Key Points
-
Deep brain stimulation (DBS) has shown remarkable therapeutic benefits for patients with otherwise treatment-resistant movement and affective disorders, such as chronic pain, Parkinson's disease, tremor and dystonia. Yet the precise mechanisms of action for DBS remain uncertain. Here we give an up-to-date overview of the principles of DBS, its neural mechanisms and its potential future applications.
-
DBS directly changes brain activity in a controlled manner and, unlike those of lesioning techniques, its effects are reversible. Furthermore, DBS is the only neurosurgical technique that allows blinded studies. New targets for DBS have been discovered through the use of translational research, and in particular through highly successful rodent and primate models of movement disorders.
-
DBS of both the normal and the diseased brain fundamentally depends on a number of parameters, including the physiological properties of the brain tissue, which may change with disease state, the stimulation parameters, including amplitude and temporal characteristics, and the geometric configuration of the electrode and the surrounding tissue.
-
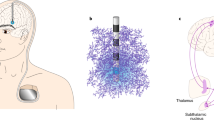
In vivo neurophysiological recordings of activity from a deep brain electrode in one brain region, made while stimulating another brain region, have shown that DBS modulates the pathological oscillatory activity between brain regions.
-
Results from animal experiments have also shown that DBS appears to elicit neurotransmitter release in downstream brain structures, although there is conflicting evidence from human studies.
-
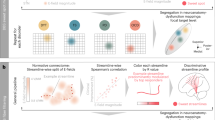
Functional neuroimaging methods can be used to elucidate the whole-brain responses elicited by DBS, and on the whole they have confirmed the findings from recordings in other animals. However, methods such as functional MRI should be used with extreme caution, as they entail significant risks to the patient. Owing to its non-invasive nature and high spatial and temporal resolution, magnetoencepholography (MEG) is currently one of the most promising techniques for elucidating the neural mechanisms affected by DBS.
-
On balance, the evidence so far suggests that the most likely mode of action for DBS is through stimulation-induced modulation of brain activity, where the modulation comes about through the local effects of the DBS electrode on the neural activity in the DBS target, which is passed on to mono- and poly-synaptic network connections.
-
Future applications of DBS include finding more effective brain targets and delivering individualized closed-loop demand-driven stimulation. It will also be important to develop robust translational models of affective disorders. Overall, combining DBS with whole-brain neuroimaging methods like MEG has the makings of a powerful and sophisticated tool for unravelling the fundamental mechanisms of normal and abnormal human brain function.
Abstract
Deep brain stimulation (DBS) has shown remarkable therapeutic benefits for patients with otherwise treatment-resistant movement and affective disorders. This technique is not only clinically useful, but it can also provide new insights into fundamental brain functions through direct manipulation of both local and distributed brain networks in many different species. In particular, DBS can be used in conjunction with non-invasive neuroimaging methods such as magnetoencephalography to map the fundamental mechanisms of normal and abnormal oscillatory synchronization that underlie human brain function. The precise mechanisms of action for DBS remain uncertain, but here we give an up-to-date overview of the principles of DBS, its neural mechanisms and its potential future applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fritsch, G. & Hitzig, E. Über die elektrische Erregbarkeit des Grosshirns. Arch. Anat. Physiol. 37, 300–332 (1870).
Gildenberg, P. L. Evolution of neuromodulation. Stereotact. Funct. Neurosurg. 83, 71–79 (2005). A good historical overview of neuromodulation.
Owen, S. L., Green, A. L., Stein, J. F. & Aziz, T. Z. Deep brain stimulation for the alleviation of post-stroke neuropathic pain. Pain 120, 202–206 (2006).
Marchand, S., Kupers, R. C., Bushnell, M. C. & Duncan, G. H. Analgesic and placebo effects of thalamic stimulation. Pain 105, 481–488 (2003).
Bittar, R. G. et al. Deep brain stimulation for movement disorders and pain. J. Clin. Neurosci. 12, 457–463 (2005).
Krack, P. et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N. Engl. J. Med. 349, 1925–1934 (2003).
Koller, W. C., Lyons, K. E., Wilkinson, S. B. & Pahwa, R. Efficacy of unilateral deep brain stimulation of the VIM nucleus of the thalamus for essential head tremor. Mov. Disord. 14, 847–850 (1999).
Rehncrona, S. et al. Long-term efficacy of thalamic deep brain stimulation for tremor: double-blind assessments. Mov. Disord. 18, 163–170 (2003).
Vidailhet, M. et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N. Engl. J. Med. 352, 459–467 (2005).
Ranck, J. B. Jr. Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 98, 417–440 (1975). Excellent review of the underlying principles of electrical stimulation of neural tissue.
McIntyre, C. C., Mori, S., Sherman, D. L., Thakor, N. V. & Vitek, J. L. Electric field and stimulating influence generated by deep brain stimulation of the subthalamic nucleus. Clin. Neurophysiol. 115, 589–595 (2004).
Holsheimer, J., Demeulemeester, H., Nuttin, B. & de Sutter, P. Identification of the target neuronal elements in electrical deep brain stimulation. Eur. J. Neurosci. 12, 4573–4577 (2000).
Nowak, L. G. & Bullier, J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. II. Evidence from selective inactivation of cell bodies and axon initial segments. Exp. Brain Res. 118, 489–500 (1998).
Volkmann, J., Herzog, J., Kopper, F. & Deuschl, G. Introduction to the programming of deep brain stimulators. Mov. Disord. 17, S181–S187 (2002).
McIntyre, C. C., Savasta, M., Kerkerian-Le Goff, L. & Vitek, J. L. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin. Neurophysiol. 115, 1239–1248 (2004). Review of the potential neural mechanisms for DBS, with evidence from neurophysiology and computer modelling.
Hashimoto, T., Elder, C. M., Okun, M. S., Patrick, S. K. & Vitek, J. L. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J. Neurosci. 23, 1916–1923 (2003).
Anderson, M. E., Postupna, N. & Ruffo, M. Effects of high-frequency stimulation in the internal globus pallidus on the activity of thalamic neurons in the awake monkey. J. Neurophysiol. 89, 1150–1160 (2003).
Dostrovsky, J. O. et al. Microstimulation-induced inhibition of neuronal firing in human globus pallidus. J. Neurophysiol. 84, 570–574 (2000).
Pralong, E. et al. Effect of deep brain stimulation of GPI on neuronal activity of the thalamic nucleus ventralis oralis in a dystonic patient. Neurophysiol. Clin. 33, 169–173 (2003).
Brown, P. et al. Effects of stimulation of the subthalamic area on oscillatory pallidal activity in Parkinson's disease. Exp. Neurol. 188, 480–490 (2004). Provided an important demonstration that DBS can modulate pathological oscillatory activity between the cortex and the basal ganglia.
Windels, F. et al. Effects of high frequency stimulation of subthalamic nucleus on extracellular glutamate and GABA in substantia nigra and globus pallidus in the normal rat. Eur. J. Neurosci. 12, 4141–4146 (2000).
Boulet, S. et al. Subthalamic stimulation-induced forelimb dyskinesias are linked to an increase in glutamate levels in the substantia nigra pars reticulata. J. Neurosci. 26, 10768–10776 (2006).
Perlmutter, J. S. et al. Blood flow responses to deep brain stimulation of thalamus. Neurology 58, 1388–1394 (2002).
Hershey, T. et al. Cortical and subcortical blood flow effects of subthalamic nucleus stimulation in PD. Neurology 61, 816–821 (2003).
Kringelbach, M. L. et al. Deep brain stimulation for chronic pain investigated with magnetoencephalography. Neuroreport 18, 223–228 (2007).
Kringelbach, M. L. et al. Deep brain stimulation and chronic pain mapped with MEG. Soc. Neurosci. Abstr. 782.1 (2006). Provided the first demonstration of the feasibility of using MEG to map the whole-brain effects of DBS.
Perlmutter, J. S. & Mink, J. W. Deep brain stimulation. Annu. Rev. Neurosci. 29, 229–257 (2006).
Beal, M. F. Experimental models of Parkinson's disease. Nature Rev. Neurosci. 2, 325–334 (2001).
Benazzouz, A. et al. Effect of high-frequency stimulation of the subthalamic nucleus on the neuronal activities of the substantia nigra pars reticulata and ventrolateral nucleus of the thalamus in the rat. Neuroscience 99, 289–295 (2000).
Maurice, N., Thierry, A. M., Glowinski, J. & Deniau, J. M. Spontaneous and evoked activity of substantia nigra pars reticulata neurons during high-frequency stimulation of the subthalamic nucleus. J. Neurosci. 23, 9929–9936 (2003).
Degos, B. et al. Neuroleptic-induced catalepsy: electrophysiological mechanisms of functional recovery induced by high-frequency stimulation of the subthalamic nucleus. J. Neurosci. 25, 7687–7696 (2005).
Kita, H., Tachibana, Y., Nambu, A. & Chiken, S. Balance of monosynaptic excitatory and disynaptic inhibitory responses of the globus pallidus induced after stimulation of the subthalamic nucleus in the monkey. J. Neurosci. 25, 8611–8619 (2005).
Filali, M., Hutchison, W. D., Palter, V. N., Lozano, A. M. & Dostrovsky, J. O. Stimulation-induced inhibition of neuronal firing in human subthalamic nucleus. Exp. Brain Res. 156, 274–281 (2004).
Brown, P. et al. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease. J. Neurosci. 21, 1033–1038 (2001).
Weinberger, M. et al. Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson's disease. J. Neurophysiol. 96, 3248–3256 (2006).
Windels, F. et al. Influence of the frequency parameter on extracellular glutamate and γ-aminobutyric acid in substantia nigra and globus pallidus during electrical stimulation of subthalamic nucleus in rats. J. Neurosci. Res. 72, 259–267 (2003).
Windels, F., Carcenac, C., Poupard, A. & Savasta, M. Pallidal origin of GABA release within the substantia nigra pars reticulata during high-frequency stimulation of the subthalamic nucleus. J. Neurosci. 25, 5079–5086 (2005).
Stefani, A. et al. Subthalamic stimulation activates internal pallidus: evidence from cGMP microdialysis in PD patients. Ann. Neurol. 57, 448–452 (2005).
Hilker, R. et al. Deep brain stimulation of the subthalamic nucleus does not increase the striatal dopamine concentration in parkinsonian humans. Mov. Disord. 18, 41–48 (2003).
Meissner, W. et al. Deep brain stimulation of subthalamic neurons increases striatal dopamine metabolism and induces contralateral circling in freely moving 6-hydroxydopamine-lesioned rats. Neurosci. Lett. 328, 105–108 (2002).
Lauritzen, M. Reading vascular changes in brain imaging: is dendritic calcium the key? Nature Rev. Neurosci. 6, 77–85 (2005).
Logothetis, N. K. & Wandell, B. A. Interpreting the BOLD signal. Annu. Rev. Physiol. 66, 735–769 (2004).
Rezai, A. R. et al. Thalamic stimulation and functional magnetic resonance imaging: localization of cortical and subcortical activation with implanted electrodes. Technical note. J. Neurosurg. 90, 583–590 (1999).
Uitti, R. J. et al. Magnetic resonance imaging and deep brain stimulation. Neurosurgery 51, 1423–1428 (2002).
Sakatani, K., Katayama, Y., Yamamoto, T. & Suzuki, S. Changes in cerebral blood oxygenation of the frontal lobe induced by direct electrical stimulation of thalamus and globus pallidus: a near infrared spectroscopy study. J. Neurol. Neurosurg. Psychiatr. 67, 769–773 (1999).
Georgi, J. C., Stippich, C., Tronnier, V. M. & Heiland, S. Active deep brain stimulation during MRI: a feasibility study. Magn. Reson. Med. 51, 380–388 (2004).
Stefurak, T. et al. Deep brain stimulation for Parkinson's disease dissociates mood and motor circuits: a functional MRI case study. Mov. Disord. 18, 1508–1516 (2003).
Hilker, R. et al. Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: evidence from a FDG–PET study in advanced Parkinson's disease. J. Cereb. Blood Flow Metab. 24, 7–16 (2004).
Fukuda, M. et al. Networks mediating the clinical effects of pallidal brain stimulation for Parkinson's disease: a PET study of resting-state glucose metabolism. Brain 124, 1601–1609 (2001).
May, A. et al. Hypothalamic deep brain stimulation in positron emission tomography. J. Neurosci. 26, 3589–3593 (2006).
Mayberg, H. S. et al. Deep brain stimulation for treatment-resistant depression. Neuron 45, 651–660 (2005).
Hämäläinen, M., Hari, R., Ilmoniemi, R. J. & Knuutila, J. Magnetoencephalography — theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev. Mod. Phys. 65, 1–93 (1993).
Van Veen, B. D., van Drongelen, W., Yuchtman, M. & Suzuki, A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng. 44, 867–880 (1997).
Hillebrand, A. & Barnes, G. R. A quantitative assessment of the sensitivity of whole-head MEG to activity in the adult human cortex. Neuroimage 16, 638–650 (2002).
Ioannides, A. A. et al. MEG tomography of human cortex and brainstem activity in waking and REM sleep saccades. Cereb. Cortex 14, 56–72 (2004).
Kringelbach, M. L. The orbitofrontal cortex: linking reward to hedonic experience. Nature Rev. Neurosci. 6, 691–702 (2005).
Ray, N. J. et al. Using magnetoencephalography to investigate deep brain stimulation for cluster headache. Biomed. Imaging Interv. J. 4 Mar 2007 (doi:10.2349/biij.3.1.e25).
Schnitzler, A. & Gross, J. Normal and pathological oscillatory communication in the brain. Nature Rev. Neurosci. 6, 285–296 (2005).
Vitek, J. L. Mechanisms of deep brain stimulation: excitation or inhibition. Mov. Disord. 17, S69–S72 (2002).
Beurrier, C., Bioulac, B., Audin, J. & Hammond, C. High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J. Neurophysiol. 85, 1351–1356 (2001).
McIntyre, C. C., Grill, W. M., Sherman, D. L. & Thakor, N. V. Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J. Neurophysiol. 91, 1457–1469 (2004).
Urbano, F. J., Leznik, E. & Llinas, R. R. Cortical activation patterns evoked by afferent axons stimuli at different frequencies: an in vitro voltage sensitive dye imaging study. Thalamus Relat. Syst. 1, 371–378 (2002).
Montgomery, E. B. Jr & Baker, K. B. Mechanisms of deep brain stimulation and future technical developments. Neurol. Res. 22, 259–266 (2000).
Burns, R. S. et al. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc. Natl Acad. Sci. USA 80, 4546–4550 (1983). Provided the first demonstration of the highly sucessful MPTP animal model of Parkinson's disease.
Langston, J. W., Ballard, P., Tetrud, J. W. & Irwin, I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219, 979–980 (1983).
Bergman, H., Wichmann, T. & DeLong, M. R. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science 249, 1436–1438 (1990).
Aziz, T. Z., Peggs, D., Sambrook, M. A. & Crossman, A. R. Lesion of the subthalamic nucleus for the alleviation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism in the primate. Mov. Disord 6, 288–292 (1991). Together with reference 66, this classic study showed that lesions of the STN alleviate the symptoms of parkinsonian MPTP-treated monkeys.
Limousin, P. et al. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet 345, 91–95 (1995). One of the first studies to demonstrate that DBS of the STN can help human patients.
Voon, V., Moro, E., Saint-Cyr, J. A., Lozano, A. M. & Lang, A. E. Psychiatric symptoms following surgery for Parkinson's disease with an emphasis on subthalamic stimulation. Adv. Neurol. 96, 130–147 (2005).
Pahwa, R., Wilkinson, S. B., Overman, J. & Lyons, K. E. Preoperative clinical predictors of response to bilateral subthalamic stimulation in patients with Parkinson's disease. Stereotact. Funct. Neurosurg. 83, 80–83 (2005).
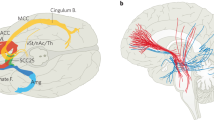
Matsumura, M. The pedunculopontine tegmental nucleus and experimental parkinsonism. A review. J. Neurol. 252, IV5–IV12 (2005).
Garcia-Rill, E., Houser, C. R., Skinner, R. D., Smith, W. & Woodward, D. J. Locomotion-inducing sites in the vicinity of the pedunculopontine nucleus. Brain Res. Bull. 18, 731–738 (1987).
Mogenson, G. J. & Wu, M. Differential effects on locomotor activity of injections of procaine into mediodorsal thalamus and pedunculopontine nucleus. Brain Res. Bull. 20, 241–246 (1988).
Brudzynski, S. M., Houghton, P. E., Brownlee, R. D. & Mogenson, G. J. Involvement of neuronal cell bodies of the mesencephalic locomotor region in the initiation of locomotor activity of freely behaving rats. Brain Res. Bull. 16, 377–381 (1986).
Crossman, A. R., Mitchell, I. J. & Sambrook, M. A. Regional brain uptake of 2-deoxyglucose in N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism in the macaque monkey. Neuropharmacology 24, 587–591 (1985).
Munro-Davies, L., Winter, J., Aziz, T. Z. & Stein, J. Kainate acid lesions of the pedunculopontine region in the normal behaving primate. Mov. Disord. 16, 150–151 (2001).
Aziz, T. Z., Davies, L., Stein, J. & France, S. The role of descending basal ganglia connections to the brain stem in parkinsonian akinesia. Br. J. Neurosurg. 12, 245–249 (1998).
Kojima, J. et al. Excitotoxic lesions of the pedunculopontine tegmental nucleus produce contralateral hemiparkinsonism in the monkey. Neurosci. Lett. 226, 111–114 (1997).
Nandi, D., Aziz, T. Z., Giladi, N., Winter, J. & Stein, J. F. Reversal of akinesia in experimental parkinsonism by GABA antagonist microinjections in the pedunculopontine nucleus. Brain 125, 2418–2430 (2002). Exciting study that shows that microinjection of bicuculline into the PPN of parkinsonian MPTP-treated primates results in significant improvement of akinesia.
Nandi, D., Liu, X., Winter, J. L., Aziz, T. Z. & Stein, J. F. Deep brain stimulation of the pedunculopontine region in the normal non-human primate. J. Clin. Neurosci. 9, 170–174 (2002).
Jenkinson, N., Nandi, D., Miall, R. C., Stein, J. F. & Aziz, T. Z. Pedunculopontine nucleus stimulation improves akinesia in a Parkinsonian monkey. Neuroreport 15, 2621–2624 (2004). First study to demonstrate that DBS in the PPN of parkinsonian MPTP-treated monkeys can alleviate akinesia.
Mazzone, P. et al. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson's disease. Neuroreport 16, 1877–1881 (2005). One of the first studies to show that low frequency DBS in the human PPN helps with the symptoms of Parkinson's disease.
Plaha, P. & Gill, S. G. Bilateral deep brain stimulation of the pedunculopontine nucleus for idiopathic Parkinson's disease. Neuroreport 16, 1883–1887 (2005). One of the first studies to show that DBS in the human PPN helps with gait disturbance and postural instability in Parkinson's disease.
Stefani, A. et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson's disease. Brain 130, 1596–1607 (2007).
Berridge, K. C. Food reward: brain substrates of wanting and liking. Neurosci. Biobehav. Rev. 20, 1–25 (1996).
Heath, R. G. Psychiatry. Annu. Rev. Med. 5, 223–236 (1954).
Olds, J. & Milner, P. Positive reinforcement produced by electrical stimulation of the septal area and other regions of rat brain. J. Comp. Physiol. Psychol. 47, 419–427 (1954).
Cardinal, R. N., Parkinson, J. A., Hall, J. & Everitt, B. J. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 26, 321–352 (2002).
Robbins, T. W. & Everitt, B. J. Limbic–striatal memory systems and drug addiction. Neurobiol. Learn. Mem. 78, 625–636 (2002).
Peciña, S., Smith, K. S. & Berridge, K. C. Hedonic hot spots in the brain. Neuroscientist 12, 500–511 (2006).
Schlaepfer, T. E. et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 11 Apr 2007 (doi: 10.1038/sj.npp.1301408). Provided an exciting demonstration that DBS of the nucleus accumbens alleviates anhedonia in treatment-resistant depression.
Krack, P. et al. Mirthful laughter induced by subthalamic nucleus stimulation. Mov. Disord. 16, 867–875 (2001).
Bejjani, B. P. et al. Transient acute depression induced by high-frequency deep-brain stimulation. N. Engl. J. Med. 340, 1476–1480 (1999).
Temel, Y. et al. Deep brain stimulation of the thalamus can influence penile erection. Int. J. Impot. Res. 16, 91–94 (2004).
Cummings, J. L. Depression and Parkinson's disease: a review. Am. J. Psychiatry 149, 443–454 (1992).
Steckler, T., Inglis, W., Winn, P. & Sahgal, A. The pedunculopontine tegmental nucleus: a role in cognitive processes? Brain Res. Brain Res. Rev. 19, 298–318 (1994).
Tass, P. A. et al. Obsessive–compulsive disorder: development of demand-controlled deep brain stimulation with methods from stochastic phase resetting. Neuropsychopharmacology 28, S27–S34 (2003).
Tsubokawa, T. et al. Deep-brain stimulation in a persistent vegetative state: follow-up results and criteria for selection of candidates. Brain Inj. 4, 315–327 (1990).
Fahn, S. & Elton, R. L. in Recent Developments in Parkinson's Disease. Vol. 2 (eds Fahn, S., Marsden, C. D., Goldstein, M. & Calne, D. B.) 153 (Macmillan Publishers Ltd, New York, 1987).
Siegfried, J. & Lippitz, B. Bilateral chronic electrostimulation of ventroposterolateral pallidum: a new therapeutic approach for alleviating all parkinsonian symptoms. Neurosurgery 35, 1126–1129 (1994).
Benabid, A. L. et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J. Neurosurg. 84, 203–214 (1996).
Jenkinson, N., Nandi, D., Aziz, T. Z. & Stein, J. F. Pedunculopontine nucleus: a new target for deep brain stimulation for akinesia. Neuroreport 16, 1875–1876 (2005).
Jenkinson, N., Nandi, D., Oram, R., Stein, J. F. & Aziz, T. Z. Pedunculopontine nucleus electric stimulation alleviates akinesia independently of dopaminergic mechanisms. Neuroreport 17, 639–641 (2006).
Kumar, R., Dagher, A., Hutchison, W. D., Lang, A. E. & Lozano, A. M. Globus pallidus deep brain stimulation for generalized dystonia: clinical and PET investigation. Neurology 53, 871–874 (1999).
Bittar, R. G. et al. Deep brain stimulation for generalised dystonia and spasmodic torticollis. J. Clin. Neurosci. 12, 12–16 (2005).
Krauss, J. K., Yianni, J., Loher, T. J. & Aziz, T. Z. Deep brain stimulation for dystonia. J. Clin. Neurophysiol. 21, 18–30 (2004).
Hassler, R. The influence of stimulations and coagulations in the human thalamus on the tremor at rest and its physiopathologic mechanism. Proc. Sec. Int. Cong. Neuropathol. 2, 637–642 (1955).
Lenz, F. A. et al. Single unit analysis of the human ventral thalamic nuclear group. Tremor-related activity in functionally identified cells. Brain 117, 531–543 (1994).
Krack, P., Pollak, P., Limousin, P., Benazzouz, A. & Benabid, A. L. Stimulation of subthalamic nucleus alleviates tremor in Parkinson's disease. Lancet 350, 1675 (1997).
Schuurman, P. R. et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N. Engl. J. Med. 342, 461–468 (2000).
Sydow, O., Thobois, S., Alesch, F. & Speelman, J. D. Multicentre European study of thalamic stimulation in essential tremor: a six year follow up. J. Neurol. Neurosurg. Psychiatr. 74, 1387–1391 (2003).
Temel, Y., Blokland, A., Steinbusch, H. W. & Visser-Vandewalle, V. The functional role of the subthalamic nucleus in cognitive and limbic circuits. Prog. Neurobiol. 76, 393–413 (2005). An important review of the role of the STN not only in movement but also in affective processes.
Pool, J. L., Clark, W. D., Hudson, P. & Lombardo, M. in Hypothalamic–Hypophysial Interrelationships (eds Fields, W. S., Guillemin, R. & Carton, C. A.) 114–124 (Charles C. Thomas Ltd, Illinois USA, 1956).
Mazars, G., Roge, R. & Mazars, Y. Results of the stimulation of the spinothalamic fasciculus and their bearing on the physiopathology of pain. Rev. Prat. 103, 136–138 (1960).
Hosobuchi, Y., Adams, J. E. & Rutkin, B. Chronic thalamic stimulation for the control of facial anesthesia dolorosa. Arch. Neurol. 29, 158–161 (1973). First successful use of chronic stimulation of the somatosensory thalamus for the treatment of denervation facial pain, anesthesia dolorosa.
Mazars, G., Merienne, L. & Ciolocca, C. Intermittent analgesic thalamic stimulation. Preliminary note. Rev. Neurol. (Paris) 128, 273–279 (1973).
Hosobuchi, Y., Adams, J. E. & Linchitz, R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science 197, 183–186 (1977).
Richardson, D. E. & Akil, H. Long term results of periventricular gray self-stimulation. Neurosurgery 1, 199–202 (1977).
Richardson, D. E. & Akil, H. Pain reduction by electrical brain stimulation in man. Part 1: acute administration in periaqueductal and periventricular sites. J. Neurosurg. 47, 178–183 (1977).
Richardson, D. E. & Akil, H. Pain reduction by electrical brain stimulation in man. Part 2: chronic self-administration in the periventricular gray matter. J. Neurosurg. 47, 184–194 (1977).
Coffey, R. J. Deep brain stimulation for chronic pain: results of two multicenter trials and a structured review. Pain Med. 2, 183–192 (2001).
Hamani, C. et al. Deep brain stimulation for chronic neuropathic pain: long-term outcome and the incidence of insertional effect. Pain 125, 188–196 (2006).
Krauss, J. K., Pohle, T., Weigel, R. & Burgunder, J. M. Deep brain stimulation of the centre median-parafascicular complex in patients with movement disorders. J. Neurol. Neurosurg. Psychiatr. 72, 546–548 (2002).
Tronnier, V. M. in Deep Brain Stimulation (ed. Simpson, B. A.) 211–236 (Elsevier, Amsterdam, 2003).
Nandi, D., Aziz, T., Carter, H. & Stein, J. Thalamic field potentials in chronic central pain treated by periventricular gray stimulation — a series of eight cases. Pain 101, 97–107 (2003).
Owen, S. L. F. et al. Deep brain stimulation for neuropathic pain. Neuromodulation 9, 100–106 (2006).
Green, A. L., Owen, S. L., Davies, P., Moir, L. & Aziz, T. Z. Deep brain stimulation for neuropathic cephalalgia. Cephalalgia 26, 561–567 (2006).
Bittar, R. G., Otero, S., Carter, H. & Aziz, T. Z. Deep brain stimulation for phantom limb pain. J. Clin. Neurosci. 12, 399–404 (2005).
Franzini, A., Ferroli, P., Leone, M. & Broggi, G. Stimulation of the posterior hypothalamus for treatment of chronic intractable cluster headaches: first reported series. Neurosurgery 52, 1095–1099; discussion 1099–101 (2003).
Leone, M., Franzini, A., Broggi, G., May, A. & Bussone, G. Long-term follow-up of bilateral hypothalamic stimulation for intractable cluster headache. Brain 127, 2259–2264 (2004).
Andy, O. J. & Jurko, F. Thalamic stimulation effects on reactive depression. Appl. Neurophysiol. 50, 324–329 (1987).
Jimenez, F. et al. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery 57, 585–593 (2005).
Nuttin, B. J. et al. Long-term electrical capsular stimulation in patients with obsessive–compulsive disorder. Neurosurgery 52, 1263–1272 (2003).
Visser-Vandewalle, V. et al. Chronic bilateral thalamic stimulation: a new therapeutic approach in intractable Tourette syndrome. Report of three cases. J. Neurosurg. 99, 1094–1100 (2003).
Ackermans, L. et al. Deep brain stimulation in Tourette's syndrome: two targets? Mov. Disord. 21, 709–713 (2006).
Beric, A. et al. Complications of deep brain stimulation surgery. Stereotact. Funct. Neurosurg. 77, 73–78 (2001).
Hariz, M. I. Complications of deep brain stimulation surgery. Mov. Disord. 17, S162–S166 (2002).
Bejjani, B. P. et al. Aggressive behavior induced by intraoperative stimulation in the triangle of Sano. Neurology 59, 1425–1427 (2002).
Kulisevsky, J. et al. Mania following deep brain stimulation for Parkinson's disease. Neurology 59, 1421–1424 (2002).
Nowak, L. G. & Bullier, J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. I. Evidence from chronaxie measurements. Exp. Brain Res. 118, 477–488 (1998).
Miocinovic, S. et al. Stereotactic neurosurgical planning, recording, and visualization for deep brain stimulation in non-human primates. J. Neurosci. Methods 162, 32–41 (2007).
Hammond, C., Bergman, H. & Brown, P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci. 25 May 2007 (doi:10.1016/j.tins.2007.05.004).
Bartholow, R. Experimental investigations into the functions of the human brain. Am. J. Med. Sci. 67, 305–313 (1874).
Horsley, V. & Clarke, R. H. The structure and functions of the cerebellum examined by a new method. Brain 31, 45–124 (1908). A classic study that pioneered the stereotaxic frame (for animal use), which has since become central to the neurosurgical procedures for DBS.
Spiegel, E. A., Wycis, H. T., Marks, M. & Lee, A. J. Stereotaxic apparatus for operations on the human brain. Science 106, 349–350 (1947). First study to adapt the stereotaxic frame for human use.
Hassler, R., Riechert, T., Mundinger, F., Umbach, W. & Ganglberger, J. A. Physiological observations in stereotaxic operations in extrapyramidal motor disturbances. Brain 83, 337–350 (1960).
Bechtereva, N. P., Bondartchuk, A. N. & Smirnov, V. M. Therapeutic electrostimulations of the deep brain structures. Vopr. Neirokhir. 1, 7–12 (1972).
Cooper, I. S. Effect of chronic stimulation of anterior cerebellum on neurological disease. Lancet 1, 206 (1973).
Cooper, I. S., Riklan, M., Amin, I., Waltz, J. M. & Cullinan, T. Chronic cerebellar stimulation in cerebral palsy. Neurology 26, 744–753 (1976).
Brice, J. & McLellan, L. Suppression of intention tremor by contingent deep-brain stimulation. Lancet 1, 1221–1222 (1980).
Merienne, L. & Mazars, G. Treatment of various dyskinesias by intermittent thalamic stimulation. Neurochirurgie 28, 201–206 (1982).
Benabid, A. L., Pollak, P., Louveau, A., Henry, S. & de Rougemont, J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl. Neurophysiol. 50, 344–346 (1987).
Benabid, A. L. et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 337, 403–406 (1991).
Siegfried, J. & Lippitz, B. Chronic electrical stimulation of the VL-VPL complex and of the pallidum in the treatment of movement disorders: personal experience since 1982. Stereotact. Funct. Neurosurg. 62, 71–75 (1994).
Acknowledgements
The authors were funded by the Medical Research Council, the Norman Collisson Foundation, TrygFonden Charitable Foundation and the Charles Wolfson Charitable Trust.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (movie)
This clip shows the effects of deep brain stimulation of the pedunculopontine nucleus in a patient with Parkinson's disease. (Clip courtesy of P. Silburn, T. Coyne and R. Wilcox). (WMV 2064 kb)
Supplementary information S2 (movie)
This clip shows the effects of deep brain stimulation of the internal globus pallidus in a patient with dystonia. (WMV 561 kb)
Supplementary information S3 (movie)
This clip shows the effects of deep brain stimulation in the periventricular/periaqueductal grey in a patient with chronic pain in a phantom limb. (WMV 897 kb)
Supplementary information S4 (movie)
This clip shows the effects of deep brain stimulation of the subthalamic nucleus on tremor in a patient with Parkinson's disease. (WMV 2070 kb)
Related links
Related links
DATABASES
OMIM
FURTHER INFORMATION
Glossary
- Dystonia
-
A movement disorder that leads to involuntary sustained muscle contractions, causing distorted posturing of the foot, leg or arm.
- Neural elements
-
The cell body, myelinated axons, dendrites and supporting glial cells.
- Bradykinesia
-
The slowing of, and difficulty in initiating, movement that is characteristic of Parkinson's disease.
- Frequency band
-
Neural oscillations have been classified into different frequency bands: delta (1–3 Hz), theta (4–7 Hz), alpha (8–13 Hz), beta (14–30 Hz), gamma (30–80 Hz), fast (80–200 Hz) and ultra fast (200–600 Hz).
- Open-loop stimulator
-
An open-loop stimulator is a simple type of non-feedback controller whereby the input into the brain region is determined using only the current behavioural state and a model of the system.
- 6-hydroxydopamine
-
(6-OHDA). The first agent used to model Parkinson's disease. Injection of 6-OHDA into the substantia nigra causes it to selectively accumulate in dopamine neurons, and then kill them as a result of toxicity that is thought to involve the generation of free radicals. 6-OHDA can produce non-specific damage to other neurons.
- 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
-
(MPTP). A neurotoxin that causes degeneration of dopaminergic neurons in the substantia nigra and hence is used to study the pathophysiology of Parkinson's disease.
- Local field potential
-
(LFP). The extracellular voltage fluctuations that reflect the sum of events in the dendrites of a local neuronal population.
- Positron emission tomography
-
(PET). A medical imaging technique that uses injected radiolabelled tracer compounds, in conjunction with mathematical reconstruction methods, to produce a three-dimensional image, or map, of functional processes in the body (such as glucose metabolism, blood flow or receptor distributions).
- Magnetic resonance imaging
-
(MRI). A non-invasive method used to obtain images of living tissue. It uses radio-frequency pulses and magnetic field gradients; the principle of nuclear magnetic resonance is used to reconstruct images of tissue characteristics (for example, proton density or water diffusion parameters).
- Blood-oxygen-level-dependent signal
-
(BOLD signal). Local changes in the proportion of oxygenated blood in the brain, as measured by functional MRI. This proportion changes in response to neural activity, therefore the BOLD signal, or haemodynamic response, indicates the location and magnitude of neural activity.
- Diffusion tensor imaging
-
(DTI). A technique based on MRI developed in the mid-1990s in which the diffusion constants of water molecules are measured along many (>six) orientations and diffusion anisotropy is characterized. It is used to visualize the location, orientation and anisotropy of the brain's white-matter tracts, and is sensitive to the directional parameters of water diffusion in the brain.
- Essential tremor
-
The most common neurological movement disorder. Symptoms include involuntary rhythmic movements of the limbs, head or neck.
- Magnetoencephalography
-
(MEG). A non-invasive neuroimaging technique that detects the changing magnetic fields associated with brain activity on the timescale of milliseconds.
- Beamforming method
-
A signal processing technique that uses receptor arrays to detect signals (for example, in MEG). These techniques can be used to determine the brain sources of signals measured from SQUIDS.
- Antidromic impulse
-
Conduction opposite the normal, orthodromic direction, whereby an action potential moves from the starting point of the depolarization towards the axons of the neuron.
- Lenticular fasciculus
-
The output pathway of the basal ganglia that originates in the globus pallidus and terminates in the thalamus.
- (14C)2-deoxyglucose
-
A glucose analogue that is used in imaging techniques carried out on experimental animals in order to estimate the level of neural activity in specific brain regions. The (14C)2-deoxyglucose is administered to the animals and subsequently taken up and trapped by active neurons.
Rights and permissions
About this article
Cite this article
Kringelbach, M., Jenkinson, N., Owen, S. et al. Translational principles of deep brain stimulation. Nat Rev Neurosci 8, 623–635 (2007). https://doi.org/10.1038/nrn2196
Issue Date:
DOI: https://doi.org/10.1038/nrn2196
This article is cited by
-
Janus microparticles-based targeted and spatially-controlled piezoelectric neural stimulation via low-intensity focused ultrasound
Nature Communications (2024)
-
Monolithic silicon for high spatiotemporal translational photostimulation
Nature (2024)
-
Brain stimulation for patients with multiple sclerosis: an umbrella review of therapeutic efficacy
Neurological Sciences (2024)
-
Neuronal and synaptic adaptations underlying the benefits of deep brain stimulation for Parkinson's disease
Translational Neurodegeneration (2023)
-
Hybrid graphene electrode for the diagnosis and treatment of epilepsy in free-moving animal models
NPG Asia Materials (2023)