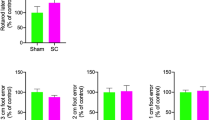
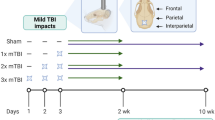
Abstract
Traumatic brain injury (TBI) is a major cause of mortality and morbidity. Various attempts have been made to replicate clinical TBI using animal models. The fluid-percussion model (FP) is one of the oldest and most commonly used models of experimentally induced TBI. Both central (CFP) and lateral (LFP) variations of the model have been used. Developed initially for use in larger species, the standard FP device was adapted more than 20 years ago to induce consistent degrees of brain injury in rodents. Recently, we developed a microprocessor-controlled, pneumatically driven instrument, micro-FP (MFP), to address operational concerns associated with the use of the standard FP device in rodents. We have characterized the MFP model with regard to injury severity according to behavioral and histological outcomes. In this protocol, we review the FP models and detail surgical procedures for LFP. The surgery involves tracheal intubation, craniotomy and fixation of Luer fittings, and induction of injury. The surgical procedure can be performed within 45–50 min.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Centers for Disease Control and Prevention. Get the Stats on Traumatic Brain Injury in the United States. Centers for Disease Control and Prevention, Atlanta, Georgia, USA. <http://www.cdc.gov/traumaticbraininjury/pdf/BlueBook_factsheet-a.pdf> (2010).
French, L.M. & Parkinson, G.W. Assessing and treating veterans with traumatic brain injury. J. Clin. Psychol. 64, 1004–1013 (2008).
Hoge, C.W. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N. Engl. J. Med. 358, 453–463 (2008).
Ellemberg, D., Henry, L.C., Macciocchi, S.N., Guskiewicz, K.M. & Broglio, S.P. Advances in sport concussion assessment: from behavioral to brain imaging measures. J. Neurotrauma 26, 2365–2382 (2009).
Denny-Brown, D. & Russell, W.R. Experimental cerebral concussion. Brain 64, 93–164 (1941).
Cernak, I. Animal models of head trauma. NeuroRx. 2, 410–422 (2005).
David, S. & Aguayo, A.J. Axonal regeneration after crush injury of rat central nervous system fibres innervating peripheral nerve grafts. J. Neurocytol. 14, 1–12 (1985).
Park, H.J. et al. Redistribution of facial nerve motor neurons after recovery from nerve crushing injury in the gerbil. Acta Otolaryngol. 115, 273–275 (1995).
Finnie, J.W. Pathology of experimental traumatic craniocerebral missile injury. J. Comp. Pathol. 108, 93–101 (1993).
Carey, M.E. Experimental missile wounding of the brain. Neurosurg. Clin. N. Am. 6, 629–642 (1995).
Goldman, H. et al. Cerebrovascular changes in a rat model of moderate closed-head injury. J. Neurotrauma 8, 129–144 (1991).
Marmarou, A. et al. A new model of diffuse brain injury in rats. Part I: pathophysiology and biomechanics. J. Neurosurg. 80, 291–330 (1994).
Foda, M.A. & Marmarou, A. A new model of diffuse brain injury in rats. Part II: morphological characterization. J. Neurosurg. 80, 301–313 (1994).
Cernak, I. et al. The pathobiology of moderate diffuse traumatic brain injury as identified using a new experimental model of injury in rats. Neurobiol. Dis. 17, 29–43 (2004).
Finnie, J.W. & Blumbergs, P.C. Traumatic brain injury. Vet. Pathol. 39, 679–689 (2002).
Blumbergs, P.C. et al. Diffuse axonal injury in head trauma. J. Neurol. Neurosurg. Psychiatry 52, 838–841 (1989).
Finnie, J.W. et al. Traumatic axonal injury in lambs: a model for paediatric axonal damage. J. Clin. Neurosci. 6, 38–42 (1999).
Betz, A.L. et al. Brain edema: a classification based on blood–brain barrier integrity. Cerebrovasc. Brain Metab. Rev. 1, 133–154 (1989).
Smith, D.H. et al. Progressive atrophy and neuron death for one year following brain trauma in the rat. J. Neurotrauma 14, 715–727 (1997).
DeKosky, S.T. et al. Secondary injury after head trauma: subacute and long-term mechanisms. Semin. Clin. Neuropsychiatry 3, 176–185 (1998).
Hamm, R.J. Neurobehavioral assessment of outcome following traumatic brain injury in rats: an evaluation of selected measures. J. Neurotrauma 18, 1207–1216 (2001).
Meythaler, J.M. et al. Current concepts: diffuse axonal injury-associated traumatic brain injury. Arch. Phys. Med. Rehabil. 82, 1461–1471 (2001).
Marguiles, S.S. & Thibault, L.E. An analytical model of traumatic diffuse brain injury. J. Biomech. Eng. 111, 241–249 (1989).
Cernak, I. et al. Blast injury from explosives munitions. J. Trauma 47, 96–103 (1999).
Cernak, I. et al. Ultrastructural and functional characteristics of blast injury-induced neurotrauma. J. Trauma 50, 695–706 (2001).
Saljo, A. et al. Blast exposure causes redistribution of phosphorylated neurofilament subunits in neurons of the adult rat brain. J. Neurotrauma 17, 719–726 (2000).
Millen, J.E. et al. A comparison of physiological responses to percussive brain trauma in dogs and sheep. J. Neurosurg. 62, 587–591 (1985).
Hayes, R.L. et al. A new model of concussive brain injury in the cat produced by extradural fluid volume loading: II. Physiological and neuropathological observations. Brain Inj. 1, 93–112 (1987).
Pfenninger, E.G. et al. Early changes of intracranial pressure, perfusion pressure, and blood flow after acute head injury. Part 1: an experimental study of the underlying pathophysiology. J. Neurosurg. 70, 774–779 (1989).
Marmarou, A. & Shima, K. Comparative studies of edema produced by fluid percussion injury with lateral and central modes of injury in cats. Adv. Neurol. 52, 233–236 (1990).
Thibault, L.E. et al. Biomechanical aspects of a fluid percussion model of brain injury. J. Neurotrauma 9, 311–322 (1992).
Härtl, R. et al. Early white blood cell dynamics after traumatic brain injury: effects on the cerebral microcirculation. J. Cereb. Blood Flow Metab. 17, 1210–1220 (1997).
McIntosh, T.K. et al. Traumatic brain injury in the rat: characterization of a midline fluid-percussion model. Cent. Nerv. Syst. Trauma 4, 119–134 (1987).
Dixon, C.E. et al. A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 67, 110–119 (1987).
Lindgren, S. & Rinder, L. Experimental studies in head injury. I. Some factors influencing results of model experiments. Biophysik 2, 320–329 (1965).
Lindgren, S. & Rinder, L. Experimental studies in head injury. II. Pressure propagation in 'percussion concussion'. Biophysik 3, 174–180 (1966).
Lindgren, S. & Rinder, L. Production and distribution of intracranial and intraspinal pressure changes at sudden extradural fluid volume input in rabbits. Acta Physiol. Scand. 76, 340–351 (1969).
Sullivan, H.G. et al. Fluid-percussion model of mechanical brain injury in the cat. J. Neurosurg. 45, 520–534 (1976).
McIntosh, T.K. et al. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience 28, 233–244 (1989).
Thompson, H.J. et al. Lateral fluid percussion brain injury: a 15-year review and evaluation. J. Neurotrauma 22, 42–75 (2005).
Morales, D.M. et al. Experimental models of traumatic brain injury: do we really need to build a better mousetrap? Neuroscience 136, 971–989 (2005).
Stalhammer, D. A new model of concussive brain injury in the cat produced by extradural fluid volume loading: I. Biomechanical properties. Brain Inj. 1, 73–91 (1987).
Schmidt, R.H. & Grady, M.S. Regional patterns of blood–brain barrier breakdown following central and lateral fluid percussion injury in rodents. J. Neurotrauma 10, 415–430 (1993).
Raghupathi, R. et al. Cellular responses to traumatic brain injury. Brain Pathol. 5, 437–442 (1995).
Graham, D.I. et al. Tissue tears in the white matter after lateral fluid percussion brain injury in the rat: relevance to human brain injury. Acta Neuropathol. (Berl) 99, 117–124 (2000).
Hilton, D.L., Jr. et al. Early assessment of neurologic deficits in the fluid percussion model of brain injury. J. Neurotrauma 10, 121–133 (1993).
Hamm, R.J. et al. Selective cognitive impairment following traumatic brain injury in rats. Behav. Brain Res. 59, 169–173 (1993).
Cortez, S.C. et al. Experimental fluid percussion brain injury: vascular disruption and neuronal and glial alterations. Brain Res. 482, 271–282 (1989).
Hicks, R. et al. Temporal and spatial characterization of neuronal injury following lateral fluid-percussion brain injury in the rat. Acta Neuropathol. 91, 236–246 (1996).
Pierce, J.E. et al. Immunohistochemical characterization of alterations in the distribution of amyloid precursor proteins and beta-amyloid peptide after experimental brain injury in the rat. J. Neurosci. 16, 1083–1090 (1996).
Bramlett, H.M. & Dietrich, W.D. Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathol. 103, 607–614 (2002).
Vink, R. et al. Small shifts in craniotomy position in the lateral fluid percussion injury model are associated with differential lesion development. J. Neurotrauma 18, 839–847 (2001).
Graham, D.I. et al. Novel aspects of the neuropathology of the vegetative state after blunt head injury. Prog. Brain Res. 150, 445–455 (2005).
Hilton, G.D. et al. Roscovitine reduces neuronal loss, glial activation, and neurologic deficits after brain trauma. J. Cereb. Blood Flow Metab. 28, 1845–1859 (2008).
Knoblach, S.M. & Faden, A.I. Administration of either anti-intercellular adhesion molecule-1 or a nonspecific control antibody improves recovery after traumatic brain injury in the rat. J. Neurotrauma 19, 115–125 (2002).
Faden, A.I. et al. Novel diketopiperazine enhances motor and cognitive recovery after traumatic brain injury in rats and shows neuroprotection in vitro and in vivo. J. Cereb. Blood Flow Metab. 23, 342–354 (2003).
Faden, A.I. Comparison of single and combination drug treatment strategies in experimental brain trauma. J. Neurotrauma 10, 91–100 (1993).
Yakovlev, A.G. et al. Activation of CPP32-like caspases contributes to neuronal apoptosis and neurological dysfunction after traumatic brain injury. J. Neurosci. 17, 7415–7424 (1997).
Di Giovanni, S. et al. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc. Natl. Acad. Sci. USA 102, 8333–8338 (2005).
Saatman, K.E. et al. Insulin-like growth factor-I (IGF-I) improves both neurological motor and cognitive outcome following experimental brain injury. Exp. Neurol. 147, 418–427 (1997).
Pierce, J.E. et al. Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience 87, 359–369 (1998).
Floyd, C.L. et al. Craniectomy position affects Morris water maze performance and hippocampal cell loss after parasagittal fluid percussion. J. Neurotrauma 19, 303–316 (2002).
Alessandri, B. et al. Cyclosporin A improves brain tissue oxygen consumption and learning/memory performance after lateral fluid percussion injury in rats. J. Neurotrauma 19, 829–841 (2002).
Liu, S. et al. Protective effect of galanin on behavioral deficits in experimental traumatic brain injury. J. Neurotrauma 11, 73–82 (2009).
Saatman, K.E. et al. Calpain inhibitor AK295 attenuates motor and cognitive deficits following experimental brain injury in the rat. Proc. Natl. Acad. Sci. USA 93, 3428–3433 (1996).
Lyeth, B.G. et al. Group I metabotropic glutamate antagonist reduces acute neuronal degeneration and behavioral deficits after traumatic brain injury in rats. Exp. Neurol. 169, 191–199 (2001).
Smith, D.H. et al. Evaluation of memory dysfunction following experimental brain injury using the Morris water maze. J. Neurotrauma 8, 259–269 (1991).
Fox, G.B. et al. Sustained sensory/motor and cognitive deficits with neuronal apoptosis following controlled cortical impact brain injury in the mouse. J. Neurotrauma 15, 599–614 (1998).
Bramlett, H.M. et al. Secondary hypoxia following moderate fluid percussion brain injury in rats exacerbates sensorimotor and cognitive deficits. J. Neurotrauma 16, 1035–1047 (1999).
Loane, D.J. et al. Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat. Med. 15, 377–379 (2009).
O'Connor, C.A. et al. Interaction between anesthesia, gender, and functional outcome task following diffuse traumatic brain injury in rats. J. Neurotrauma 20, 533–541 (2003).
Hogg, S. et al. Mild traumatic lesion of the right parietal cortex in the rat: characterization of a conditioned freezing deficit and its reversal by dizocilpine. Behav. Brain Res. 93, 157–165 (1998).
Sönmez, U. et al. Neuroprotective effects of resveratrol against traumatic brain injury in immature rats. Neurosci. Lett. 420, 133–137 (2007).
Albensi, B.M. et al. Diffusion and high resolution MRI of traumatic brain injury in rats: time course and correlation with histology. Exp. Neurol. 162, 61–72 (2000).
Andrews, J.S. et al. Performance of four different rat strains in the autoshaping, two-object discrimination, and swim maze tests of learning and memory. Physiol. Behav. 57, 785–790 (1995).
Tan, A.A. et al. Strain differences in response to traumatic brain injury in Long-Evans compared to Sprague-Dawley rats. J. Neurotrauma 26, 539–548 (2009).
Avila, M.A. et al. L-arginine decreases fluid-percussion injury-induced neuronal nitrotyrosine immunoreactivity in rats. J. Cereb. Blood Flow Metab. 28, 1733–1741 (2008).
Leonard, J.R. et al. Fluid percussion injury causes disruption of the septohippocampal pathway in the rat. Exp. Neurol. 143, 177–187 (1997).
Hare, G.M.T. et al. Severe hemodilutional anemia increases cerebral tissue injury following acute neurotrauma. J. Appl. Physiol. 103, 1021–1029 (2007).
Lifshitz, J. et al. Acute cognitive improvement after lateral fluid percussion brain injury recovers by 1 month: evaluation by conditioned fear response. Behav. Brain Res. 177, 347–357 (2007).
Phillips, J.J. et al. Glutamate antagonism during secondary deafferentation enhances cognition and axo-dendritic integrity after traumatic brain injury. Hippocampus 8, 390–401 (1998).
Prins, M.L. et al. Fluid percussion brain injury in the developing and adult rat: a comparative study of mortality, morphology, intracranial pressure and mean arterial blood pressure. Brain Res. Dev. Brain Res. 95, 272–282 (1996).
Hoh, T. et al. Complex behavioral strategy and reversal learning in the water maze without NMDA receptor-dependent long-term potentiation. J. Neurosci. 19, RC2 (1999).
Vorhees, C.V. & Williams, M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 1, 848–858 (2006).
Acknowledgements
This study was supported by the National Institutes of Health Grant no. NS052568. We would like to thank D.J. Loane for his helpful comments on the paper.
Author information
Authors and Affiliations
Contributions
The project was conceived by A.I.F and was supported by the National Institutes of Health Grant no. NS052568 to A.I.F. The study was designed by A.I.F., B.A.S. and G.D.H. D.N.Z. originally developed the model. G.D.H. and S.V.K. equally contributed to the paper in terms of conducting the study, compiling the results and writing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kabadi, S., Hilton, G., Stoica, B. et al. Fluid-percussion–induced traumatic brain injury model in rats. Nat Protoc 5, 1552–1563 (2010). https://doi.org/10.1038/nprot.2010.112
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2010.112
This article is cited by
-
White and gray matter integrity evaluated by MRI-DTI can serve as noninvasive and reliable indicators of structural and functional alterations in chronic neurotrauma
Scientific Reports (2024)
-
Blood glutamate scavenging as a novel glutamate-based therapeutic approach for post-traumatic brain injury anxiety and social impairment
Translational Psychiatry (2023)
-
Effect of stress on the rehabilitation performance of rats with repetitive mild fluid percussion-induced traumatic brain injuries
Cognitive Neurodynamics (2023)
-
A novel simple traumatic brain injury mouse model
Chinese Neurosurgical Journal (2022)
-
Traumatic Brain Injury Models in Zebrafish (Danio rerio)
Neuroscience and Behavioral Physiology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.