Abstract
Anxiety disorders are commonly associated with increased generalization of fear from a stress- or trauma-associated environment to a neutral context or environment. Differences in context-associated memory in males and females may contribute to increased susceptibility to anxiety disorders in women. Here we examined sex differences in context fear generalization and its neural correlates. We observed stronger context fear conditioning and more generalization of fear to a similar context in females than males. In addition, context preexposure increased fear conditioning in males and decreased generalization in females. Accordingly, males showed stronger cFos activity in dorsal hippocampus during memory retrieval and context generalization, whereas females showed preferential recruitment of basal amygdala. Together, these findings are consistent with previous research showing that hippocampal activity correlates with reduced context fear generalization. Differential competition between hippocampus and amygdala-dependent processes may thus contribute to sex differences in retrieval of context fear and greater generalization of fear-associated memory.
Similar content being viewed by others
INTRODUCTION
Women are more susceptible to disorders of fear and anxiety than men, with the prevalence of anxiety disorders and posttraumatic stress disorder (PTSD) two- to three-fold higher in women (Kessler et al, 1995, 2012). Whereas normal fear responses are triggered by trauma-associated contexts, in disorders such as PTSD, fear is also elicited in neutral or safe contexts (Kindt, 2014; Lissek et al, 2010, 2014; Lopresto et al, 2015). Excessive generalization of fear may contribute to increased susceptibility to disorders of fear and anxiety. Therefore, in this project we examined whether females show stronger generalization of context fear, and how neural correlates of retrieval and generalization differ between females and males.
One potential mechanism for generalization of context-dependent fear is the failure to learn a complete representation of a context associated with an aversive event, resulting in an inability to distinguish between a dangerous context and a neutral place (Rudy and O’Reilly, 1999; Westbrook et al, 1994). Alternatively, failure to retrieve detailed context information at remote timepoints also results in increased generalization of fear (Wiltgen and Silva, 2007; Wiltgen et al, 2010). Separate studies have demonstrated that females have slower acquisition of context representations (Wiltgen et al, 2001), more rapid loss of context specificity with time (Lynch et al, 2013), and are less adept at pattern separation compared with males (Yagi et al, 2015). Therefore, altered formation or retrieval of context representations in females may result in increased generalization of context fear memories.
Learning about context is a critical contributor to sex differences in context fear conditioning, where males are reported as showing stronger context fear conditioning (Maren et al, 1994; Mizuno et al, 2012; Gresack et al, 2009) and more activation of hippocampus (Gresack et al, 2009; Kudo et al, 2004; Maren et al, 1994) during acquisition and consolidation of context fear conditioning compared with females. Given the role of dorsal hippocampus in context representations (Biedenkapp and Rudy, 2007; Wiltgen et al, 2010; Zelikowsky et al, 2014), and the requirement of context representation for context fear conditioning (Barrientos et al, 2002), these findings suggest that males have stronger encoding of context representations and thus more precise context fear memory.
In contrast, additional studies demonstrate that sex differences in context fear conditioning are more complex than a failure of females to acquire context representations or context–shock associations. First, there is limited evidence for stronger fear conditioning in males compared with females. Many studies fail to show sex differences in context fear conditioning (Dachtler et al, 2011; Kosten et al, 2006), or only observe sex differences under specific experimental conditions such as very short context exposures (Wiltgen et al, 2001) or in specific strains (Pryce et al, 1999). Other studies show stronger context fear conditioning in females (Moore et al, 2010; Ris et al, 2005), or do not use sex as a variable in analyses (Temme et al, 2014). Second, sex differences in retrieval of fear-associated memories suggest that this process, and not memory formation, causes differential fear responses at test. In females, for example, hippocampal estradiol causes decreased retrieval of hippocampal-dependent memory (Lynch et al, 2014), whereas in males testosterone suppressed amygdala activation (Chen et al, 2014). This pattern of results suggests that females shift away from hippocampal processing during retrieval of fear-associated memories, and males shift toward hippocampal processing. Given competition between hippocampus and basal amygdala in context fear conditioning (Biedenkapp and Rudy, 2009) and the importance of basal amygdala in context fear conditioning (Amano et al, 2012; Jin and Maren, 2015; Matus-Amat et al, 2007), females might shift toward basal amygdala activation during memory retrieval. Sex-biased patterns of hippocampus and amygdala mechanisms during retrieval may thereby result in greater generalization of context fear in females compared with males.
Here we examined whether males and females differ in foreground context fear conditioning and context generalization, as well as how the sexes differ in activation of hippocampus and basal amygdala after memory retrieval. We used foreground context fear conditioning alone to examine sex differences in generalization, and context fear conditioning after preexposure to the training context to manipulate the strength of context representations. To control for nonspecific effects of handling and exploration of a novel context on context generalization, we used preexposure to a similar context. We demonstrated that in context fear conditioning alone, females exhibited stronger fear conditioning and more generalization of context fear than males. Preexposure to the training context reduced generalization in females. In addition, we observed weaker cFos activation in dorsal hippocampus, and stronger activation in basal amygdala during memory retrieval in females compared with males.
MATERIALS AND METHODS
Animals
The 9-week-old C57BL/6 mice (72 males, 73 females) from Envigo (Indianapolis, IN) were individually housed throughout experiments with standard diet and water ad libitum. Individual housing in males is required to reduce fighting-induced stress (Meakin et al, 2013), and is consistent with both previous fear conditioning studies (see, eg, Van Craenendonck and Ver Donck, 2014; Radulovic et al, 1998; Tanaka et al, 2014; Tronson et al, 2009) and University of Michigan Institutional Care and use Committee policies on management of fighting in mice, and does not increase variance in either sex (Prendergast et al, 2014). Because of independent social structures of both male and female mice (Becker and Koob, 2016), individual housing is ecologically appropriate for both sexes. The colony room was adjacent to behavioral testing rooms and maintained at 20±2 °C with a 12 h 0700 : 1900 h light/dark cycle (lights on at 0700 h). All mice were acclimated to the colony room for at least 7 days before experiments began. All experimenters in this study were women (Sorge et al, 2014). The University of Michigan Committee on the Use and Care of Animals approved all experimental methods performed in this research.
Apparatus
Training and testing conditions were performed in conditioning chambers (9 3/4′′ × 12 3/4′′ × 9 3/4′′; MedAssociates, VT), enclosed in sound-attenuating cubicles, equipped with a NIR camera (VID-CAM-MONO-2A). Grid floor rods were connected to a shock generator. Male and female mice were tested in separate chambers and chambers were cleaned between each animal with either 70% ethanol or 1% acetic acid. Video Freeze software (MedAssociates) automatically scored freezing and locomotor activity. Two experimenters, blind to experimental conditions, hand scored freezing to verify automatic scoring.
Three different contexts were used: (1) Training context: context A (CxtA) consisted of a rectangular box with white walls, lights on, an evenly sized grid floor (36 stainless steel rods, 1/8′′ diameter, spaced 1/4′′ apart), and 70% ethanol odor. (2) Generalization context: context B (CxtB) had curved white walls, house lights off, the same floors, and ethanol odor as in CxtA. (3) Distinct context: context C (CxtC) consisted of black angled walls, house lights off, staggered grid floors with alternating 1/8′′ and 3/16′′ grid rods, and 1% acetic acid odor.
Context Fear Conditioning
Foreground context fear conditioning was conducted as previously described (Tronson et al, 2009). Briefly, mice were placed in CxtA for 3 min, followed by delivery of a 2 s, 0.8 mA footshock. Mice were then replaced in their home cage and returned to the colony room.
Retrieval and Generalization Tests
At 24 h after training, mice were placed into CxtA or CxtB for 3 min and immediately returned to the colony room. Freezing was assessed during this time. Mice were retested at 24 h intervals first in the reverse context (CxtB or CxtA) and subsequently in CxtC (ie, test order ABC or BAC; Figure 1). All behavioral experiments used a group size of n=8 males and n=8 females per test order.
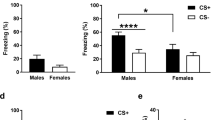
Females show more generalization of context fear than males. (a) Experimental design. (b) Females showed significantly greater freezing than males in the training context (CxtA), and more generalization to CxtB. Both males and females showed low levels of freezing in CxtC. There was no difference in freezing between different test orders (ABC, white background; BAC, dotted background). (c) Males (striped bars) had higher discrimination scores (DS) than females (white bars). Error bars represent SEM. *P<0.05, **P<0.01, ***P<0.001 cf CxtA. #P<0.05, ##P<0.01 cf females.
Context Preexposure
To determine whether prior exposure to training or test contexts decreased generalization, mice were placed in either CxtA (Figure 2) or CxtB (Figure 3) for 10 min and returned to their home cage. At 24 h after context exposure, mice were fear conditioned in CxtA and tested in CxtA, B, and C, as described above.
Preexposure to CxtA reduces generalization of context fear in females. (a) Experimental design. (b) Preexposure to CxtA effectively reduced context generalization to CxtB in females, only with ABC test order (ABC, white background; BAC, dotted background). Males showed increased freezing in CxtA compared with non-preexposed males (P<0.001). (c) Both males (striped bars) and females (white bars) showed strong discrimination when tested first in CxtA (ABC test order), whereas BAC females showed significantly more generalization. Error bars represent SEM. *P<0.05, ***P<0.001 cf CxtA. ^P<0.05, cf ABC test order.
Preexposure to CxtB increases generalization of context fear in males. (a) Experimental design. (b) There were no sex differences in freezing in context. Both males and females showed generalization between CxtA and CxtB. Test order did not affect freezing or generalization (ABC, white background; BAC, dotted background). (c) Discrimination scores did not differ between males (lined bars) and females (white bars). Error bars represent SEM. *P<0.05, **P<0.01, ***P<0.001 cf CxtA.
Estrous Cycle
Estrous samples were collected at approximately the same time each day, 1 h before behavioral experiments. To determine estrous phase in female mice, wet vaginal smears were taken in 5 μl of distilled deionized water. Fluid was pipetted 3–4 times and dropped on slides. Light microscopy was used to assess vaginal cytology and determine estrous stage (Caligioni, 2009).
Discrimination Score (DS)
We calculated a DS to compare context generalization across experiments, taking into account variability in freezing. We used the following formula: (CxtA−CxtB)/(CxtA−CxtC), where CxtA, CxtB, and CxtC represent freezing in each context. This formula compares the difference in freezing in the similar contexts (CxtA and CxtB) with difference in freezing in distinct contexts (CxtA and CxtC). Because others have demonstrated good discrimination between two distinctive contexts (eg, Cxts A and C) (Wiltgen et al, 2010), freezing in CxtC represents freezing to nonspecific factors including transportation and handling (Rudy and O’Reilly, 2001) that trigger some retrieval of prior experience and thus small increases in freezing. In addition, experimentally, tests in CxtC controlled for different levels of freezing between the sexes in a novel context. Including these data in the discrimination score performs the same function mathematically.
cFos Immunohistochemical Analysis
Mice were deeply anesthetized (avertin, 480 mg/kg, i.p.) and transcardially perfused with 4% paraformaldehyde 1 h after fear conditioning (naive: n=4 per sex, fear conditioning: male n=4, female n=5), or after retrieval in CxtA or CxtB (naive n=4, CxtA n=6, CxtB: n=6 per sex). Standard immunohistochemistry protocols were used, as previously described (Tronson et al, 2009). Briefly, sections were incubated with anti-cFos (EMD Millipore; 1 : 6250), goat anti-mouse secondary (VectorLabs; 1 : 200), and DAB chromogen (Sigma Aldrich, St Louis, MO). Quantification of cFos+ cells was conducted by investigators blind to experimental group in the following brain regions: hippocampal CA1, CA3, DG, and basal amygdala (BA that included both basolateral and basomedial amygdala; see Amano et al, 2012). Sections for all brain regions were cut at 40 μM and selected at the same level (−1.79 mm AP relative to bregma, Paxinos and Franklin, 2013). Regions for quantification were defined by ImageJ (NIH, Bethesda, MD) and held constant across animals. cFos+ counts were normalized to the number of cells in naive animals.
Statistical Analysis
All statistical analyses were conducted using SPSS v23. Three-way repeated measures ANOVA (Context × Order × Sex) was used to determine sex differences in generalization of context fear. Separate two-way analyses were conducted on the discrimination score (Sex × Order), and to compare discrimination scores or freezing of females and males between experiments (Sex × Preexposure). For cFos experiments we used two-way (Sex × Context) ANOVA to determine sex differences in cFos+ cells after retrieval or consolidation of context fear. A two-way multivariate ANOVA (Estrous × Preexposure) was used to determine the effect of estrous on context fear conditioning. The post hoc tests with Bonferroni corrections for multiple tests were used to further examine significant effects in each experiment. The 95% confidence intervals are reported for post hoc tests. Partial η2 (ηp2) is reported as effect size estimate (0.01, 0.06, and 0.14 as small, medium, and large, respectively), as calculated by SPSS.
RESULTS
Sex Differences in Context Fear Conditioning
After single trial context fear conditioning, females showed more freezing in the training context (CxtA) compared with males (Figure 1b) (Sex: F(1, 28)=12.29, P=0.002, ηp2=0.31; ABC: P=0.041 (95% CI: 0.86–39.14); BAC: P=0.027 (95% CI: 2.61–40.89)).
Estrous Cycle Did Not Affect Context Fear Conditioning
Context fear conditioning in females was not affected by estrous phase on training day. In the context fear conditioning alone condition, females showed similar freezing in CxtA (F(3, 12)=1.50, P=0.265), CxtB (F(3, 12)=1.06, P=0.40), or CxtC (F(3, 12)=1.04, P=0.41) regardless of estrous phase (proestrous n=3, estrous n=4, metestrous n=5, diestrous n=4). To ensure adequate statistical power, we examined the impact of estrous cycle across all behavioral experiments. Freezing was not affected by estrous at training in any test context (Main Effect Estrous: CxtA: F(3, 48)=0.643, P=0.59, CxtB: F(3, 48) =1.873, P=0.152; CxtC: F(3, 48)=0.28, P=0.84) or in any preexposure condition (Preexposure × Estrous interaction, CxtA: F(6, 48)=0.76, P=0.61; CxtB: F(6, 48) =1.74, P=0.14; CxtC: F(6, 48)=1.31, P=0.28).
Sex Differences in Generalization of Context Fear
Females showed greater generalization of fear between CxtA and CxtB compared with males (Context × Sex interaction: F(2, 56)=5.62, P<0.003, ηp2=0.17), with similar freezing levels in CxtA and CxtB regardless of test order (ABC: P=0.073; BAC: P=0.104), and significantly less freezing in CxtC (ABC: P<0.0001 (95% CI: 26.52–49.73); BAC: P<0.0001 (95% CI: 24.02–47.23); Figure 1b). In contrast, males showed good discrimination of context fear with significantly less freezing in CxtB (ABC: P=0.001 (95% CI: 4.27–28.98); BAC: P=0.029 (95% CI: 1.52–26.23)) and less freezing in CxtC (ABC: P=0.003 (95% CI: 6.52–29.73); BAC: P<0.0001 (95% CI: 17.39–40.61)) compared with CxtA (Figure 1b).
Both males and females showed low levels of freezing in CxtC, and this did not differ between the sexes (ABC: P=1.00 (95% CI: −15.76 to 15.76); BAC P=0.06 (95% CI: −0.64 to 30.89); Figure 1b). Generalization between similar contexts by females is thus because of its similarity with the training context and not a nonspecific aversion to novel contexts.
To determine whether generalization by females was a function of stronger fear in CxtA, we calculated DS. Here, males had a significantly higher DS (DS=0.78) than females (DS=0.1) (F(1, 28)=5.58, P=0.016, ηp2=0.18), demonstrating stronger generalization of fear from CxtA to CxtB in females (Figure 1c).
Preexposure to Training Context Reduced Fear Generalization in Females
Preexposure to the training context decreased generalization of context fear in females. After preexposure to CxtA and training in the same context, both male and female mice showed more freezing in CxtA than CxtB or CxtC (Context: F(2, 56)=249.87, P<0.0001, ηp2=0.90); Figure 2b), with no sex differences in freezing or context generalization (Sex F(1, 28)<1; Sex × Context F(2, 56)<1). Preexposure to CxtA resulted in discrimination between CxtA and CxtB by females (ABC: P<0.0001 (95% CI: 40.36–60.15); BAC: P=0.048 (95% CI: 0.11–19.90), cf CxtA). Males continued to show little generalization between CxtA and CxtB (ABC: P<0.0001 (95% CI: 29.73–49.52); BAC: P<0.0001 (95% CI: 15.86–35.65) cf CxtA); Figure 2b).
When CxtB was tested before CxtA, both males and females showed stronger context generalization compared with animals tested in CxtA first (Context × Order: F(2, 56)=19.57, P<0.0001, ηp2=0.41), and this effect was more pronounced in females (Context × Sex × Order: F(2, 56)=3.97, P=0.024, ηp2=0.12; Figure 2b). Analysis of discrimination scores confirmed these test order effects (Order: F(1, 28)=37.77, P<0.0001; Females: P<0.0001 (95% CI: 0.44–0.91); Males: P=0.009 (95% CI: 0.09–0.55); Sex × Order: F(1, 28)=4.81, P=0.037, ηp2=0.15; Figure 2c).
Compared with the previous experiment, preexposure to CxtA significantly increased the discrimination scores of females (P=0.018 (95% CI: 0.08–0.80)) but not males (P=0.41; Figures 1c and 2c). Preexposure to the training context thus reduced context fear generalization in females.
Preexposure to CxtA eliminated the sex differences in freezing in CxtA (Figure 2a), with only males exhibiting more freezing after preexposure compared with context fear conditioning alone (2-way ANOVA PreExp: F(1, 60)=22.51, P<0.0001, ηp2=0.27; Sex: F(1, 60)=7.34, P=0.009, ηp2=0.11; PreExp × Sex: F(1, 60)=6.14, P=0.002, ηp2=0.10; males: P<0.001 (95% CI: 17.68–40.45); females: P=0.114).
Preexposure to CxtB Increased Generalization of Context Fear in Males
After preexposure to CxtB, there were no sex differences in freezing or context generalization (Context F(2, 56)=190.98, P<0.0001, ηp2=0.87; Sex: largest F(1, 28)<1; Figure 3a). Both males and females showed a small but significant decrease of freezing in CxtB compared with CxtA (Females: ABC: P=0.002 (95% CI: 7.40–29.35), BAC: P=0.033 (95% CI: 1.03–22.98); Males: ABC: P=0.002 (95% CI: 6.90–28.85), BAC: P<0.0001 (95% CI: 11.53–33.48)) and substantially less freezing in CxtC (all P<0.0001; Figure 3b). Discrimination scores did not differ between Context, Order, or Sex (largest F=1.02, P=0.32), demonstrating no sex differences in context generalization after preexposure to CxtB (Figure 3c).
In comparison to context fear conditioning alone, preexposure to CxtB resulted in higher freezing to CxtA, but only in males (PreExp: F(1, 60)=11.09, P=0.001, ηp2=0.16; Sex: F(1, 60)=5.64, P=0.021, ηp2=0.09; PreExp × Sex: F(1, 60)=7.70, P=0.007, ηp2=0.11). In males, freezing increased after preexposure to CxtB in both CxtA (P<0.0001 (95% CI: 13.28–36.22)) and CxtB (P=0.001 (95% CI: 8.35–31.27); Figures 1b and 3b). Accordingly, context generalization was high in males preexposed to CxtB, with lower discrimination scores compared with fear conditioning alone (P=0.044 (95% CI: 0.01–0.736); Figures 1c and 3c). Preexposure to CxtB did not alter generalization in females (P=0.29).
Sex Differences in Dorsal Hippocampus cFos Activation after Context Fear Memory Retrieval
We observed more cFos+ cells in males compared with females in CA1 (Figure 4a), CA3 (Figure 4b), and DG (Figure 4c) after retrieval of context fear conditioning with significant main effects of sex in all regions (CA1: F(1, 26)=4.6, P=0.041, ηp2=0.15; CA3: F(1, 26)=2.90, P=0.101; DG: F(1, 26)=7.36, P=0.012, ηp2=0.22) and a main effect of test in CA1 and CA3 (CA1: F(1, 26)=7.32, P=0.003, ηp2=0.36; CA3: F(2, 26)=3.43, P=0.047, ηp2=0.21; DG: F(2, 26)=1.16, P=0.33). Males exhibited strong activation of cFos after test in either training (CxtA) or generalization context (CxtB) compared with naive in both CA1 (CxtA: P=0.002 (95% CI: 1.47–4.26); CxtB: P=0.013 (95% CI: 1.15–3.94)) and CA3 (CxtA: P=0.020 (95% CI: 0.30–3.27); CxtB: P=0.039 (95% CI: 0.09–3.05)). In contrast, females showed a significant increase in cFos only in CA1 after test in CxtA (CA1: CxtA: P=0.05 (95% CI: −0.04 to 5.03); CA3: CxtA P=0.27) but not CxtB (CA1: CxtB: P=0.661; CA3: CxtB: P=0.51). In CA1, cFos+ cells were similar for both males and females in the training context (CA1: CxtA: P=0.136), and significantly greater in males in the generalization context (CA1: CxtB: P=0.020 (95% CI: 0.55–5.97)). These findings suggest that CA1 is activated during retrieval of context for both males and females but a similar context recruits cFos in this region only in males (Figure 4a).
Sex differences in dorsal hippocampus and amygdala cFos activity during retrieval and generalization of context fear. (a) Both males and females show increased cFos+ cells in CA1 following test in CxtA. Only males show increases in CA1 during generalization test in CxtB. (b) Males but not females show increased cFos positive cells in CA3 during tests in CxtA and CxtB. (c) In DG, males but not females show increased cFos+ cells during memory retrieval in CxtA. (d) In basal amygdala, females showed increased cFos+ cells after retrieval in CxtA and generalization test in CxtB. All data are normalized to naive levels. +P=0.05, *P<0.05, **P<0.01, ***P<0.001 cf same sex naive. Representative images from each group show cFos+ cells.
Similarly, in DG, cFos+ cells were increased in males, only after test in CxtA (Sex: F(1, 26)=7.36, P=0.012, ηp2=0.22; Test: F(2, 26)=1.16, P=0.33; interaction: F(2, 26)=1.94, P=0.17; CxtA: P=0.025 (95% CI: 0.09–1.23); CxtB: P=0.27), whereas females showed no increases in cFos (CxtA: P=0.69; CxtB: P=0.54; Figure 4c).
Sex Differences in Basal Amygdala cFos Activation after Context Fear Memory Retrieval
In the basal amygdala (Figure 4d), females but not males showed an increased number of cFos+ cells (Sex: F(1, 26)=10.74, P=0.003, ηp2=0.29; Condition F(2, ,26)=9.19, P=0.001, ηp2=0.41; Interaction: F(2, 26)=2.14, P=0.14). In females, testing in CxtA (P<0.0001 (95% CI: 1.47–4.26)) or CxtB (P=0.001 (95% CI: 1.15–3.94)) resulted in increased cFos+ cells compared with naives. In contrast, no such activation was observed in males (CxtA: P=0.18; CxtB: P=0.15). After both tests, the number of cFos+ cells in basal amygdala was significantly higher in females compared with males (CxtA: P=0.003 (95% CI: 0.76–3.26); CxtB: P=0.012 (95% CI: 0.38–2.88); Figure 4d).
Together, these results suggest that males and females differentially recruit fear memory-associated brain regions during retrieval, with males showing preferential cFos activation of hippocampus and females preferentially activating cFos in amygdala.
Males and Females Show Similar cFos Activation in Dorsal Hippocampus and Amygdala during Memory Consolidation
After training, cFos+ cells were increased in males and females in CA1 (Training: F(1,14)=18.92, P=0.001, ηp2=0.58; Sex: F(1, 14)<1; Sex × Training: F(1, 14)<1; Figure 5a) and in basal amygdala (Training: F(1, 14)=19.55, P=0.001, ηp2=0.58; Sex: F(1, 14)<1; Sex × Training: F(1, 14)<1; Figure 5d) after context fear conditioning. The post hoc tests confirmed increased cFos in both females (CA1: P=0.005 (95% CI: 0.70–3.30); BA: P=0.003 (95% CI: 0.69–2.74)) and males (CA1: P=0.012 (95% CI: 0.50–3.41); BA: P=0.017 (95% CI: 0.31–2.60)). These findings suggest that males and females show similar activation of cFos in hippocampus and amygdala during consolidation of context fear conditioning.
No sex differences in dorsal hippocampus and amygdala cFos activity during consolidation of context fear conditioning. (a) Both males and females show more cFos+ cells in CA1 after context fear conditioning. (b, c) Neither males nor females show elevated cFos in CA3 (b) or DG (c) after fear conditioning. (d) Males and females show similar cFos activation in basal amygdala after context fear conditioning. All data are normalized to naive levels. Error bars indicate SEM. BA, basal amygdala; CFC, context fear conditioning; DG, dentate gyrus. *P<0.05, **P<0.01 cf same sex naive.
DISCUSSION
Here we demonstrated that females show greater generalization of context fear conditioning than males as well as sex-specific patterns of retrieval-induced cFos in hippocampus and amygdala. Together with previous findings demonstrating that hippocampal activation correlates with less generalization of context fear in males (Wiltgen et al, 2010) because of its role in retrieval of detailed information about context (Gafford et al, 2013), our results suggest that males and females utilize different neural correlates or molecular mechanisms in retrieval of context-associated memories.
Our observation that females showed less context specificity of context fear conditioning than males is consistent with studies demonstrating sex differences in learning and consolidation of context representations (Wiltgen et al, 2001, 2010) and in hippocampal mechanisms of context fear conditioning (Antunes-Martins et al, 2007; Dachtler et al, 2011; Mizuno et al, 2006, 2007; Moore et al, 2010). Our data further demonstrate that sex differences in context fear-associated memory and hippocampal activity occur even when both males and females show strong context fear responses. These findings add to the growing consideration of sex differences as complex and nuanced. Previous studies of context fear conditioning have demonstrated male-specific mechanisms of context fear conditioning (Antunes-Martins et al, 2007; Dachtler et al, 2011; Mizuno et al, 2006; Moore et al, 2010). This is the first paper to observe both male- and female-biased neural correlates of fear-associated memory retrieval, thereby providing some initial insight into how (Maney, 2016; De Vries, 2004) males and females differ in this task.
Sex differences in context fear conditioning in this task cannot be simply attributed to less efficient formation of context representations in females. Preexposure to the training context appeared to enhance context fear conditioning in males, consistent with prior evidence that stronger context representations are required for formation of context–shock associations (Fanselow, 1990; Rudy and O’Reilly, 1999). In contrast, females did not show better context fear conditioning after context preexposure, but instead decreased generalization between similar contexts. These results demonstrate that both males and females require additional time to learn detailed representations of context but might use context information in different ways. Whereas females are biased toward increased freezing in ambiguous contexts, males are biased toward increased freezing only in contexts that are strongly associated with aversive events. These findings raise the intriguing possibility that sex differences in generalization between similar contexts are due to differences in retrieval of context information.
Supporting the role of retrieval mechanisms in context generalization, in females, preexposure to the training context decreased generalization in a test-order-specific manner—ie, only when tested first in the training context. This has several implications: first, learning about CxtA before context fear conditioning is not sufficient to reduce context generalization as female mice tested in test order CxtB and then CxtA continued to generalize. Second, as females tested in test order CxtA and then CxtB show robust discrimination, retrieval in CxtA and postretrieval processing such as extinction may contribute to reduced ambiguity and thus decreased fear in subsequent exposures to a similar context.
Counter to our expectations that preexposure to CxtB would not affect context generalization, we observed increased generalization in males, suggesting that retrieval of the CxtA representation is less specific than after context fear conditioning alone. This effect is somewhat surprising, as learning about CxtB (with no shock) and CxtA (paired with shock) should result in no impairment, and potentially enhance, context discrimination. Our results are consistent, however, with findings that preexposure to a context followed by an immediate footshock in a different box results in conditioned fear to the preexposure context (Bae et al, 2015; Rudy and O’Reilly, 2001). Our data extend these findings to show that this overgeneralization effect occurs even when male mice are given sufficient time to form a conjunctive hippocampal representation of the training context. In contrast, preexposure to CxtB in males, similarly to CxtA, increased freezing levels to that of females. These data suggest that after preexposure to CxtB, males are unable to retrieve a sufficiently distinct memory of the training context to suppress freezing in a similar context.
Our data exclude the possibility that increased generalization by females is due to more defensive behaviors in novel contexts. First, males and females did not differ in freezing to a very different context, despite its novelty. Second, after preexposure to the training context, males and females showed equivalent low levels of freezing to the novel CxtB. Third, after preexposure to CxtB, both males and females showed strong freezing in CxtB, despite its familiarity. Finally, recent data suggest that in contrast to showing more freezing, females are more likely to show active behavioral responses to shock-associated stimuli (Gruene et al, 2015a). Stronger generalization in females, therefore, cannot be explained by a greater propensity to express freezing responses in novel situations and must therefore be due to sex differences in memory processes.
In accordance with a retrieval-based account of context fear generalization, we observed sex differences in cFos activation in hippocampus and basal amygdala after memory retrieval, but not consolidation. Specifically, we observed that males had stronger activation in dorsal hippocampus after tests in both CxtA and CxtB, whereas females had greater amygdala activation. That cFos activity did not correlate with levels of freezing behavior in either males or females is consistent with previous work demonstrating that hippocampal cFos production does not drive freezing behavior (Tronson et al, 2009; Wiltgen et al, 2010). Sex differences in hippocampal cFos activation suggest that males and females utilize either different underlying molecular mechanisms or an overlapping subset of brain regions in the retrieval of memories for fear-associated contexts.
It is noteworthy that cFos activation does not only reflect memory retrieval. In the hippocampus, cFos has been strongly linked with novelty detection (Radulovic and Tronson, 2010; Radulovic et al, 1998; Yochiy et al, 2012) and habituates with repeated exposure to a context (Radulovic et al, 1998; Tronson et al, 2009). Within this conceptual framework, our data suggest that females retrieve a strong context representation in CxtA, where less hippocampal cFos represents recognition that CxtA is familiar. Furthermore, it suggests that failure to upregulate cFos in CxtB reflects recognition of the similarity between contexts by females, thus triggering fear responses in CxtB. In contrast, robust cFos in both CxtA and CxtB by males suggests that both contexts are perceived as somewhat new, resulting in less freezing compared with females. This interpretation is also consistent with previous findings demonstrating an inverse relationship between hippocampal cFos levels and generalization of context-related fear (Wiltgen et al, 2010), where freezing to a generalization context is high when cFos is low (ie, recognized as similar to the training context), and freezing is low when cFos is high (ie, the generalization context is perceived as distinct from the training context and therefore novel). This conceptual framework also suggests that sex differences in hippocampal and amygdalar cFos levels during retrieval and generalization of context fear conditioning suggest that males and females are either using different processing strategies or using different circuit or molecular mechanisms during retrieval of context-associated fear.
Given that hippocampus is associated with detailed memory for context (Gafford et al, 2013) whereas amygdala is associated with emotional information (Cahill et al, 2001) and memory ‘gist’ (Adolphs et al, 2001), these findings also suggest that males and females show biases in retrieval of affective and contextual information. This possibility parallels findings from spatial memory tasks where sex differences are reliably observed in the kind of information and strategies preferentially used (Chai and Jacobs, 2010; Keeley et al, 2013; Rodríguez et al, 2010; Yagi et al, 2015). Whether males and females similarly show different dominant strategies for context fear conditioning remains unknown.
Sex differences in recruitment of brain regions during retrieval may result from differential competition between memory systems. It is well known that during aversive memory tasks, for example, hippocampal activity can result in suppression of amygdala processes, and vice versa (Biedenkapp and Rudy, 2009; McDonald and White, 1995; Mcintyre et al, 2002). In females, activation of amygdala may therefore modulate hippocampal activity, resulting in greater defensive responses and less accurate retrieval of detailed context information. Recent evidence, however, suggests that (in males) optogenetic stimulation of dentate gyrus cells tagged during consolidation of fear conditioning is sufficient to retrieve a context fear memory (Liu et al, 2012), in part via connections with basal amygdala (Ryan et al, 2015). That hippocampal–amygdala interactions can either act cooperatively or competitively further suggests that different circuits or molecular mechanisms activated in males and females may result in very different behavioral outcomes.
Other brain regions may also be differentially required for retrieval of context fear memories in males and females and involved in competition between memory systems. For example, retrosplenial cortex is required for retrieval of recent and remote context fear memories in males (Corcoran et al, 2011; Cowasange et al, 2014). In addition, anterior cingulate cortex is implicated in retrieval of remote (Frankland et al, 2004), and medial prefrontal cortex (mPFC) in retrieval of recent (Zelikowsky et al, 2013) context fear memories. The role of mPFC in modulation of amygdala activation (Quirk et al, 2003; Sotres-Bayon et al, 2012) and hippocampus (Jin and Maren, 2015), possibly via nucleus reuniens (Varela et al, 2015; Xu and Südhof, 2013), as well as sex differences in mPFC activation during extinction of fear (Gruene et al, 2015b) make this brain region particularly interesting for mediating differential competition between memory systems and context generalization in males and females.
Differential effects of sex and stress hormones on hippocampal and amygdalar function may also mediate sex differences in mechanisms of memory retrieval. In females, hippocampal estrogens regulate generalization of passive avoidance (Lynch et al, 2014, 2016), whereas in males, testosterone decreases amygdala activity during memory retrieval (Chen et al, 2014). Corticosterone alters hippocampus activation (Bohacek et al, 2015; Conrad et al, 2004), competition between memory systems (Beck and Luine, 2010), and is differentially increased during retrieval of fear-associated memories in males and females (Daviu et al, 2014). Sex and stress hormones may thereby contribute to sex differences in retrieval-induced hippocampal and amygdalar mechanisms, and greater generalization of context fear in females.
The initial consolidation of memory determines, in part, which cells store the memory trace (Josselyn et al, 2015) and thus which brain regions are required during memory retrieval, suggesting that sex differences in memory consolidation should also be evident. Nevertheless, we observed that dorsal hippocampus was activated during consolidation of context fear conditioning in both males and females. This is consistent with findings that both males and females activate hippocampus during consolidation of context fear but recruit different molecular mechanisms in this region (Dachtler et al, 2011; Keiser and Tronson, 2016; Mizuno et al, 2007). It remains unclear how sex differences in mechanisms of memory consolidation contribute to subsequent retrieval and generalization of context fear.
These findings add to growing evidence for sex differences in context fear conditioning and, more generally, sex differences in memory processes. Studies of context fear conditioning have observed impaired context fear conditioning in females compared with males (Gresack et al, 2009; Maren et al, 1994), no differences (Dachtler et al, 2011; Moore et al, 2010), or differences dependent on protocol (Wiltgen et al, 2001). In contrast, we found that females have stronger context fear conditioning and concurrently more context generalization. What is consistent across these studies is that they show differential utilization of hippocampus in females compared with males. This has been exhibited in the form of less hippocampal plasticity (Maren et al, 1994), recruitment of plasticity-related signaling compared with males (Gresack et al, 2009), and more time required for context representations and context fear conditioning (Wiltgen et al, 2001). In these studies, females show less—or delayed—context fear conditioning. Our findings extend this literature to show that males and females differ in context generalization and hippocampal activity during retrieval even under conditions in which females show stronger context fear context fear conditioning than males. Additional studies have demonstrated sex-specific involvement of glutamatergic (Dachtler et al, 2011) and GABAergic (Moore et al, 2010) receptors, and diverging signal transduction mechanisms and gene expression in the hippocampus (Antunes-Martins et al, 2007; Mizuno et al, 2006, 2012) during learning and consolidation of context fear conditioning. We additionally demonstrate that males and females differentially recruit hippocampal mechanisms and open the possibility that amygdalar-dependent mechanisms also exhibit sex differences in memory retrieval.
Together, our data demonstrate sex differences in retrieval of context fear conditioning, where females show more context generalization and more amygdala activation, and males show strong context discrimination and greater hippocampal activation. Sex differences in memory retrieval have important implications for post-retrieval memory processes, including extinction and memory reconsolidation, and important caveats for their use in treatments for disorders of anxiety and PTSD. Finally, given the role of fear generalization in these disorders (Lissek et al, 2014), our findings suggest that differential circuit activation during anxiety and recall of trauma-associated memories may contribute to increased generalization in women, and higher risk of these disorders in women.
Funding and disclosure
The authors declare no conflict of interest.
References
Adolphs R, Denburg NL, Tranel D (2001). The amygdala’s role in long-term declarative memory for gist and detail. Behav Neurosci 115: 983–992.
Amano T, Duvarci S, Popa D, Pare D (2012). The fear circuit revisited: contributions of the basal amygdala nuclei to conditioned fear. J Neurosci 31: 15481–15489.
Antunes-Martins A, Mizuno K, Irvine EE, Lepicard EM, Giese KP (2007). Sex-dependent up-regulation of two splicing factors, Psf and Srp20, during hippocampal memory formation. Learn Mem 14: 693–702.
Bae SE, Holmes NM, Westbrook RF (2015). False context fear memory in rats. Learn Mem 22: 519–526.
Barrientos RM, O’Reilly RC, Rudy JW (2002). Memory for context is impaired by injecting anisomycin into dorsal hippocampus following context exploration. Behav Brain Res 134: 299–306.
Beck KD, Luine VN (2010). Evidence for sex-specific shifting of neural processes underlying learning and memory followingstress. Physiol Behav 99: 204–211.
Becker JB, Koob GF (2016). Sex differences in animal models: focus on addiction. Pharmacol Rev 68: 242–263.
Biedenkapp JC, Rudy JW (2007). Context preexposure prevents forgetting of a contextual fear memory: implication for regional changes in brain activation patterns associated with recent and remote memory tests. Learn Mem 14: 200–203.
Biedenkapp JC, Rudy JW (2009). Hippocampal and extrahippocampal systems compete for control of contextual fear: role of ventral subiculum and amygdala. Learn Mem 16: 38–45.
Bohacek J, Manuella F, Roszkowski M, Mansuy IM (2015). Hippocampal gene expression induced by cold swim stress depends on sex and handling. Psychoneuroendocrinology 52: 1–12.
Cahill L, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C et al (2001). Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiol Learn Mem 75: 1–9.
Caligioni C (2009). Assessing reproductive status/stages in mice. Curr Protoc Neurosci Appendix 4, Appendix 4I, A.4I.1-A.4I.8.
Chai XJ, Jacobs LF (2010). Effects of cue types on sex differences in human spatial memory. Behav Brain Res 208: 336–342.
Chen L-S, Tzeng W-Y, Chuang J-Y, Cherng CG, Gean P-W, Yu L (2014). Roles of testosterone and amygdaloid LTP induction in determining sex differences in fear memory magnitude. Horm Behav 66: 498–508.
Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL et al (2004). Acute stress impairs spatial memory in male but not female rats: influence of estrous cycle. Pharmacol Biochem Behav 78: 569–579.
Corcoran KA, Donnan MD, Tronson NC, Guzmán YF, Gao C, Jovasevic V et al (2011). Retrieval of recent and remote context fear memory. J Neurosci 31: 11655–11659.
Cowasange KK, Shuman T, Dillingham BC, Chang A, Golshani P, Mayford M (2014). Direct reactivation of a coherent neocortical memory of context. Neuron 84: 432–441.
Dachtler J, Fox KD, Good MA (2011). Gender specific requirement of GluR1 receptors in contextual conditioning but not spatial learning. Neurobiol Learn Mem 96: 461–467.
Daviu N, Andero R, Armario A, Nadal R (2014). Sex differences in the behavioural and hypothalamic-pituitary-adrenal response to contextual fear conditioning in rats. Horm Behav 66: 713–723.
De Vries GJ (2004). Minireview: sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology 145: 1063–1068.
Fanselow MS (1990). Factors governing one-trial contextual conditioning. Anim Learn Behav 18: 264–270.
Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ (2004). The involvement of the anterior cingulate cortex in remote contextual fear memory. Science 304: 881–883.
Gafford GM, Parsons RG, Helmstetter FJ (2013). Memory accuracy predicts hippocampal mTOR pathway activation following retrieval of contextual fear memory. Hippocampus 23: 842–847.
Gresack JE, Schafe G, Orr P, Frick K (2009). Sex differences in contextual fear conditioning are associated with differential ventral hippocampal extracellular signal-regulated kinase activation. Neuroscience 159: 451–467.
Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM (2015a). Sexually divergent expression of active and passive conditioned fear responses in rats. Elife 4: 1–9.
Gruene TM, Roberts E, Thomas V, Ronzio A, Shansky RM (2015b). Sex-specific neuroanatomical correlates of fear expression in prefrontal-amygdala circuits. Biol Psychiatry 78: 186–193.
Jin J, Maren S (2015). Fear renewal preferentially activates ventral hippocampal neurons projecting to both amygdala and prefrontal cortex in rats. Sci Rep 5: 8388.
Josselyn SA, Köhler S, Frankland PW (2015). Finding the engram. Nat Publ Gr 16: 521–534.
Keeley RJ, Tyndall AV, Scott GA, Saucier DM (2013). Sex difference in cue strategy in a modified version of the morris water task: correlations between brain and behaviour. PLoS One 8: e69727.
Keiser AA, Tronson NC (2016). Molecular mechanisms of memory in males and females. In: Shansky RM, 1st edn. Sex Differences in the Central Nervous System. Elseiver Academic Press: Boston, chapter 2, pp 27–51.
Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU (2012). Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res 21: 169–184.
Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB (1995). Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 52: 1048–1060.
Kindt M (2014). A behavioural neuroscience perspective on the aetiology and treatment of anxiety disorders. Behav Res Ther 62: 24–36.
Kosten TA, Lee HJ, Kim JJ (2006). Early life stress impairs fear conditioning in adult male and female rats. Brain Res 1087: 142–150.
Kudo K, Qiao C-X, Kanba S, Arita J (2004). A selective increase in phosphorylation of cyclic AMP response element-binding protein in hippocampal CA1 region of male, but not female, rats following contextual fear and passive avoidance conditioning. Brain Res 1024: 233–243.
Lissek S, Kaczkurkin AN, Rabin S, Geraci M, Pine DS, Grillion C (2014). Generalized anxiety disorder is associated with overgeneralization of classically conditioned-fear. Biol Psychiatry 75: 909–915.
Lissek S, Rabin S, Heller R, Lukenbaugh D, Geraci M, Pine DS et al (2010). Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. Am J Psychiatry 167: 47–55.
Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K et al (2012). Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484: 381–387.
Lopresto D, Schipper P, Homberg JR (2015). Neural circuits and mechanisms involved in fear generalization: implications for the pathophysiology and treatment of posttraumatic stress disorder. Neurosci Biobehav Rev 60: 31–42.
Lynch JF, Cullen PK, Jasnow AM, Riccio DC (2013). Sex differences in the generalization of fear as a function of retention intervals. Learn Mem 20: 628–632.
Lynch JF, Dejanovic D, Winiecki P, Mulvany J, Ortiz S, Riccio DC et al (2014). Activation of ERβ modulates fear generalization through an effect on memory retrieval. Horm Behav 66: 421–429.
Lynch JF, Winiecki P, Vanderhoof T, Riccio DC, Jasnow AM (2016). Hippocampal cytosolic estrogen receptors regulate fear generalization in females. Neurobiol Learn Mem 130: 83–92.
Maney DL (2016). Perils and pitfalls of reporting sex differences. Phil Trans R Soc 3711: 20150119.
Maren S, De Oca B, Fanselow MS (1994). Sex differences in hippocampal long-term potentiation (LTP) and pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res 661: 25–34.
Matus-Amat P, Higgins EA, Sprunger D, Wright-Hardesty K, Rudy JW (2007). The role of dorsal hippocampus and basolateral amygdala NMDA receptors in the acquisition and retrieval of context and contextual fear memories. Behav Neurosci 121: 721–731.
McDonald RJ, White NM (1995). Information acquired by the hippocampus interferes with acquisition of the amygdala-based conditioned-cue preference in the rat. Hippocampus 5: 189–197.
Mcintyre CK, Pal SN, Marriott LK, Gold PE (2002). Competition between memory systems: acetylcholine release in the hippocampus correlates negatively with good performance on an amygdala-dependent task. J Neurosci 22: 1171–1176.
Meakin LB, Sugiyama T, Galea GL, Browne WJ, Lanyon LE, Price JS (2013). Male mice housed in groups engage in frequent fighting and show a lower response to additional bone loading than females or individually housed males that do not fight. Bone 54: 113–117.
Mizuno K, Antunes-Martins A, Ris L, Peters M, Godaux E, Giese KP (2007). Calcium/calmodulin kinase kinase beta has a male-specific role in memory formation. Neuroscience 145: 393–402.
Mizuno K, Dempster E, Mill J, Giese KP (2012). Long-lasting regulation of hippocampal Bdnf gene transcription after contextual fear conditioning. Genes Brain Behav 11: 651–659.
Mizuno K, Ris L, Sánchez-Capelo A, Godaux E, Giese KP (2006). Ca2+/calmodulin kinase kinase alpha is dispensable for brain development but is required for distinct memories in male, though not in female, mice. Mol Cell Biol 26: 9094–9104.
Moore MD, Cushman J, Chandra D, Homanics GE, Olsen RW, Fanselow MS (2010). Trace and contextual fear conditioning is enhanced in mice lacking the alpha4 subunit of the GABA(A) receptor. Neurobiol Learn Mem 93: 383–387.
Paxinos G, Franklin KBJ (2013) The Mouse Brain in Stereotaxic Coordinates 4th edn Elsevier: San Diego, CA.
Prendergast BJ, Onishi KG, Zucker I (2014). Neuroscience and biobehavioral reviews female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev 40: 1–5.
Pryce CR, Lehmann J, Feldon J (1999). Effect of sex on fear conditioning is similar for context and discrete CS in Wistar, Lewis and Fischer rat strains. Pharmacol Biochem Behav 64: 753–759.
Quirk GJ, Likhtik E, Pelletier JG, Paré D (2003). Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 23: 8800–8807.
Radulovic J, Kammermeier J, Spiess J (1998). Relationship between fos production and classical fear conditioning: effects of novelty, latent inhibition, and unconditioned stimulus preexposure. J Neurosci 18: 7452–7461.
Radulovic J, Tronson NC (2010). Molecular specificity of multiple hippocampal processes governing fear extinction. Rev Neurosci 21: 1–17.
Ris L, Angelo M, Plattner F, Capron B, Errington M, Bliss T et al (2005). Sexual dimorphisms in the effect of low-level p25 expression on synaptic plasticity and memory. Eur J Neurosci 21: 3023–3033.
Rodríguez CA, Torres A, Mackintosh NJ, Chamizo VD (2010). Sex differences in the strategies used by rats to solve a navigation task. J Exp Psychol Anim Behav Process 36: 395–401.
Rudy JW, O’Reilly RC (1999). Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behav Neurosci 113: 867–880.
Rudy JW, O’Reilly RC (2001). Conjunctive representations, the hippocampus, and contextual fear conditioning. Cogn Affect Behav Neurosci 1: 66–82.
Ryan TJ, Roy DS, Pignatelli M, Arons A, Tonegawa S (2015). Engram cells retain memory under retrograde amnesia. Science 348: 1007–1013.
Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH et al (2014). Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods 11: 629–632.
Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E (2012). Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 76: 804–812.
Tanaka KZ, Pevzner A, Hamidi AB, Nakazawa Y, Graham J (2014). Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron 84: 347–354.
Temme SJ, Bell RZ, Pahumi R, Murphy GG (2014). Comparison of inbred mouse substrains reveals segregation of maladaptive fear phenotypes. Neurosci Behav 8: 1–9.
Tronson NC, Schrick C, Guzman YF, Huh KH, Deepak P, Penzes P et al (2009). Mediating conditioning and extinction of contextual fear. J Neurosci 29: 3387–3394.
Van Craenendonck H, Ver Donck L (2014). Cued fear conditioning: minimizing “contextual leftover”-freezing by maximizing changes of context. In: Spink AJ, van den Broek EL, Loijens LWS, Woloszynowska-Fraser M, Noldus LPJJ (eds). Proceedings of Measuring Behavior. Wageningen: The Netherlands, pp 373–375.
Varela C, Kumar S, Yang JY, Wilson MA (2015). Anatomical substrates for direct interactions between hippocampus, medial prefrontal cortex, and the thalamic nucleus reuniens. Brain Struct Funct 219: 911–929.
Westbrook RF, Good AJ, Kiernan MJ (1994). Effects of the interval between exposure to a novel environment and the occurrence of shock on the freezing responses of rats. Q J Exp Psychol J Exp Psychol 47b: 427–446.
Wiltgen BJ, Sanders MJ, Fanselow MS, Behne NS (2001). Sex differences, context preexposure, and the immediate shock deficit in pavlovian context conditioning with mice. Behav Neurosci 115: 26–32.
Wiltgen BJ, Silva AJ (2007). Memory for context becomes less specific with time. Learn Mem 14: 313–317.
Wiltgen BJ, Zhou M, Cai Y, Balaji J, Karlson M, Parivash S et al (2010). The hippocampus plays a selective role in the retrieval of detailed context memories. Curr Biol 20: 1336–1344.
Xu W, Südhof TC (2013). A neural circuit for memory specificity and generalization. Science 339: 1290–1295.
Yagi S, Chow C, Lieblich SE, Galea LA (2015). Sex and strategy use matters for pattern separation, adult neurogenesis, and immediate early gene expression in the hippocampus. Hippocampus 101: 87–101.
Yochiy A, Britto LRG, Hunziker MHL (2012). Novelty, but not operant aversive learning, enhances Fos and Egr-1 expression in the medial prefrontal cortex and hippocampal areas of rats. Behav Neurosci 126: 826–834.
Zelikowsky M, Bissiere S, Hast TA, Bennett RZ, Abdipranoto A (2013). Prefrontal microcircuit underlies contextual learning after hippocampal loss. Proc Natl Acad Sci USA 110: 9938–9943.
Zelikowsky M, Hersman S, Chawla MK, Barnes CA, Fanselow MS (2014). Neuronal ensembles in amygdala, hippocampus, and prefrontal cortex track differential components of contextual fear. J Neurosci 34: 8462–8466.
Acknowledgements
We thank Elissa Donzis, Alexandra Rubin, and Ilana Bruckman for their excellent technical assistance; and Katie Collette, Daria Tchessalova, Caitlin Posillico, Lining Pan, and Rosa Garcia-Hernandez for their extremely helpful comments on and discussions of this manuscript. This research was funded by MH093459 to NCT and DOD NDSEG to AAK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keiser, A., Turnbull, L., Darian, M. et al. Sex Differences in Context Fear Generalization and Recruitment of Hippocampus and Amygdala during Retrieval. Neuropsychopharmacol 42, 397–407 (2017). https://doi.org/10.1038/npp.2016.174
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2016.174
This article is cited by
-
Pharmacological diacylglycerol lipase inhibition impairs contextual fear extinction in mice
Psychopharmacology (2024)
-
Sex differences in learning and performing the Go/NoGo tasks
Biology of Sex Differences (2023)
-
Proteasome-independent K63 polyubiquitination selectively regulates ATP levels and proteasome activity during fear memory formation in the female amygdala
Molecular Psychiatry (2023)
-
CB1R blockade unmasks TRPV1-mediated contextual fear generalization in female, but not male rats
Neuropsychopharmacology (2023)
-
A sex-specific thermogenic neurocircuit induced by predator smell recruiting cholecystokinin neurons in the dorsomedial hypothalamus
Nature Communications (2023)