Key Points
-
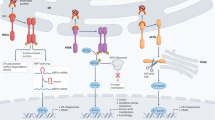
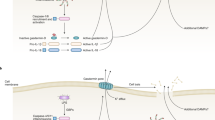
Many different components facilitate autocrine purinergic signalling, including pannexin 1 hemichannels (which facilitate ATP release), P2X and P2Y receptors (which respond to ATP), ectonucleotidases (which hydrolyse ATP to adenosine), P1 receptors (which respond to adenosine) and nucleoside transporters and adenosine deaminase (which remove adenosine).
-
Different immune cells express distinct purinergic signalling components, and this has an important role in providing signal amplification following cell activation. The positive autocrine feedback loops mediated by purinergic signalling are essential for gradient sensing by phagocytes and antigen recognition by T cells.
-
When released from damaged, dying and apoptotic cells, ATP can serve as a danger signal that stimulates the NLRP3 (NOD-, LRR- and pyrin domain-containing 3) inflammasome, promotes chemotaxis of microglia, and boosts activation of other immune cell types that are recruited to sites of inflammation and tissue damage. High ATP concentrations at sites of inflammation trap neutrophils and other phagocytes by interfering with their autocrine purinergic chemotaxis signalling systems.
-
ATP release and autocrine purinergic signalling can amplify activation signals in immune cells but can also downregulate immune cell responses, either by activating suppressive P2 receptors or through adenosine formation and activation of suppressive A2A receptors.
-
A growing arsenal of pharmacological agents is available to modulate purinergic signalling in immune cells. The most widely investigated drugs target P1 receptors or the molecular processes that control the availability of the P1 receptor ligand adenosine.
Abstract
Stimulation of almost all mammalian cell types leads to the release of cellular ATP and autocrine feedback through a diverse array of purinergic receptors. Depending on the types of purinergic receptors that are involved, autocrine signalling can promote or inhibit cell activation and fine-tune functional responses. Recent work has shown that autocrine signalling is an important checkpoint in immune cell activation and allows immune cells to adjust their functional responses based on the extracellular cues provided by their environment. This Review focuses on the roles of autocrine purinergic signalling in the regulation of both innate and adaptive immune responses and discusses the potential of targeting purinergic receptors for treating immune-mediated disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Burnstock, G., Fredholm, B. B., North, R. A. & Verkhratsky, A. The birth and postnatal development of purinergic signalling. Acta Physiol. 199, 93–147 (2010). This is a comprehensive review by the pioneers in the field who describe the development and growth of the purinergic signalling field from its beginning, with the initial discovery of ATP, to its current widespread scope that encompasses a wide range of research fields.
Ralevic, V. & Burnstock, G. Receptors for purines and pyrimidines. Pharmacol. Rev. 50, 413–492 (1998). This review offers an in-depth view of the molecular and pharmacological properties of the known purinergic receptors.
Zimmermann, H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch. Pharmacol. 362, 299–309 (2000). This review by a leader in the field provides detailed information on the various ectoenzyme families that facilitate the hydrolysis of extracellular ATP and related nucleotides.
Burnstock, G. Unresolved issues and controversies in purinergic signalling. J. Physiol. 586, 3307–3312 (2008).
Corriden, R. & Insel, P. A. Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci. Signal. 3, re1 (2010).
Abbracchio, M. P., Burnstock, G., Verkhratsky, A. & Zimmermann, H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 32, 19–29 (2009).
Bours, M. J., Swennen, E. L., Di Virgilio, F., Cronstein, B. N. & Dagnelie, P. C. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 112, 358–404 (2006). This comprehensive review article provides a summary of the evidence that purinergic signalling systems are involved in the regulation of virtually all immune cell populations.
Filippini, A., Taffs, R. E. & Sitkovsky, M. V. Extracellular ATP in T lymphocyte activation: possible role in effector functions. Proc. Natl Acad. Sci. USA 87, 8267–8271 (1990). This work first demonstrated ATP release from stimulated T cells.
Schenk, U. et al. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci. Signal. 1, ra6 (2008). This paper elucidated the important role of pannexin 1 in facilitating ATP release, which drives the autocrine purinergic signalling events that are required for T cell activation.
Yip, L. et al. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J. 23, 1685–1693 (2009).
Woehrle, T. et al. Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T cell activation at the immune synapse. Blood 116, 3475–3484 (2010). This paper showed that pannexin 1 and two key P2X receptors translocate to the immune synapse and contribute to calcium influx in response to TCR stimulation.
Chen, Y. et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314, 1792–1795 (2006). This paper was the first report to indicate that neutrophil chemotaxis is regulated by autocrine purinergic signalling.
Elliott, M. R. et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461, 282–286 (2009). This paper showed that ATP released from apoptotic cells serves as a find-me signal and induces the recruitment of monocytes.
Trautmann, A. Extracellular ATP in the immune system: more than just a “danger signal”. Sci. Signal. 2, pe6 (2009).
Praetorius, H. A. & Leipziger, J. ATP release from non-excitable cells. Purinergic Signal. 5, 433–446 (2009). This review article provides a thorough overview of the various ATP release mechanisms that are involved in purinergic signalling as well as the methods available to assess ATP release.
Abbracchio. et al. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 58, 281–341 (2006).
Burnstock, G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol. Rev. 58, 58–86 (2006).
Yegutkin, G. G. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim. Biophys. Acta 1783, 673–694 (2008). This review article provides a comprehensive overview of the complex enzymatic systems that regulate extracellular concentrations of the ligands of purinergic receptors.
Di Virgilio, F. Purinergic mechanism in the immune system: a signal of danger for dendritic cells. Purinergic Signal. 1, 205–209 (2005).
Ravichandran, K. S. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J. Exp. Med. 207, 1807–1817 (2010).
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010).
Burnstock, G. Purinergic signalling and disorders of the central nervous system. Nature Rev. Drug Discov. 7, 575–590 (2008).
Dahl, G. & Locovei, S. Pannexin: to gap or not to gap, is that a question? IUBMB Life 58, 409–419 (2006).
Laird, D. W. Life cycle of connexins in health and disease. Biochem. J. 394, 527–543 (2006).
MacVicar, B. A. & Thompson, R. J. Non-junction functions of pannexin-1 channels. Trends Neurosci. 33, 93–102 (2010).
Eltzschig, H. K. et al. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ. Res. 99, 1100–1108 (2006).
Chen, Y. et al. Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci. Signal. 3, ra45 (2010). This paper shows that autocrine purinergic signalling is an essential event in neutrophil activation.
Beldi, G. et al. The role of purinergic signaling in the liver and in transplantation: effects of extracellular nucleotides on hepatic graft vascular injury, rejection and metabolism. Front. Biosci. 13, 2588–2603 (2008).
Gray, J. H., Owen, R. P. & Giacomini, K. M. The concentrative nucleoside transporter family, SLC28. Pflugers Arch. 447, 728–734 (2004).
Baldwin, S. A. et al. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 447, 735–743 (2004).
Franco, R., Valenzuela, A., Lluis, C. & Blanco, J. Enzymatic and extraenzymatic role of ecto-adenosine deaminase in lymphocytes. Immunol. Rev. 161, 27–42 (1998).
Cristalli, G. et al. Adenosine deaminase: functional implications and different classes of inhibitors. Med. Res. Rev. 21, 105–128 (2001).
Kameoka, J., Tanaka, T., Nojima, Y., Schlossman, S. F. & Morimoto, C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science 261, 466–469 (1993).
Ciruela, F. et al. Adenosine deaminase affects ligand-induced signalling by interacting with cell surface adenosine receptors. FEBS Lett. 380, 219–223 (1996).
Herrera, C. et al. Adenosine A2B receptors behave as an alternative anchoring protein for cell surface adenosine deaminase in lymphocytes and cultured cells. Mol. Pharmacol. 59, 127–134 (2001).
Franco, R., Pacheco, R., Gatell, J. M., Gallart, T. & Lluis, C. Enzymatic and extraenzymatic role of adenosine deaminase 1 in T-cell–dendritic cell contacts and in alterations of the immune function. Crit. Rev. Immunol. 27, 495–509 (2007).
Zavialov, A. V. et al. Human adenosine deaminase 2 induces differentiation of monocytes into macrophages and stimulates proliferation of T helper cells and macrophages. J. Leukoc. Biol. 88, 279–290 (2010).
Apasov, S. G. & Sitkovsky, M. V. The extracellular versus intracellular mechanisms of inhibition of TCR-triggered activation in thymocytes by adenosine under conditions of inhibited adenosine deaminase. Int. Immunol. 11, 179–189 (1999).
Apasov, S. G., Blackburn, M. R., Kellems, R. E., Smith, P. T. & Sitkovsky, M. V. Adenosine deaminase deficiency increases thymic apoptosis and causes defective T cell receptor signaling. J. Clin. Invest. 108, 131–141 (2001).
Molina-Arcas, M., Casado, F. J. & Pastor-Anglada, M. Nucleoside transporter proteins. Curr. Vasc. Pharmacol. 7, 426–434 (2009).
King, A. E., Ackley, M. A., Cass, C. E., Young, J. D. & Baldwin, S. A. Nucleoside transporters: from scavengers to novel therapeutic targets. Trends Pharmacol. Sci. 27, 416–425 (2006).
Kichenin, K., Pignede, G., Fudalej, F. & Seman, M. CD3 activation induces concentrative nucleoside transport in human T lymphocytes. Eur. J. Immunol. 30, 366–370 (2000).
Soler, C. et al. Regulation of nucleoside transport by lipopolysaccharide, phorbol esters, and tumor necrosis factor-α in human B-lymphocytes. J. Biol. Chem. 273, 26939–26945 (1998).
Soler, C. et al. Macrophages require different nucleoside transport systems for proliferation and activation. FASEB J. 15, 1979–1988 (2001).
Smith, C. L., Pilarski, L. M., Egerton, M. L. & Wiley, J. S. Nucleoside transport and proliferative rate in human thymocytes and lymphocytes. Blood 74, 2038–2042 (1989).
Jarvis, M. F. & Khakh, B. S. ATP-gated P2X cation-channels. Neuropharmacology 56, 208–215 (2009).
Janetopoulos, C. & Firtel, R. A. Directional sensing during chemotaxis. FEBS Lett. 582, 2075–2085 (2008). This article provides an overview of the current knowledge of the signalling requirements that are needed for efficient chemotaxis.
Choudhuri, K. & van der Merwe, P. A. Molecular mechanisms involved in T cell receptor triggering. Semin. Immunol. 19, 255–261 (2007).
Corriden, R. et al. Ecto-nucleoside triphosphate diphosphohydrolase 1 (E-NTPDase1/CD39) regulates neutrophil chemotaxis by hydrolyzing released ATP to adenosine. J. Biol. Chem. 283, 28480–28486 (2008).
Junger, W. G. Purinergic regulation of neutrophil chemotaxis. Cell. Mol. Life Sci. 65, 2528–2540 (2008).
Cronstein, B. N. Adenosine, an endogenous anti-inflammatory agent. J. Appl. Physiol. 76, 5–13 (1994).
Fredholm, B. B. Purines and neutrophil leukocytes. Gen. Pharmacol. 28, 345–350 (1997).
Ward, P. A., Cunningham, T. W., McCulloch, K. K. & Johnson, K. J. Regulatory effects of adenosine and adenine nucleotides on oxygen radical responses of neutrophils. Lab. Invest. 58, 438–447 (1988).
Zhang, Y., Palmblad, J. & Fredholm, B. B. Biphasic effect of ATP on neutrophil functions mediated by P2U and adenosine A2A receptors. Biochem. Pharmacol. 51, 957–965 (1996).
de la Harpe, J. & Nathan, C. F. Adenosine regulates the respiratory burst of cytokine-triggered human neutrophils adherent to biologic surfaces. J. Immunol. 143, 596–602 (1989).
Meshki, J., Tuluc, F., Bredetean, O., Ding, Z. & Kunapuli, S. P. Molecular mechanism of nucleotide-induced primary granule release in human neutrophils: role for the P2Y2 receptor. Am. J. Physiol. Cell Physiol. 286, C264–C271 (2004).
Verghese, M. W., Kneisler, T. B. & Boucheron, J. A. P2U agonists induce chemotaxis and actin polymerization in human neutrophils and differentiated HL60 cells. J. Biol. Chem. 271, 15597–15601 (1996).
Kronlage, M. et al. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci. Signal. 3, ra55 (2010). This report demonstrates that macrophage chemotaxis is regulated by autocrine purinergic signalling.
Orr, A. G., Orr, A. L., Li, X. J., Gross, R. E. & Traynelis, S. F. Adenosine A2A receptor mediates microglial process retraction. Nature Neurosci. 12, 872–878 (2009).
Kukulski, F. et al. Extracellular ATP and P2 receptors are required for IL-8 to induce neutrophil migration. Cytokine 46, 166–170 (2009).
Lecut, C. et al. P2X1 ion channels promote neutrophil chemotaxis through Rho kinase activation. J. Immunol. 183, 2801–2909 (2009).
Honda, S. et al. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J. Neurosci. 21, 1975–1982 (2001). This is the first report demonstrating chemotaxis of microglia towards ATP in an ATP concentration gradient field.
Inoue, K. Purinergic systems in microglia. Cell. Mol. Life Sci. 65, 3074–3080 (2008).
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nature Neurosci. 8, 752–758 (2005).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005).
Haynes, S. E. et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nature Neurosci. 9, 1512–1519 (2006).
Koizumi, S. et al. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature 446, 1091–1095 (2007).
McCloskey, M. A., Fan, Y. & Luther, S. Chemotaxis of rat mast cells toward adenine nucleotides. J. Immunol. 163, 970–977 (1999).
Liu, Q. H. et al. Expression and a role of functionally coupled P2Y receptors in human dendritic cells. FEBS Lett. 445, 402–408 (1999).
Schnurr, M. et al. ATP gradients inhibit the migratory capacity of specific human dendritic cell types: implications for P2Y11 receptor signaling. Blood 102, 613–620 (2003).
Müller, T. et al. The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy 65, 1545–1553 (2010).
Piccini, A. et al. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1β and IL-18 secretion in an autocrine way. Proc. Natl Acad. Sci. USA 105, 8067–8072 (2008).
Kukulski, F. et al. Endothelial P2Y2 receptor regulates LPS-induced neutrophil transendothelial migration in vitro. Mol. Immunol. 47, 991–999 (2010).
Ben Yebdri, F., Kukulski, F., Tremblay, A. & Sévigny, J. Concomitant activation of P2Y2 and P2Y6 receptors on monocytes is required for TLR1/2-induced neutrophil migration by regulating IL-8 secretion. Eur. J. Immunol. 39, 2885–2894 (2009).
Hyman, M. C. et al. Self-regulation of inflammatory cell trafficking in mice by the leukocyte surface apyrase CD39. J. Clin. Invest. 119, 1136–1149 (2009).
McDonald, B. et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330, 362–366 (2010). This article demonstrates that chemotaxis of neutrophils to inflammatory sites involves a complex network of short-distance and long-distance signals, but shows that ATP released at inflammatory sites is not directly involved in neutrophil chemotaxis.
Chekeni, F. B. et al. Pannexin 1 channels mediate 'find-me' signal release and membrane permeability during apoptosis. Nature 467, 863–867 (2010). This paper describes the mechanisms by which apoptotic cells release cellular ATP that serves as a danger signal.
Valitutti, S., Müller, S., Cella, M., Padovan, E. & Lanzavecchia, A. Serial triggering of many T-cell receptors by a few peptide–MHC complexes. Nature 375, 148–151 (1995).
van der Merwe, P. A. The TCR triggering puzzle. Immunity 14, 665–668 (2001).
Ma, Z. & Finkel, T. H. T cell receptor triggering by force. Trends Immunol. 31, 1–6 (2010). This review article presents evidence that mechanical forces, which are known to induce ATP release from many cell types, may have an important role in signal amplification at the immune synapse.
Canaday, D. H. et al. ATP and control of intracellular growth of mycobacteria by T cells. Infect. Immun. 70, 6456–6459 (2002).
Into, T., Okada, K., Inoue, N., Yasuda, M. & Shibata, K. Extracellular ATP regulates cell death of lymphocytes and monocytes induced by membrane-bound lipoproteins of Mycoplasma fermentans and Mycoplasma salivarium. Microbiol. Immunol. 46, 667–675 (2002).
Di Virgilio, F. et al. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood 97, 587–600 (2001).
Wang, L., Jacobsen, S. E., Bengtsson, A. & Erlinge, D. P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol. 5, 16 (2004).
Leal, D. B. et al. Characterization of NTPDase (NTPDase1; ectoapyrase; ecto-diphosphohydrolase; CD39; EC3.6.1.5) activity in human lymphocytes. Biochim. Biophys. Acta 1721, 9–15 (2005).
Lee, D. H., Park, K. S., Kong, I. D., Kim, J. W. & Han, B. G. Expression of P2 receptors in human B cells and Epstein-Barr virus-transformed lymphoblastoid cell lines. BMC Immunol. 7, 22 (2006).
Beldi, G. et al. Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice. Hepatology 51, 1702–1711 (2010).
Padeh, S., Cohen, A. & Roifman, C. M. ATP-induced activation of human B lymphocytes via P2-purinoceptors. J. Immunol. 146, 1626–1632 (1991).
Sakowicz-Burkiewicz, M., Kocbuch, K., Grden, M., Szutowicz, A. & Pawelczyk, T. Adenosine 5′-triphosphate is the predominant source of peripheral adenosine in human B lymphoblasts. J. Physiol. Pharmacol. 61, 491–499 (2010).
Gorini, S. et al. ATP secreted by endothelial cells blocks CX3CL1-elicited natural killer cell chemotaxis and cytotoxicity via P2Y11 receptor activation. Blood 116, 4492–4500 (2010).
Sumi, Y. et al. Adrenergic receptor activation involves ATP release and feedback through purinergic receptors. Am. J. Physiol. Cell Physiol. 299, C1118–C1126 (2010).
Peter, C., Wesselborg, S. & Lauber, K. Molecular suicide notes: last call from apoptosing cells. J. Mol. Cell. Biol. 2, 78–80 (2010).
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010).
Franchi, L., Warner, N., Viani, K. & Nuñez, G. Function of Nod-like receptors in microbial recognition and host defense. Immunol. Rev. 227, 106–128 (2009).
Ghiringhelli, F. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nature Med. 15, 1170–1178 (2009).
Boeynaems, J. M. & Communi, D. Modulation of inflammation by extracellular nucleotides. J. Invest. Dermatol. 126, 943–944 (2006).
Di Virgilio, F., Boeynaems, J. M. & Robson, S. C. Extracellular nucleotides as negative modulators of immunity. Curr. Opin. Pharmacol. 9, 507–713 (2009).
Kaufmann, A. et al. “Host tissue damage” signal ATP promotes non-directional migration and negatively regulates Toll-like receptor signaling in human monocytes. J. Biol. Chem. 280, 32459–32467 (2005).
Duhant, X. et al. Extracellular adenine nucleotides inhibit the activation of human CD4+ T lymphocytes. J. Immunol. 169, 15–21 (2002).
Chen, Y., Shukla, A., Namiki, S., Insel, P. A. & Junger, W. G. A putative osmoreceptor system that controls neutrophil function through the release of ATP, its conversion to adenosine, and activation of A2 adenosine and P2 receptors. J. Leukoc. Biol. 76, 245–253 (2004).
Chen, Y., Hashiguchi, N., Yip, L. & Junger, W. G. Hypertonic saline enhances neutrophil elastase release through activation of P2 and A3 receptors. Am. J. Physiol. Cell Physiol. 290, C1051–C1059 (2006).
Yip, L. et al. Hypertonic stress regulates T-cell function by the opposing actions of extracellular adenosine triphosphate and adenosine. Shock 27, 242–250 (2007).
Mizumoto, N. et al. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nature Med. 8, 358–365 (2002).
Stagg, J. & Smyth, M. J. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene 29, 5346–5358 (2010).
Otha, A. et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc. Natl Acad. Sci. USA 103, 13132–13137 (2006).
Ohtsuka, T. et al. Ecto-5′-nucleotidase (CD73) attenuates allograft airway rejection through adenosine 2A receptor stimulation. J. Immunol. 185, 1321–1329 (2010).
Sevigny, C. P. et al. Activation of adenosine 2A receptors attenuates allograft rejection and alloantigen recognition. J. Immunol. 178, 4240–4249 (2007).
Deaglio, S. et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204, 1257–1265 (2007).
Sun, X. et al. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology 139, 1030–1040 (2010).
Inoue, Y., Chen, Y., Hirsh, M. I., Yip, L. & Junger, W. G. A3 and P2Y2 receptors control the recruitment of neutrophils to the lungs in a mouse model of sepsis. Shock 30, 173–177 (2008).
Fredholm, B. B. Adenosine receptors as drug targets. Exp. Cell Res. 316, 1284–1288 (2010).
Price, M. J. Bedside evaluation of thienopyridine antiplatelet therapy. Circulation 119, 2625–2632 (2009).
Kam, P. C. & Nethery, C. M. The thienopyridine derivatives (platelet adenosine diphosphate receptor antagonists), pharmacology and clinical developments. Anaesthesia 58, 28–35 (2003).
Jacobson, K. A. & Gao, Z. G. Adenosine receptors as therapeutic targets. Nature Rev. Drug Discov. 5, 247–264 (2006).
Baraldi, P. G., Tabrizi, M. A., Gessi, S. & Borea, P. A. Adenosine receptor antagonists: translating medicinal chemistry and pharmacology into clinical utility. Chem. Rev. 108, 238–263 (2008).
Haskó, G., Linden, J., Cronstein, B. & Pacher, P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nature Rev. Drug Discov. 7, 759–770 (2008). This review article summarizes the roles of P1 receptors in immune disorders and discusses possible therapeutic strategies for targeting these receptors.
Cronstein, B. N. Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharmacol. Rev. 57, 163–172 (2005).
Cronstein, B. N., Naime, D. & Ostad, E. The anti-inflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J. Clin. Invest. 92, 2675–2682 (1993).
Baharav, E. et al. Antiinflammatory effect of A3 adenosine receptor agonists in murine autoimmune arthritis models. J. Rheumatol. 32, 469–476 (2005).
Bar-Yehuda, S. et al. The anti-inflammatory effect of A3 adenosine receptor agonists: a novel targeted therapy for rheumatoid arthritis. Expert Opin. Investig. Drugs 16, 1601–1613 (2007).
Silverman, M. H. et al. Clinical evidence for utilization of the A3 adenosine receptor as a target to treat rheumatoid arthritis: data from a phase II clinical trial. J. Rheumatol. 35, 41–48 (2008).
Corriden, R., Insel, P. A. & Junger, W. G. A novel method using fluorescence microscopy for real-time assessment of ATP release from individual cells. Am. J. Physiol. Cell Physiol. 293, C1420–C1425 (2007).
Pellegatti, P., Falzoni, S., Pinton, P., Rizzuto, R. & Di Virgilio, F. A novel recombinant plasma membrane-targeted luciferase reveals a new pathway for ATP secretion. Mol. Biol. Cell 16, 3659–3665 (2005).
Pellegatti, P. et al. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS ONE 3, e2599 (2008).
Acknowledgements
I acknowledge with great appreciation the work of my many colleagues in this field, even though much of their important work could not be cited here. I also thank my co-workers and colleagues. Special gratitude goes to Y. Chen, L. Yip, T. Woehrle, Y. Inoue, Y. Sumi and N. Hashiguchi, and to my close collaborators S. Robson and P. Insel. I also acknowledge the major funding sources that have supported the work in my laboratory: US National Institutes of Health grants GM-51477, GM-60475, AI-072287, AI-080582 and Congressionally Directed Medical Research Programs grant PR043034.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Immune synapse
-
A large junctional structure that is formed at the cell surface between a T cell and an antigen-presenting cell. It is also known as the supramolecular activation cluster. Important molecules that are involved in T cell activation — including the T cell receptor, numerous signal-transduction molecules and molecular adaptors — accumulate in an orderly manner at this site. Immune synapses are now known to also form between other types of immune cells; for example, between dendritic cells and natural killer cells.
- Find-me signal
-
A signal emitted by dying cells to promote the recruitment of scavenger cells, which clear the apoptotic cell body.
- Inflammasome
-
A large multiprotein complex comprising an NLR (NOD-like receptor), the adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD; also known as PYCARD) and pro-caspase 1. The assembly of the inflammasome leads to the activation of caspase 1, which cleaves pro-interleukin-1β (pro-IL-1β) and pro-IL-18 to generate the active pro-inflammatory cytokines.
- Connexin and pannexin hemichannels
-
Channels in the cell membrane formed from either connexin or pannexin molecules. Two hemichannels on adjacent cells can interact to form a gap junction, which allows intercellular communication by enabling the exchange of cytosolic molecules between the adjoining cells. In individual cells, connexin and pannexin hemichannels can facilitate the release of cellular ATP into the extracellular space.
- Inside-out signalling
-
The process by which intracellular signalling mechanisms result in the activation of cell surface receptors, such as integrins. By contrast, outside-in signalling is the process by which ligation of a cell surface receptor activates signalling pathways inside the cell.
Rights and permissions
About this article
Cite this article
Junger, W. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol 11, 201–212 (2011). https://doi.org/10.1038/nri2938
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri2938
This article is cited by
-
The P2X7 receptor in mucosal adaptive immunity
Purinergic Signalling (2024)
-
Deoxynivalenol triggers the expression of IL-8-related signaling cascades and decreases protein biosynthesis in primary monocyte-derived cells
Mycotoxin Research (2024)
-
Adenosine metabolized from extracellular ATP ameliorates organ injury by triggering A2BR signaling
Respiratory Research (2023)
-
CD73 mediates the therapeutic effects of endometrial regenerative cells in concanavalin A-induced hepatitis by regulating CD4+ T cells
Stem Cell Research & Therapy (2023)
-
Roles of extracellular adenosine triphosphate on the functions of periodontal ligament cells
BDJ Open (2023)