 143–152 (2001).
Mantella, R. C., Vollmer, R. R., Li, X. & Amico, J. A. Female oxytocin-deficient mice display enhanced anxiety-related behavior. Endocrinology 144, 2291–2296 (2003).
Harris, B. et al. Maternity blues and major endocrine changes: cardiff puerperal mood and hormone study II. BMJ 308, 949–953 (1994).
Zonana, J. & Gorman, J. M. The neurobiology of postpartum depression. CNS Spectr. 10, 792–799 (2005).
Forman, D. R. et al. Effective treatment for postpartum depression is not sufficient to improve the developing mother-child relationship. Dev. Psychopathol. 19, 585–602 (2007).
Kammerer, M., Taylor, A. & Glover, V. The HPA axis and perinatal depression: a hypothesis. Arch. Womens Ment. Health 9, 187–196 (2006).
Magiakou, M. A. et al. The maternal hypothalamic-pituitary-adrenal axis in the third trimester of human pregnancy. Clin. Endocrinol. 44, 419–428 (1996).
Torner, L. et al. In vivo release and gene upregulation of brain prolactin in response to physiological stimuli. Eur. J. Neurosci. 19, 1601–1608 (2004).
Bloch, M. et al. Effects of gonadal steroids in women with a history of postpartum depression. Am. J. Psychiatry 157, 924–930 (2000). This is an elegant hypothesis-driven study in which the authors show that women who experienced postpartum depression were more vulnerable to developing low mood after experimental progesterone and estrogen withdrawal.
Pedersen, C. A. et al. Antenatal thyroid correlates of postpartum depression. Psychoneuroendocrinology 32, 235–245 (2007).
Vasudevan, N., Ogawa, S. & Pfaff, D. Estrogen and thyroid hormone receptor interactions: physiological flexibility by molecular specificity. Physiol. Rev. 82, 923–944 (2002).
Smith, J. W., Seckl, J. R., Evans, A. T., Costall, B. & Smythe, J. W. Gestational stress induces post-partum depression-like behaviour and alters maternal care in rats. Psychoneuroendocrinology 29, 227–244 (2004).
Brummelte, S., Pawluski, J. L. & Galea, L. A. High post-partum levels of corticosterone given to dams influence postnatal hippocampal cell proliferation and behavior of offspring: a model of post-partum stress and possible depression. Horm. Behav. 50, 370–382 (2006).
Boccia, M. L. et al. Repeated long separations from pups produce depression-like behavior in rat mothers. Psychoneuroendocrinology 32, 65–71 (2007).
Eser, D. et al. Neuroactive steroids and affective disorders. Pharmacol. Biochem. Behav. 84, 656–666 (2006).
Majewska, M. D., Ford-Rice, F. & Falkay, G. Pregnancy-induced alterations of GABAA receptor sensitivity in maternal brain: an antecedent of post-partum 'blues'? Brain Res. 482, 397–401 (1989).
Frye, C. A. & Walf, A. A. Hippocampal 3α,5α-THP may alter depressive behavior of pregnant and lactating rats. Pharmacol. Biochem. Behav. 78, 531–540 (2004).
Nappi, R. E. Serum allopregnanolone in women with postpartum “blues”. Obstet. Gynecol. 97, 77–80 (2001).
Pearson Murphy, B. E., Steinberg, S. I., Hu, F. Y. & Allison, C. M. Neuroactive ring A-reduced metabolites of progesterone in human plasma during pregnancy: elevated levels of 5α-dihydroprogesterone in depressed patients during the latter half of pregnancy. J. Clin. Endocrinol. Metab. 86, 5981–5987 (2001).
Epperson, C. N. et al. Preliminary evidence of reduced occipital GABA concentrations in puerperal women: a 1H-MRS study. Psychopharmacology (Berl.) 186, 425–433 (2006).
Altemus, M. et al. Changes in cerebrospinal fluid neurochemistry during pregnancy. Biol. Psychiatry 56, 386–392 (2004).
Smith, S. S. et al. Withdrawal from 3α-OH-5α-pregnan-20-one using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor α4 subunit in association with increased anxiety. J. Neurosci. 18, 5275–5284 (1998).
M'Baïlara, K. et al. Decreased brain tryptophan availability as a partial determinant of post-partum blues. Psychoneuroendocrinology 31, 407–413 (2006).
Maes, M., Ombelet, W., Verkerk, R., Bosmans, E. & Scharpe, S. Effects of pregnancy and delivery on the availability of plasma tryptophan to the brain: relationships to delivery-induced immune activation and early post-partum anxiety and depression. Psychol. Med. 31, 847–858 (2001).
Coyle, N., Jones, I., Robertson, E., Lendon, C. & Craddock, N. Variation at the serotonin transporter gene influences susceptibility to bipolar affective puerperal psychosis. Lancet 356, 1490–1491 (2000).
Whitby, D. H. & Smith, K. M. The use of tricyclic antidepressants and selective serotonin reuptake inhibitors in women who are breastfeeding. Pharmacotherapy 25, 411–425 (2005).
Matsumoto, K., Puia, G., Dong, E. & Pinna, G. GABAA receptor neurotransmission dysfunction in a mouse model of social isolation-induced stress: possible insights into a non-serotonergic mechanism of action of SSRIs in mood and anxiety disorders. Stress 10, 3–12 (2007).
van Broekhoven, F. & Verkes, R. J. Neurosteroids in depression: a review. Psychopharmacology (Berl.) 165, 97–110 (2003).
Moses-Kolko, E. L. et al. Serotonin 1A receptor reductions in postpartum depression: a positron emission tomography study. Fertil. Steril. 80, 554–549 (2007).
de Weerth, C. & Buitelaar, J. K. Physiological stress reactivity in human pregnancy: a review. Neurosci. Biobehav. Rev. 29, 295–312 (2005).
Kammerer, M., Adams, D., Castelberg, B. v. & Glover, V. Pregnant women become insensitive to cold stress. BMC Pregnancy Childbirth 2, 8 (2002).
Nierop, A. et al. Prolonged salivary cortisol recovery in second-trimester pregnant women and attenuated salivary α-amylase responses to psychosocial stress in human pregnancy. J. Clin. Endocrinol. Metab. 91, 1329–1335 (2006).
Nierop, A., Bratsikas, A., Zimmermann, R. & Ehlert, U. Are stress-induced cortisol changes during pregnancy associated with postpartum depressive symptoms? Psychosom. Med. 68, 931–937 (2006).
Magiakou, M. A. et al. Hypothalamic corticotropin-releasing hormone suppression during the postpartum period: implications for the increase in psychiatric manifestations at this time. J. Clin. Endocrinol. Metab. 81, 1912–1917 (1996).
Ma, S., Shipston, M. J., Morilak, D. & Russell, J. A. Reduced hypothalamic vasopressin secretion underlies attenuated adrenocorticotropin stress responses in pregnant rats. Endocrinology 146, 1626–1637 (2005).
da Costa, A. P., Ma, X., Ingram, C. D., Lightman, S. L. & Aguilera, G. Hypothalamic and amygdaloid corticotropin-releasing hormone (CRH) and CRH receptor-1 mRNA expression in the stress-hyporesponsive late pregnant and early lactating rat. Brain Res. Mol. Brain Res. 91, 119–130 (2001).
Heuser, I., Yassouridis, A. & Holsboer, F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J. Psychiatr. Res. 28, 341–356 (1994).
Keck, M. E. et al. Vasopressin mediates the response of the combined dexamethasone/CRH test in hyper-anxious rats: implications for pathogenesis of affective disorders. Neuropsychopharmacology 26, 94–105 (2002).
Herman, J. P. & Cullinan, W. E. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 20, 78–84 (1997). This is a detailed neuroanatomical study that dissects the pathways that are involved in processing different types of stressor and the activation of the HPA axis.
Brunton, P. J., Bales, J. & Russell, J. A. Neuroendocrine stress but not feeding responses to centrally administered neuropeptide Y are suppressed in pregnant rats. Endocrinology 147, 3737–3745 (2006).
Bates, S. H. et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421, 856–859 (2003).
Ladyman, S. R. & Grattan, D. R. Suppression of leptin receptor messenger ribonucleic acid and leptin responsiveness in the ventromedial nucleus of the hypothalamus during pregnancy in the rat. Endocrinology 146, 3868–3874 (2005). Addressing the problem of the causes of leptin resistance in pregnancy, this paper provides evidence for changes in leptin signalling in the ventromedial nucleus (the satiety centre).
Seeber, R. M., Smith, J. T. & Waddell, B. J. Plasma leptin-binding activity and hypothalamic leptin receptor expression during pregnancy and lactation in the rat. Biol. Reprod. 66, 1762–1767 (2002).
Johnstone, L. E. & Higuchi, T. Food intake and leptin during pregnancy and lactation. Prog. Brain Res. 133, 215–227 (2001).
Mistry, A. M. & Romsos, D. R. Intracerebroventricular leptin administration reduces food intake in pregnant and lactating mice. Exp. Biol. Med. 227, 616–619 (2002).
Ladyman, S. R. & Grattan, D. R. Region-specific reduction in leptin-induced phosphorylation of signal transducer and activator of transcription-3 (STAT3) in the rat hypothalamus is associated with leptin resistance during pregnancy. Endocrinology 145, 3704–3711 (2004).
Naef, L. & Woodside, B. Prolactin/leptin interactions in the control of food intake in rats. Endocrinology 148, 5977–5983 (2007). Pursuant to reference 168, this study provides evidence that lactogens are responsible for inducing leptin resistance in pregnancy.
Grueso, E., Rocha, M. & Puerta, M. Plasma and cerebrospinal fluid leptin levels are maintained despite enhanced food intake in progesterone-treated rats. Eur. J. Endocrinol. 144, 659–665 (2001).
Garcia, M. C. et al. Hypothalamic levels of NPY, MCH, and prepro-orexin mRNA during pregnancy and lactation in the rat: role of prolactin. FASEB J. 17, 1392–1400 (2003).
Rocha, M., Bing, C., Williams, G. & Puerta, M. Pregnancy-induced hyperphagia is associated with increased gene expression of hypothalamic agouti-related peptide in rats. Regul. Pept. 114, 159–165 (2003).
Oberto, A., Mele, P., Zammaretti, F., Panzica, G. & Eva, C. Evidence of altered neuropeptide Y content and neuropeptide Y1 receptor gene expression in the hypothalamus of pregnant transgenic mice. Endocrinology 144, 4826–4830 (2003).
Chen, S. W., Rodriguez, L., Davies, M. F. & Loew, G. H. The hyperphagic effect of 3α-hydroxylated pregnane steroids in male rats. Pharmacol. Biochem. Behav. 53, 777–782 (1996).
Sahu, A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front. Neuroendocrinol. 24, 225–253 (2003).
Blevins, J. E., Schwartz, M. W. & Baskin, D. G. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R87–R96 (2004).
Douglas, A. J., Johnstone, L. E. & Leng, G. Neuroendocrine mechanisms of change in food intake during pregnancy: a potential role for brain oxytocin. Physiol. Behav. 91, 352–365 (2007).
Brunton, P. J. & Russell, J. A. Hypothalamic-pituitary-adrenal responses to centrally administered orexin-A are suppressed in pregnant rats. J. Neuroendocrinol. 15, 633–637 (2003).
Acknowledgements
This work was supported by grants from the Biotechnology and Biological Sciences Research Council (BBSRC) UK.
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- Physical stressor
-
A category of stressor (including infection and injury) that poses a real threat to homeostasis or survival and automatically activates the HPA axis.
- Cholecystokinin
-
(CCK). A peptide hormone from the gastrointestinal tract that stimulates digestion and signals satiety to the brain.
- Interleukin-1β
-
(IL1β). A pro-inflammatory cytokine that is produced by macrophage cells in response to infection.
- Endogenous opioids
-
Peptides, including dynorphins, endomorphins, endorphins and enkephalins, that are produced naturally in the body and, like morphine, act through opioid receptors.
- Neuroactive steroid
-
A steroid derivative that rapidly alters neuronal excitability through interaction with neurotransmitter-gated ion channels.
- Allosteric modulator
-
A molecule that binds to a receptor at a regulatory site that is distinct from the active ligand-binding site to influence the receptor's function.
- Endocannabinoid
-
A signalling molecule that is structurally related to tetrahydrocannabinol (the main active substance found in cannabis). Endocannabinoids released by neurons act through cannabinoid receptors to modulate synaptic input.
- Lactogen
-
A peptide hormone that stimulates milk production (for example, prolactin and placental lactogen).
- Mitral cell
-
A type of neuron that is located in the olfactory system and that processes and transmits information from primary olfactory sensory neurons to other brain regions.
- Transcriptome
-
The set of all mRNAs produced in a cell or a population of cells.
Rights and permissions
About this article
Cite this article
Brunton, P., Russell, J. The expectant brain: adapting for motherhood. Nat Rev Neurosci 9, 11–25 (2008). https://doi.org/10.1038/nrn2280
Issue Date:
DOI: https://doi.org/10.1038/nrn2280
This article is cited by
-
Do Parental Hormone Levels Synchronize During the Prenatal and Postpartum Periods? A Systematic Review
Clinical Child and Family Psychology Review (2024)
-
Adolescent stress impairs postpartum social behavior via anterior insula-prelimbic pathway in mice
Nature Communications (2023)
-
Microglia depletion facilitates the display of maternal behavior and alters activation of the maternal brain network in nulliparous female rats
Neuropsychopharmacology (2023)
-
The case of poor postpartum mental health: a consequence of an evolutionary mismatch – not of an evolutionary trade-off
Biology & Philosophy (2023)
-
Interaction of the pre- and postnatal environment in the maternal immune activation model
Discover Mental Health (2023)