Key Points
-
Molecular chaperones have key roles in protein quality control and recovery from stress conditions. They assist folding and unfolding and prevent or reverse aggregation of a wide range of substrates, but their actions decline with age, leading to late onset misfolding diseases.
-
The major chaperone systems of the cell, namely heat shock protein 60 (HSP60), HSP70, HSP90 and HSP100, use the energy of ATP binding and hydrolysis to carry out their actions, which include stabilizing non-native proteins, unfolding misfolded proteins or folded proteins targeted for proteolysis as well as providing conditions that are favourable for folding.
-
HSP70 and HSP90 are highly interactive, open structures with many exposed binding sites for co-chaperones that regulate their functions in various of biological pathways. By contrast, HSP60 and HSP100 are self-contained, with internalized active sites and few cooperating partners.
-
Chaperones are extremely flexible and dynamic machines. In order to act on their substrates, their domains rotate up to 100° and are displaced by up to 50 Å or more.
-
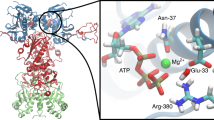
ATP binding and hydrolysis influence the accessibility and dynamics of binding sites for non-native proteins. For example, the lid subdomain of the substrate-binding domain in HSP70 is fully opened upon interaction with the ATPase domain, whereas HSP60 converts an open ring into an enclosed container for protein folding.
Abstract
Molecular chaperones are diverse families of multidomain proteins that have evolved to assist nascent proteins to reach their native fold, protect subunits from heat shock during the assembly of complexes, prevent protein aggregation or mediate targeted unfolding and disassembly. Their increased expression in response to stress is a key factor in the health of the cell and longevity of an organism. Unlike enzymes with their precise and finely tuned active sites, chaperones are heavy-duty molecular machines that operate on a wide range of substrates. The structural basis of their mechanism of action is being unravelled (in particular for the heat shock proteins HSP60, HSP70, HSP90 and HSP100) and typically involves massive displacements of 20–30 kDa domains over distances of 20–50 Å and rotations of up to 100°.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gidalevitz, T., Prahlad, V. & Morimoto, R. I. The stress of protein misfolding: from single cells to multicellular organisms. Cold Spring Harb. Perspect. Biol. 3, a009704 (2011).
Taylor, R. C. & Dillin, A. Aging as an event of proteostasis collapse. Cold Spring Harb. Perspect. Biol. 3, a004440 (2011).
Korennykh, A. & Walter, P. Structural basis of the unfolded protein response. Annu. Rev. Cell Dev. Biol. 28, 251–277 (2012).
Hartl, F. U., Bracher, A. & Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 475, 324–332 (2011).
Bukau, B., Weissman, J. & Horwich, A. Molecular chaperones and protein quality control. Cell 125, 443–451 (2006).
Mayer, M. P. Gymnastics of molecular chaperones. Mol. Cell 39, 321–331 (2010).
Kampinga, H. H. & Craig, E. A. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nature Rev. Mol Cell. Biol. 11, 579–592 (2010).
Zuiderweg, E. R. et al. Allostery in the Hsp70 chaperone proteins. Top. Curr. Chem. 328, 99–153 (2013).
Young, J. C. Mechanisms of the Hsp70 chaperone system. Biochem. Cell Biol. 88, 291–300 (2010).
Sharma, S. K., De los Rios, P., Christen, P., Lustig, A. & Goloubinoff, P. The kinetic parameters and energy cost of the Hsp70 chaperone as a polypeptide unfoldase. Nature Chem. Biol. 6, 914–920 (2010).
Rothnie, A., Clarke, A. R., Kuzmic, P., Cameron, A. & Smith, C. J. A sequential mechanism for clathrin cage disassembly by 70-kDa heat-shock cognate protein (Hsc70) and auxilin. Proc. Natl Acad. Sci. USA 108, 6927–6932 (2011).
Shorter, J. The mammalian disaggregase machinery: Hsp110 synergizes with Hsp70 and Hsp40 to catalyze protein disaggregation and reactivation in a cell-free system. PLoS ONE 6, e26319 (2011). Shows, together with reference 13 and 14, that HSP110, in cooperation with the HSP70 system, exerts the disaggregase activity in metazoan cells.
Rampelt, H. et al. Metazoan Hsp70 machines use Hsp110 to power protein disaggregation. EMBO J. 31, 4221–4235 (2012). Shows that the HSP70 nucleotide exchange activity of HSP110 is essential for its role in disaggregation, whereas HSP110 ATPase and intrinsic chaperone activity are not required.
Song, Y. et al. Molecular chaperone Hsp110 rescues a vesicle transport defect produced by an ALS-associated mutant SOD1 protein in squid axoplasm. Proc. Natl Acad. Sci. USA 110, 5428–5433 (2013). Reveals that inhibition of axonal transport by misfolded, disease-associated superoxide dismutase is reversed by the HSP70–HSP110 disaggregation machinery.
Mattoo, R. U. H., Sharma, S. K., Priya, S., Finka, A. & Goloubinoff, P. Hsp110 is a bona fide chaperone using ATP to unfold stable misfolded polypeptides and reciprocally collaborate with Hsp70 to solubilize protein aggregates. J. Biol. Chem. 288, 21399–21411 (2013).
Flaherty, K. M., Deluca-Flaherty, C. & McKay, D. B. 3-dimensional structure of the ATPase fragment of a 70 k heat-shock cognate protein. Nature 346, 623–628 (1990).
Bork, P., Sander, C. & Valencia, A. An ATPase domain common to prokaryotic cell-cycle proteins, sugar kinases, actin, and Hsp70 heat-shock proteins. Proc. Natl Acad. Sci. USA 89, 7290–7294 (1992).
Zhu, X. T. et al. Structural analysis of substrate binding by the molecular chaperone DnaK. Science 272, 1606–1614 (1996).
Liu, Q. & Hendrickson, W. A. Insights into Hsp70 chaperone activity from a crystal structure of the yeast Hsp110 Sse1. Cell 131, 106–120 (2007).
Kityk, R., Kopp, J., Sinning, I. & Mayer, M. P. Structure and dynamics of the ATP-bound open conformation of Hsp70 chaperones. Mol. Cell. 48, 863–874 (2012). Obtains the crystal structure of the allosterically active, domain-docked form of HSP70 by disulphide stabilization of its ATP-bound conformation. Reveals a wide opening of the substrate-binding site.
Zhuravleva, A., Clerico, E. M. & Gierasch, L. M. An interdomain energetic tug-of-war creates the allosterically active state in Hsp70 molecular chaperones. Cell 151, 1296–1307 (2012). Shows, using methyl TROSY NMR of an HSP70 mutant defective in ATP hydrolysis, the dynamics of the domain interactions in the formation of alternative interfaces that are induced by the interdomain linker region.
Qi, R. et al. Allosteric opening of the polypeptide-binding site when an Hsp70 binds ATP. Nature Struct. Mol. Biol. 20, 900–907 (2013).
Jiang, J. et al. Structural basis of J cochaperone binding and regulation of Hsp70. Mol. Cell 28, 422–433 (2007).
Ahmad, A. et al. Heat shock protein 70 kDa chaperone/DnaJ cochaperone complex employs an unusual dynamic interface. Proc. Natl Acad. Sci. USA 108, 18966–18971 (2011).
Li, J. Z., Qian, X. G. & Sha, B. The crystal structure of the yeast Hsp40 Ydj1 complexed with its peptide substrate. Structure 11, 1475–1483 (2003).
Rodriguez, F. et al. Molecular basis for regulation of the heat shock transcription factor σ32 by the DnaK and DnaJ chaperones. Mol. Cell 32, 347–358 (2008).
Petrova, K., Oyadomari, S., Hendershot, L. M. & Ron, D. Regulated association of misfolded endoplasmic reticulum lumenal proteins with p58/DNAJc3. EMBO J. 27, 2862–2872 (2008).
Harrison, C. J., Hayer-Hartl, M., Di Liberto, M., Hartl, F. & Kuriyan, J. Crystal structure of the nucleotide exchange factor GrpE bound to the ATPase domain of the molecular chaperone DnaK. Science 276, 431–435 (1997).
Schuermann, J. P. et al. Structure of the Hsp110:Hsc70 nucleotide exchange machine. Mol. Cell 31, 232–243 (2008).
Polier, S., Dragovic, Z., Hartl, F. U. & Bracher, A. Structural basis for the cooperation of Hsp70 and Hsp110 chaperones in protein folding. Cell 133, 1068–1079 (2008).
Pratt, W. B. & Toft, D. O. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endrocrine Rev. 18, 306–360 (1997).
Li, J., Soroka, J. & Buchner, J. The Hsp90 chaperone machinery: conformational dynamics and regulation by co-chaperones. Biochim. Biophys. Acta 1823, 624–635 (2012).
Didenko, T., Duarte, A. M. S., Karagoz, G. E. & Rudiger, S. G. D. Hsp90 structure and function studied by NMR spectroscopy. Biochim. Biophys. Acta 1823, 636–647 (2012).
Johnson, J. L. Evolution and function of diverse Hsp90 homologs and cochaperone proteins. Biochim. Biophys. Acta 1823, 607–613 (2012).
Taipale, M., Jarosz, D. F. & Lindquist, S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nature Rev. Mol. Cell Biol. 11, 515–528 (2010).
Jarosz, D. F. & Lindquist, S. Hsp90 and environmental stress transform the adaptive value of natural genetic variation, Science 330, 1820–1824 (2010).
Ali, M. M. et al. Crystal structure of an Hsp90–nucleotide–p23/Sba1 closed chaperone complex. Nature 440, 1013–1017 (2006).
Krukenberg, K. A. et al. Conformational dynamics of the molecular chaperone Hsp90. Q. Rev. Biophys. 44, 229–255 (2011).
Prodromou, C. et al. The ATPase cycle of Hsp90 drives a molecular 'clamp' via transient dimerization of the N-terminal domains. EMBO J. 19, 4383–4392 (2000).
Dutta, R. & Inouye, M. GHKL, an emergent ATPase/kinase superfamily. Trends Biochem. Sci. 25, 24–28 (2000).
Mickler, M., Hessling, M., Ratzke, C., Buchner, J. & Hugel, T. The large conformational changes of Hsp90 are only weakly coupled to ATP hydrolysis. Nature Struct. Mol. Biol. 16, 281–286 (2009).
Siligardi, G. et al. Regulation of Hsp90 by the co-chaperone Cdc37p/p50cdc37. J. Biol. Chem. 277, 20151–20159 (2002).
Prodromou, C. et al. Regulation of Hsp90 ATPase activity by tetratricopeptide repeat (TPR)-domain co-chaperones. EMBO J. 18, 754–762 (1999).
Chen, S. & Smith, D. F. Hop as an adaptor in the heat shock protein 70 (Hsp70) and Hsp90 chaperone machinery. J. Biol. Chem. 273, 35194–35200 (1998).
Retzlaff, M. et al. Asymmetric activation of the Hsp90 dimer by its cochaperone Aha1. Mol. Cell 37, 344–354 (2010).
Theodoraki, M. A. & Caplan, A. J. Quality control and fate determination of Hsp90 client proteins. Biochim. Biophys. Acta 1823, 683–688 (2012).
Southworth, D. R. & Agard, D. A. Client-loading conformation of the Hsp90 molecular chaperone revealed in the cryo-EM structure of the human Hsp90:Hop complex. Mol. Cell 42, 771–781 (2011). Reveals a low-resolution cryo-EM structure of the HSP90–HOP complex and suggests how HOP alters the conformation of HSP90 to one that is poised to accept substrate and that closes upon ATP binding.
Vaughan, C. K. et al. Structure of an Hsp90–Cdc37–Cdk4 complex. Mol. Cell 23, 697–707 (2006).
Fang, L. Ricketson, D. Getubig, L. & Darimont, B. Unliganded and hormone-bound glucocorticoid receptors interact with distinct hydrophobic sites in the Hsp90 C-terminal domain. Proc. Natl Acad. Sci. USA 103, 18487–18492 (2006).
Genest, O. et al. Uncovering a region of heat shock protein 90 important for client binding in E. coli and chaperone function in yeast. Mol. Cell 49, 464–473 (2013).
Trepel, J., Mollapour, M., Giaccone, G. & Neckers, L. Targeting the dynamic HSP90 complex in cancer. Nature Rev. Cancer 10, 537–549 (2010).
Hemmingsen, S. M. et al. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature 333, 330–334 (1988).
Goloubinoff, P., Gatenby, A. A. & Lorimer, G. H. GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature 337, 44–47 (1989).
Ostermann, J., Horwich, A. L., Neupert, W. & Hartl, F. U. Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature 341, 125–130 (1989).
Horwich, A. L. & Fenton, W. A. Chaperonin-mediated protein folding: using a central cavity to kinetically assist polypeptide chain folding. Q. Rev. Biophys. 42, 83–116 (2009).
Saibil, H. R., Fenton, W. A., Clare, D. K. & Horwich, A. L. Structure and allostery of the chaperonin GroEL. J. Mol. Biol. 425, 1476–1487 (2013).
Braig, K. et al. The crystal structure of the bacterial chaperonin GroEL at 2.8 Å. Nature 371, 578–586 (1994).
Xu, Z., Horwich, A. L. & Sigler, P. B. The crystal structure of the asymmetric GroEL–GroES–(ADP)7 chaperonin complex. Nature 388, 741–750 (1997).
Farr, G. W. et al. Multivalent binding of nonnative substrate proteins by the chaperonin GroEL. Cell 100, 561–573 (2000).
Elad, N. et al. Topologies of a substrate protein bound to the chaperonin GroEL. Mol. Cell 26, 415–426 (2007).
Lin, Z., Madan, D. & Rye, H. S. GroEL stimulates protein folding through forced unfolding. Nature Struct. Mol. Biol. 15, 303–311 (2008).
Motojima, F., Chaudhry, C., Fenton, W. A., Farr, G. W. & Horwich, A. L. Substrate polypeptide presents a load on the apical domains of the chaperonin GroEL. Proc. Natl Acad. Sci. USA 101, 15005–15012 (2004).
Chaudhuri, T. K., Farr, G. W., Fenton, W. A., Rospert, S. & Horwich, A. L. GroEL/GroES-mediated folding of a protein too large to be encapsulated. Cell 107, 235–246 (2001).
Clare, D. K., Bakkes, P. J., van Heerikhuizen, H., van der Vies, S. M. & Saibil, H. R. Chaperonin complex with a newly folded protein encapsulated in the folding chamber. Nature 457, 107–110 (2009).
Clare, D. K. et al. ATP-triggered molecular mechanics of the chaperonin GroEL. Cell 149, 113–123 (2012). Determines multiple conformations of ATP-bound GroEL by single-particle cryo-EM and reveals a repertoire of domain movements that are likely to be involved in unfolding and encapsulation of non-native substrates.
Yifrach, O. & Horovitz, A. Nested cooperativity in the ATPase activity of the oligomeric chaperonin GroEL. Biochemistry 34, 5303–5308 (1995).
Miyazaki, T. et al. GroEL–substrate–GroES ternary complexes are an important transient intermediate of the chaperonin cycle. J. Biol. Chem. 277, 50621–50628 (2002).
Nojima, T., Murayama, S., Yoshida, M. & Motojima, F. Determination of the number of active GroES subunits in the fused heptamer GroES required for interactions with GroEL. J. Biol. Chem. 283, 18385–18392 (2008).
Buckle, A. M., Zahn, R. & Fersht, A. R. A structural model for GroEL–polypeptide recognition. Proc. Natl Acad. Sci. USA 94, 3571–3575 (1997).
Chen, L. & Sigler, P. B. The crystal structure of a GroEL/peptide complex: plasticity as a basis for substrate diversity. Cell 99, 757–768 (1999).
Clare, D., Bakkes, P. J., van Heerikhuizen, H., van der Vies, S. M. & Saibil, H. R. An expanded protein folding cage in the GroEL–gp31 complex. J. Mol. Biol. 358, 905–911 (2006).
Chen, D.-H. et al. Visualizing GroEL/ES in the act of encapsulating a folding protein. Cell 153, 1354–1365 (2013).
Ranson, N. A. et al. Allosteric signaling of ATP hydrolysis in GroEL–GroES complexes. Nature Struct. Mol. Biol. 13, 147–152 (2006).
Fei, X., Yang, D., LaRonde-LeBlanc, N. & Lorimer, G. H. Crystal structure of a GroEL–ADP complex in the relaxed allosteric state at 2.7 Å resolution. Proc. Natl Acad. Sci. USA 110, E2958–E2966 (2013). Determines the crystal structure of a highly asymmetric GroEL–ADP complex lacking key salt bridges. Shows that in this structure the conformations of individual subunits resemble the range of states seen by cryo-EM.
Ditzel, L. et al. Crystal structure of the thermosome, the archaeal chaperonin and homolog of CCT. Cell 93, 125–138 (1998).
Kalisman, N., Adams, C. M. & Levitt, M. Subunit order of eukaryotic TRiC/CCT chaperonin by cross-linking, mass spectrometry, and combinatorial homology modeling. Proc. Natl Acad. Sci. USA 109, 2884–2889 (2012).
Leitner, A. et al. The molecular architecture of the eukaryotic chaperonin TRiC/CCT. Structure 20, 814–825 (2012).
Clare, D. K. et al. Multiple states of a nucleotide-bound group 2 chaperonin. Structure 16, 528–534 (2008).
Zhang, J. et al. Mechanism of folding chamber closure in a group II chaperonin. Nature 463, 379–383 (2010).
Huo, Y. et al. Crystal structure of group II chaperonin in the open state. Structure 18, 1270–1279 (2010).
Cong, Y. et al. Symmetry-free cryo-EM structures of the chaperonin TRiC along its ATPase-driven conformational cycle. EMBO J. 31, 720–730 (2011).
Horovitz, A. & Willison, K. R. Allosteric regulation of chaperonins. Curr. Opin. 15, 646–651 (2005).
Bigotti, M. G. & Clarke, A. R. Cooperativity in the thermosome. J. Mol. Biol. 348, 13–26 (2005).
Neuwald, A. F., Aravind, L., Spouge, J. L. & Koonin, E. V. AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 9, 27–43 (1999).
Hanson, P. I. & Whiteheart, S. W. AAA+ proteins: have engine, will work. Nature Rev. Mol. Cell Biol. 6, 519–529 (2005).
Wang, J. et al. Crystal structures of the HslVU peptidase–ATPase complex reveal an ATP-dependent proteolysis mechanism. Structure 9, 177–184 (2001).
Effantin, G., Ishikawa, T., De Donatis, G. M., Maurizi, M. R. & Steven, A. C. Local and global mobility in the ClpA AAA+ chaperone detected by cryo-electron microscopy: functional connotations. Structure 18, 553–562 (2010).
Lander, G. C. et al. Complete subunit architecture of the proteasome regulatory particle. Nature 482, 186–191 (2012).
da Fonseca, P. C., He, J. & Morris, E. P. Molecular model of the human 26S proteasome. Mol. Cell 46, 54–66 (2012).
Cranz-Mileva, S. et al. The flexible attachment of the N-domains to the ClpA ring body allows their use on demand. J. Mol. Biol. 378, 412–424 (2008).
Zhang, T. et al. Flexible connection of the N-terminal domain in ClpB modulates substrate binding and the aggregate reactivation efficiency. Proteins 80, 2758–2768 (2012).
Sauer, R. T. & Baker, T. A. AAA+ proteases: ATP-fueled machines of protein destruction. Annu. Rev. Biochem. 80, 587–612 (2011).
Weber-Ban, E. U., Reid, B. G., Miranker, A. D. & Horwich, A. L. Global unfolding of a substrate protein by the Hsp100 chaperone ClpA. Nature 401, 90–93 (1999).
Hinnerwisch, J., Fenton, W. A., Furtak, K. J., Farr, G. W. & Horwich, A. L. Loops in the central channel of ClpA chaperone mediate protein binding, unfolding, and translocation. Cell 121, 1029–1041 (2005).
Glynn, S. E., Martin, A., Nager, A. R., Baker, T. A. & Sauer, R. T. Structures of asymmetric ClpX hexamers reveal nucleotide-dependent motions in a AAA+ protein-unfolding machine. Cell 139, 744–756 (2009).
Maillard, R. A. et al. ClpX(P) generates mechanical force to unfold and translocate its protein substrates. Cell 145, 459–469 (2011). Single-molecule force study suggests how the ATP-dependent protease ClpXP mechanically destabilizes and unfolds tagged GFP and translocates it through the pore channel in 1 nm steps.
DeLaBarre, B. & Brunger, A. T. Nucleotide dependent motion and mechanism of action of p97/VCP. J. Mol. Biol. 347, 437–452 (2005).
Niwa, H. et al. The role of the N-domain in the ATPase activity of the mammalian AAA ATPasep97/VCP. J. Biol. Chem. 287, 8561–8570 (2012).
Barthelme, D. & Sauer, R. T. Identification of the Cdc48•20S proteasome as an ancient AAA+ proteolytic machine. Science 337, 843–846 (2012).
Glover, J. R. & Lindquist, S. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell 94, 73–82 (1998).
Goloubinoff, P., Mogk, A., Zvi, A. P., Tomoyasu, T. & Bukau, B. Sequential mechanism of solubilization and refolding of stable protein aggregates by a bichaperone network. Proc. Natl Acad. Sci. USA 96, 13732–13737 (1999).
Miot, M. et al. Species-specific collaboration of heat shock proteins (Hsp) 70 and 100 in thermotolerance and protein disaggregation. Proc. Natl Acad. Sci. USA 108, 6915–6920 (2011).
Lee, S. et al. The structure of ClpB: a molecular chaperone that rescues proteins from an aggregated state. Cell 115, 229–240 (2003).
Weibezahn, J. et al. Thermotolerance requires refolding of aggregated proteins by substrate translocation through the central pore of ClpB. Cell 119, 653–665 (2004).
Seyffer, F. et al. Hsp70 proteins bind Hsp100 regulatory M domains to activate AAA+ disaggregase at aggregate surfaces. Nature Struct. Mol. Biol. 19, 1347–1355 (2012).
Rosenzweig, R., Moradi, S., Zarrine-Afsar, A., Glover, J. R. & Kay, L. E. Unraveling the mechanism of protein disaggregation through a ClpB–DnaK interaction. Science 339, 1080–1083 (2013). Proposes a model based on data from methyl TROSY NMR analysis in which the DnaK ATPase domain binds the coiled-coil domain of ClpB to activate disaggregation in this bi-chaperone system.
Wendler, P. et al. Atypical AAA+ subunit packing creates an expanded cavity for disaggregation by the protein-remodeling factor Hsp104. Cell 131, 1366–1377 (2007).
Lee, S., Sielaff, B., Lee, J. & Tsai, F. T. CryoEM structure of Hsp104 and its mechanistic implication for protein disaggregation. Proc. Natl Acad. Sci. USA 107, 8135–8140 (2010).
Wang, F. et al. Structure and mechanism of the hexameric MecA-ClpC molecular machine. Nature 471, 331–335 (2011).
Oguchi, Y. et al. A tightly regulated molecular toggle controls AAA+ disaggregase. Nature Struct. Mol. Biol. 19, 1338–1346 (2012). Uses mutations at opposite ends of the ClpB coiled-coil domain together with accessibility and interaction studies and reveals a toggle switch mechanism for regulating disaggregation activity.
Kaushal, S. & Khorana, H. G. Structure and function in rhodopsin. 7. Point mutations associated with autosomal dominant retinitis pigmentosa. Biochemistry 33, 6121–6128 (1994).
Mendes, H. F., Van der Spuy, J., Chapple, J. P. & Cheetham, M. E. Mechanisms of cell death in rhodopsin retinitis pigmentosa: implications for therapy. Trends Mol. Med. 11, 177–185 (2005).
Chiti, F. & Dobson, C. M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 75, 333–366 (2006).
Buxbaum, J. N. & Linke, R. P. A molecular history of the amyloidoses. J. Mol. Biol. 421, 142–159 (2012).
Eisenberg, D. & Jucker, M. The amyloid state of proteins in human diseases. Cell 148, 1188–1203 (2012).
Auluck, P. K., Chan, H. Y. E., Trojanowski, J. Q., Lee, V. M. Y. & Bonini, N. M. Chaperone suppression of α-synuclein toxicity in a Drosophila model for Parkinson's disease. Science 295, 865–868 (2002).
Kitamura, A. et al. Cytosolic chaperonin prevents polyglutamine toxicity with altering the aggregation state. Nature Cell Biol. 8, 1163–1170 (2006).
Tam, S., Geller, R., Spiess, C. & Frydman, J. The chaperonin TRiC controls polyglutamine aggregation and toxicity through subunit-specific interactions. Nature Cell Biol. 8, 1155–1162 (2006).
Behrends, C. et al. Chaperonin TRiC promotes the assembly of polyQ expansion proteins into nontoxic oligomers. Mol. Cell 23, 887–897 (2006).
Balch, W. E., Morimoto, R. I., Dillin, A. & Kelly, J. W. Adapting proteostasis for disease intervention. Science 319, 916–919 (2008).
Powers, E. T., Morimoto, R. I., Dillin, A., Kelly, J. W. & Balch, W. E. Biological and chemical approaches to diseases of proteostasis deficiency. Annu. Rev. Biochem. 78, 959–991 (2009).
Shiau, A. K., Harris, S. F., Southworth, D. R. & Agard, D. A. Structural analysis of E. coli hsp90 reveals dramatic nucleotide-dependent conformational rearrangements, Cell 127, 329–340 (2006).
Dollins, D. E., Warren, J. J., Immormino, R. M. & Gewirth, D. T. Structures of GRP94–nucleotide complexes reveal mechanistic differences between the hsp90 chaperones. Mol. Cell 28, 41–56 (2007).
Sousa, M. C. et al. Crystal and solution structures of an HslUV protease–chaperone complex. Cell 103, 633–643 (2000).
Acknowledgements
The author is grateful to D. Clare, C. Vaughan, J. Trapani and D. Middendorf for helpful comments on the manuscript and thanks the Wellcome Trust for funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Supplementary information
Supplementary information S1 (figure)
GroEL-ATP states. (PDF 454 kb)
Supplementary information S2 (movie)
Trajectory of ATP-induced movements in a GroEL ring.The movie is an interpolation between the structures of four experimentally determined states of GroEL, starting from apo GroEL and passing through two major intermediate states of GroEL-ATP7, in which the apical domains first tilt and then expand radially, before going through the final 100º rotation to the GroES bound conformation1-3. GroES is not shown. The movements are then shown in reverse. Helices H and I, shown in red and orange respectively, denote the substrate-binding site. The green helix in the intermediate domain contains the catalytic aspartate, and the magenta helix in the equatorial domain links the nucleotide site to the inter-ring interface. 1. Braig, K. et al.The crystal structure of the bacterial chaperonin GroEL at 2.8 Å. Nature 371, 578–586 (1994). 2. Clare, D. K. et al. ATP-triggered molecular mechanics of the chaperonin GroEL. Cell 149, 113–123 (2012). 3. Xu, Z., Horwich, A. L. & Sigler, P. B. The crystal structure of the asymmetric GroEL–GroES–(ADP)7 chaperonin complex. Nature 388, 741–750 (1997). (MOV 4868 kb)
Supplementary information S3 (movie)
Trajectory of ring closure in an archaeal group II chaperonin. An interpolation between three states of an M. maripaludis chaperonin ring1 are shown with the same colour coding as in Movie 1. This thermosome has 8 subunits per ring, and the unliganded state is very expanded. The apical domains twist and close in movements that are similar to those of GroEL, but occurring in a different sequence. The extension of the red helix forms the builtin lid that takes the place of GroES. 1. Clare, D. K. et al. Multiple states of a nucleotide-bound group 2 chaperonin. Structure 16, 528–534 (2008). (MOV 2922 kb)
Supplementary information S4 (figure)
Comparison of chaperone nucleotide binding sites. (PDF 360 kb)
Glossary
- Autophagy
-
A process in which intracellular material is enclosed in a membrane compartment and delivered to the lysosome (vacuole in yeast) for degradation and recycling of the macromolecular constituents.
- Unfolded protein response
-
(UPR). A signalling system that regulates the balance between folding capacity of the endoplasmic reticulum (ER) and protein synthesis. If misfolded proteins accumulate, this pathway triggers apoptosis.
- Heat shock proteins
-
(HSPs). The expression of these proteins is greatly enhanced by increased temperature or other stress conditions. Most chaperones are HSPs.
- Allosteric machines
-
Macromolecular complexes in which the activity is indirectly modulated by binding of an effector at a site remote from the active site. This induces shifts in the domain or subunit structure that influence the conformation of the active site.
- Amyloid
-
Protein species that form deposits consisting of fibrillar protein aggregates rich in β-sheet structure. They assemble from proteins that have unfolded or misfolded. About 20 distinct protein species are associated with particular amyloid diseases.
- Methyl transverse relaxation optimized spectroscopy
-
(methyl TROSY). A method that uses selective isotope labelling of methyl groups on protein side chains with a transverse relaxation scheme optimized for methyl groups to obtain well-resolved nuclear magnetic resonance (NMR) spectra from large protein structures far beyond the normal range obtained in NMR structure determination.
- GHKL
-
An ATP-binding superfamily that includes DNA gyrase, the molecular chaperone heat shock protein 90, the DNA-mismatch-repair enzyme MutL and His kinase, which bind ATP in a characteristic bent conformation.
Rights and permissions
About this article
Cite this article
Saibil, H. Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Biol 14, 630–642 (2013). https://doi.org/10.1038/nrm3658
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3658
This article is cited by
-
BAG5 regulates HSPA8-mediated protein folding required for sperm head-tail coupling apparatus assembly
EMBO Reports (2024)
-
Pre-treatment with IL-6 potentiates β-cell death induced by pro-inflammatory cytokines
BMC Molecular and Cell Biology (2023)
-
Simple model systems reveal conserved mechanisms of Alzheimer’s disease and related tauopathies
Molecular Neurodegeneration (2023)
-
ATF5 regulates tubulointerstitial injury in diabetic kidney disease via mitochondrial unfolded protein response
Molecular Medicine (2023)
-
Metal-enriched HSP90 nanoinhibitor overcomes heat resistance in hyperthermic intraperitoneal chemotherapy used for peritoneal metastases
Molecular Cancer (2023)