Abstract
The anterior bed nucleus of the stria terminalis (BNST) has been recognized as a critical structure in regulating trait anxiety, contextual fear memory, and appetitive behavior, and is known to be sensitive to stress manipulations. As one of the most complex structures in the central nervous system, the intrinsic circuitry of the BNST is largely unknown; however, recent technological developments have allowed researchers to begin to untangle the internal connections of the nucleus. This research has revealed the possibility of two opposing circuits, one anxiolytic and one anxiogenic, within the BNST, the relative strength of which determines the behavioral outcome. The balance of these pathways is critical in maintaining a normal physiological and behavioral state; however, stress and drugs of abuse can differentially affect the opposing circuitry within the nucleus to shift the balance to a pathological state. In this review, we will examine how stress interacts with the neuromodulators, corticotropin-releasing factor, norepinephrine, dopamine, and serotonin to affect the circuitry of the BNST as well as how synaptic plasticity in the BNST is modulated by stress, resulting in long-lasting changes in the circuit and behavioral state.
Similar content being viewed by others
INTRODUCTION
A growing body of evidence suggests that the bed nucleus of the stria terminalis (BNST) plays a crucial role in regulating trait anxiety as well as contextual fear memory formation (Sullivan et al, 2004; Kalin et al, 2005; Straube et al, 2007; Walker and Davis, 2008; Duvarci et al, 2009; Oler et al, 2009; Fox et al, 2010; Somerville et al, 2010; Zimmerman and Maren, 2011; Hott et al, 2012; Yassa et al, 2012; Sink et al, 2013; Davis and Walker, 2014). However, the BNST can be subdivided into at least 16 unique subregions and has been reported to be one of the most complex structures in the entire central nervous system (Ju et al, 1989; Dong et al, 2001b; Dong and Swanson, 2004; Larriva-Sahd, 2006; Bota et al, 2012). Indeed, recent evidence suggests that the BNST is involved in regulating appetitive as well as aversive behavior. Consequently, understanding the intrinsic circuitry of the BNST and how this circuitry may be affected by stress hormones and neurotransmitters will be key to understanding anxiety in both a normal and pathological state. The heterogeneous nature of the BNST, in which different subregions and cell types modulate anxiety in opposing ways, creates two opposing circuits, the relative strength of which determines the behavioral outcome. Stress can differentially affect the opposing circuitries within the BNST to shift the balance from an anxiolytic to an anxiogenic state.
In this review we will focus our attention on the anterior lateral group of the BNST (BNSTALG) as it is densely connected with the hypothalamus, amygdala, midbrain, and lower brainstem regions associated with autonomic function, emotional processing, reward, and pain (Dong et al, 2001b; Dong and Swanson, 2004). The BNSTALG, as defined by Dong and Swanson (2004), consists of the oval, juxtacapsular, fusiform, and rhomboid (not pictured) nuclei, as well as the undifferentiated region surrounding these nuclei termed the anterolateral area (BNSTAL; Figure 1b). Many studies also discuss the dorsal and ventral BNST (dBNST and vBNST) separately, defined as the regions dorsal and ventral to the anterior commissure. The BNST regions receive different afferents (for review see McDonald et al, 1999 and Dong et al, 2001a) and have distinct projections (Dong et al, 2000, 2001b). In addition, there is a large amount of connectivity in and between the smaller BNST nuclei (Dong et al, 2000, 2001b; Dong and Swanson, 2004; Turesson et al, 2013). The vast majority of the neurons in the BNST use γ-aminobutyric acid (GABA) as the primary neurotransmitter; however, there are also a small number of glutamatergic neurons primarily located in the vBNST (Csáki et al, 2000; Jalabert et al, 2009; Jennings et al, 2013b; Turesson et al, 2013). Finally, the neurons in the BNST also express a vast array of neuropeptides including corticotropin-releasing factor (CRF), enkephalin (ENK), neuropeptide Y (NPY), neurotensin, and somatostatin (SOM) (Walter et al, 1991). In this review, we will explore how neuromodulators affect the circuitry of the BNSTALG and interact with stress to provide dynamic control over the system. We will then discuss how stress modulates synaptic plasticity in the BNSTALG, resulting in long-lasting changes in the circuit and behavioral state.
The BNST is a complex structure composed of multiple nuclei and cell types. (a) The anterior BNST surrounds the anterior commissure (AC) medial to the internal capsule (IC). (b) The BNSTALG consists of the oval (OV), juxtacapsular (JC), and fusiform (FU) nuclei as well as the undifferentiated anterolateral region (yellow). The dorsal BNST (dBNST) is located dorsal to the AC and includes the OV, JC, and dorsal portion of the anterolateral region (cross-hatch, AD). (c) The distribution of type I–III neurons differs within each nucleus of the dBNST. The location of type I–III neurons was approximated at the time of recording. Inset indicates a bar graph showing the percentage of type I–III neurons in the OV and the AD. Neurons in the gray square were excluded from the analysis as they were considered to be in the transition zone between the OV and the AD. Notably, type III cells were only found in the OV. (d) A schematic of the proposed local circuits in the BNSTALG and the downstream consequences of activation. Different subregions and cell types modulate anxiety in opposing ways, creating two opposing circuits, an anxiogenic pathway (red) and an anxiolytic pathway (blue), the relative strength of which determines the behavior outcome. In this theoretical model, the anxiogenic and anxiolytic pathway reciprocally inhibit one another. Dashed line with perpendicular ends indicates postulated inhibitory connections; dashed line with arrow at end indicates postulated excitatory connection; solid line with perpendicular end indicates known inhibitory connection. AD, anterodorsal BNST; CRF, corticotropin-releasing factor; GABA, γ-aminobutyric acid; Glut, glutamate; PKCδ, protein kinase C-δ; vBNST, ventral BNST.
INTERACTION OF STRESS WITH NEUROMODULATORS
Corticotropin-Releasing Factor
CRF is a stress hormone that both acts in the BNSTALG to affect anxiety behavior and is produced by BNSTALG neurons (Lee and Davis, 1997; Dabrowska et al, 2013a; Cummings et al, 1983). The BNSTALG has been shown to be an important site of action for the role of CRF in potentiating anxiety-like behavior and the stress response. CRF infused into the lateral cerebral ventricle increases startle that could be blocked with either lesions of the lateral BNST or microinfusion of a CRF antagonist into the BNSTALG (Lee and Davis, 1997). In addition, intra-BNST infusion of CRF increases anxiety-like behaviors in the elevated plus maze (Sahuque et al, 2006), increases retention in an inhibitory avoidance task (Liang et al, 2001), produces a conditioned place aversion (Sahuque et al, 2006), and induces reinstatement of cocaine seeking (Erb and Stewart, 1999). The CRF-related peptides, urocortin 1, 2, and 3, may also contribute to CRF signaling in the BNST (Bale and Vale, 2004; Kormos and Gaszner, 2013; Koob, 2010). Not only does CRF act in the BNSTALG to affect anxiety-like behaviors and responses to stress, but the BNSTALG also contains CRF-producing neurons that are responsive to stress (Cummings et al, 1983; Dabrowska et al, 2013a; Day et al, 1999; Ju et al, 1989). CRF mRNA in the BNSTALG increases after exposure to corticosterone (Makino et al, 1994), acute foot-shock, and the α2 adrenoreceptor antagonist, yohimbine, a pharmacological stressor (Funk et al, 2006). Hence, it is evident that CRF plays a crucial part in the role of the BNSTALG in modulating stress and anxiety.
Importantly, CRF acts presynaptically to enhance glutamatergic transmission in the dorsal lateral BNSTALG, effectively tuning the BNSTALG to whichever inputs are carrying the CRFR1 receptor. Application of CRF onto the BNSTALG in vitro increases the frequency of spontaneous excitatory postsynaptic currents (sEPSCs); an effect that is blocked by application of the selective CRF receptor 1 antagonist, NBI27914 (NBI) (Kash et al, 2008). Moreover, withdrawal from chronic intermittent ethanol (CIE) exposure, a potent stressor, has been shown to enhance glutamatergic tone onto BNST neurons that project to the ventral tegmental area (VTA) (Silberman et al, 2013). Notably, the CIE effect was blocked with pretreatment of NBI, indicating that with CIE withdrawal, CRF acts through a CRFR1-dependent mechanism in vivo to enhance glutamatergic input onto BNSTALG neurons. Although it seems as though glutamatergic input is increased by CRF throughout the BNSTALG (including regions both dorsal and ventral to the commissure), it is unknown which specific inputs are being modulated by CRF transmission and/or whether all of the inputs are equally affected by stress. More targeted optogenetic manipulations may begin to address this issue. Interestingly, CRF has been reported to depolarize a subset of neurons in the dBNST that are thought to be local interneurons, potentially counteracting the increase in glutamatergic input into the system (Ide et al, 2013).
Although it is clear that CRF acts in the BNSTALG to affect anxiety-like behavior, the origin of CRF is unknown, as the BNSTALG contains both CRF-containing neurons and CRF fibers. The lateral division of the central nucleus of the amygdala (CeAL) also produces CRF (Day et al, 1999) and contributes to the CRF-immunoreactive fibers found in the dBNST (Sakanaka et al, 1986). It has been proposed that it is CRF from the CeAL that acts in the BNST to produce the BNST-dependent effects of CRF (Walker et al, 2009). Indeed, there is evidence for a serial flow of activation from the CeA to the BNST; morphine withdrawal induces c-fos expression in the BNST and CeA, and lesions of the CeA reduce c-fos expression in the BNST, whereas lesions of the BNST have no effect on c-fos expression in the CeA (Nakagawa et al, 2005). In a study using a cross-lesion design to examine the CRF-containing pathway from the CeA to the BNST in stress-induced reinstatement of cocaine seeking, tetrodotoxin infused into the CeA of one hemisphere and CRF antagonist infused into the BNST of the opposite hemisphere reduced stress-induced reinstatement compared with the unilateral manipulation, indicating that the actions of CRF in the BNST are, at least in part, dependent on CeA activity (Erb et al, 2001). Similarly, a unilateral lesion of the amygdala and CRF antagonist infused into the contralateral BNST reduced, but did not block, social defeat in Syrian hamsters (Jasnow et al, 2004). Importantly, in both studies, the bilateral manipulation did not block reinstatement completely, suggesting that although the CeA is one source of CRF in the BNSTALG, it is not the only source.
Recently, evidence is growing for a role in local CRF release affecting the excitability of the BNSTALG. Hence, dopamine (DA) release in the BNST has been reported to enhance excitatory transmission through an indirect action at CRFR1 receptors (Kash et al, 2008). Although it is possible that DA acts on CRF terminals from the CeA to increase CRF release in the BNST, there is also likely a direct action of DA on CRF neurons. A subpopulation of BNSTALG neurons are significantly depolarized in response to DA application (Kash et al, 2008). Significantly, preliminary single-cell RT-PCR data from our lab show that mRNA for the D1 receptor is expressed exclusively in CRF cells dorsal to the commissure. Together, these data indicate that DA may be acting on CRF neurons in the oval nucleus of the BNST to increase local CRF release, thereby enhancing excitatory transmission. In addition, the β-adrenergic receptor agonist, isoproterenol, enhances excitatory transmission in the BNST through a CRFR1-dependent mechanism (Nobis et al, 2011). In fact, using a CRF-tomato mouse line to visualize CRF cells, in vitro patch-clamp recordings showed both DA and isoproterenol depolarize CRF neurons in the BNST (Silberman et al, 2013). This physiological evidence along with the cross-lesion studies described above indicate that both CRF from the CeAL and the BNST act in the BNST to affect anxiety behavior and the response to stress. Future studies should begin to determine whether CRF from the CeAL and the BNST work together or whether they are differentially activated by specific stressors.
There are at least two distinct populations of CRF neurons in the BNSTALG: those found in the oval nucleus dorsal to the anterior commissure, and those found in the fusiform nucleus ventral to the commissure (Cummings et al, 1983; Ju et al, 1989). Intriguingly, stress can cause an increase in the expression of CRF mRNA in these nuclei; however, not every stressor causes a change in mRNA expression in both populations of CRF neurons, implying that they are functionally distinct cell populations. Dorsal and ventral CRF mRNA both increase after an intermittent foot-shock stressor, but are differentially affected by social defeat and yohimbine, with social defeat only increasing CRF mRNA in the vBNST and yohimbine only increasing that of the dBNST (Funk et al, 2006). CRF mRNA in the dBNST but not vBNST increased after chronic mild stress (Kim et al, 2006). Similarly, high levels of subcutaneous corticosterone over 14 days resulted in increased levels of CRF mRNA in dBNST but not vBNST (Makino et al, 1994). In another study, no change in CRF mRNA was observed after foot-shock alone, but an increase in CRF mRNA in the dBNST but not vBNST was observed after foot-shock in animals that have been extinguished from self-administration of heroin (Shalev et al, 2001). Finally, following hypertonic saline injection, the amount of CRF mRNA in the oval nucleus decreased, whereas it increased in the fusiform nucleus of the BNST (Watts et al, 1995) (see Table 1 for summary of these results). Beyond differences in responding to stress, the CRF neurons of the oval and fusiform nuclei may be distinct on a more fundamental level. The CRF neurons in the oval nucleus are known to be GABAergic, unlike the CRF neurons of the periventricular nucleus of the hypothalamus (PVN) that coexpress glutamate (Dabrowska et al, 2013a). However, it is still unknown whether the CRF neurons in the fusiform nucleus are glutamatergic or GABAergic. In fact, there has been significant confusion in the literature on this topic (Choi et al, 2007; Radley et al, 2009). Nevertheless, these data suggest that the CRF neurons of the oval and fusiform nucleus are distinct cell populations that differentially respond to stress.
At least three different types of neurons in the dorsal BNSTALG of the rat have been recognized based on their spiking and rectification properties and rebound depolarization in response to hyperpolarizing and depolarizing current injection: type I (regular spiking), type II (low-threshold bursting), and type III (fast inward rectifiers) (Hammack et al, 2007; Rodríguez-Sierra et al, 2013). The electrophysiological profile of neurons in the BNSTALG may be indicative of what proteins are being expressed by that cell, including CRF. In addition to the different electrophysiological properties of these neurons, single-cell RT-PCR revealed that the different cell types expressed the mRNA for distinct complements of ion channels (Hazra et al, 2011) and serotonin receptors (Hazra et al, 2012). Importantly, nearly all of the type III cells express the mRNA for CRF (Dabrowska et al, 2013a). In a transgenic mouse line in which GFP is exclusively expressed in CRF-containing neurons (Martin et al, 2010), we have preliminary data showing that the GFP cells in the BNST share many of the same electrophysiological characteristics as type III neurons in the rat. Another transgenic mouse line, a CRF-tomato reporter line, has also been used to record from CRF neurons in the BNST (Silberman et al, 2013). In this mouse, the CRF neurons in the BNST were not of a consistent electrophysiological profile, but rather, based on the voltage responses to hyperpolarizing and depolarizing current injections, there were some CRF neurons that fit into each cell-type classification as well as some that did not fit into any of the predefined cell types. This inconsistency brings up multiple questions regarding the use of cell-type classification and transgenic reporter mice. First, the cell types that were defined in the rat BNST have not been confirmed to exist in the mouse. We have observed all three cell types in the mouse, but in a different proportion from what is seen in the rat BNST, as well as some cells that do not fit into the any of the predefined cell types (unpublished observation). Therefore, we must use caution when we apply concepts shown in one species to another. In addition, it is possible that a CRF transgenic reporter line could inadvertently label more neurons than ones that express functional levels of the CRF peptide. Many cells express low (or even high) levels of an mRNA transcript without functionally expressing the corresponding protein (Tropea et al, 2001); however, the hypothalamic field has relied on measuring CRF mRNA as a proxy for CRF peptide and found these measures to be reliable (Imaki et al, 1991; Swanson and Simmons, 1989). In a reporter line, the fluorescent protein is created regardless of whether, or not, the CRF mRNA is translated into functional protein. Hence, the apparent disparity in the electrophysiological phenotype of CRF neurons may be attributed to ectopic expression in the reporter line used by Silberman et al (2013). Indeed, the expression of the tomato fluorescence seems to extend beyond the oval nucleus where immunohistochemical studies have localized CRF cells (Silberman et al, 2013; Sawchenko and Swanson, 1985; Swanson et al, 1983). However, immunohistochemistry is not without its faults and may be underrepresenting CRF protein expression in the BNST. In this case the GFP reporter line may not label all CRF neurons in the BNST. Regardless, in the rat, 95% of type III cells express the mRNA for CRF (Dabrowska et al, 2013a). Furthermore, type III neurons are only found in the region of the oval nucleus of the rat BNST, where CRF neurons are located, and not in the undifferentiated anterolateral region (Figure 1c, unpublished observation). Together, these data indicate that type III neurons in the dBNST express CRF.
CRF neurons in the dBNST also express distinct receptors and proteins. For example, in the oval nucleus, striatal-enriched protein tyrosine phosphatase (STEP; also called Ptpn5) immunoreactivity has almost total colocalization with CRF immunoreactivity, and all type III neurons express the mRNA for STEP (Dabrowska et al, 2013b). STEP is known to regulate long-term potentiation (LTP) in the amygdala (Paul et al, 2007; Yang et al, 2012), and its role in synaptic plasticity in CRF neurons in the BNST will be discussed later on in this review. Preliminary evidence from our lab suggests the D1 subtype of the DA receptor is also specifically expressed in type III CRF cells in the BNST. This is supported by the finding that DA directly depolarizes CRF neurons in the dBNST, presumably by acting through the GS-coupled D1 receptor (Silberman et al, 2013). Determining more biochemical/molecular ways in which CRF neurons in the BNST are distinct from other cell types has the potential to lead to the discovery of drugable targets that could isolate these neurons and modulate a crucial component of anxiety behavior.
Although studies have shown that CRF neurons in the BNST are responsive to stress manipulations as mentioned above, new technologies that allow us to target specific groups of cells are going to be critical in determining the role of BNST CRF neurons in anxiety behavior. A recent study has shown that distinct subregions of the BNST can have opposing actions in modulating anxiety (Kim et al, 2013). In this study, the oval nucleus was targeted with the injection of a Cre-dependent enhanced form of the halorhodopsin (eNpHR3.0) virus into the BNST of mice that express Cre in cells that express the D1 receptor (Drd1a::cre). Unsurprisingly, as CRF neurons express the D1 receptor and are localized in the oval nucleus, this mouse line shows eNpHR3.0 expression restricted to the oval nucleus of the BNST. Optogenetically inhibiting the oval CRF neurons in the BNST resulted in a decrease in anxiety-like behavior in the elevated plus maze and open field test as well as a decrease in respiratory rate, consistent with an anxiogenic role for the oval CRF neurons (Kim et al, 2013). These CRF neurons were also shown to send an inhibitory projection to the undifferentiated anterodorsal (AD) region of the BNST. Conversely, optogenetically inhibiting the AD region of the BNST resulted in an increase in anxiety-like behavior and respiratory rate, suggesting an anxiolytic role of this region. These data indicate that the oval CRF neurons could directly promote anxiety by release of CRF and indirectly by inhibiting the anxiolytic projection from the AD region of the BNST. In fact, in another study chemogenetically inhibiting CRF neurons using the DREADD (designer receptor exclusively activated by designer drugs) system caused a reduction in anxiety-like behavior (Pleil et al, 2015). CRF neurons in the BNST are thought to make local connections as well as project out of the nucleus to regions involved in emotion processing including the PVN, VTA, periaqueductal gray (PAG), dorsal potion of the dorsal raphe (DRD), and locus coeruleus (LC) (Dabrowska et al, 2011; Dabrowska and Rainnie, 2014; Meloni et al, 2006; Rodaros et al, 2007; Silberman et al, 2013; Van Bockstaele et al, 1999). These projections could contribute to the anxiogenic role of the BNST CRF neurons and/or activate a compensatory mechanism, such as a negative feedback loop, to put a brake on the anxiety response. Isolating the projections, inputs, and function of the different CRF cell populations in the BNST and how they are affected by stress will be an important step to understanding the circuit.
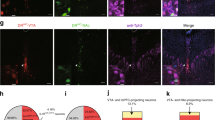
The oval CRF neurons that project out of the oval nucleus to promote anxiety-like behavior are sitting within a predominantly GABAergic nucleus, suggesting that local interneurons could provide an inhibitory control over the output of the CRF neurons. A microcircuitry for modulation of CRF neurons by local GABA neurons has recently been described in the CeAL (Haubensak et al, 2010; Sakanaka et al, 1986). The CRF neurons in the CeAL are a distinct population from neurons expressing the neuronal marker, PKC-δ (Haubensak et al, 2010). These PKC-δ+ neurons form local and reciprocal inhibitory connections with the PKC-δ− cells in the CeAL (Haubensak et al, 2010). In addition, electrically inhibiting the PKC-δ+ cells was shown to enhance fear. Similarly, PKC-δ is also expressed in the oval nucleus of the BNST, and a PKC-δ antibody labels a population of cells largely separate from those labeled by the STEP antibody (Figure 2). As STEP has been shown to colocalize with CRF cells in the BNST (Dabrowska et al, 2013b), we can infer that PKC-δ neurons in the BNST are a separate population of neurons from the CRF neurons in the oval nucleus. In fact, our recent single-cell RT-PCR data showed that only type II cells in the BNST expressed the mRNA for PKC-δ (unpublished observation). As the BNST is in many ways an extension of the central amygdala (Alheid and Heimer, 1988), it is reasonable to hypothesize the local circuitry in the BNST may mirror that of the CeA, with PKC-δ+ and CRF neurons reciprocally inhibiting one another. Whereas PKC-δ+ neurons in the CeAL represent ‘fear off’ neurons (Haubensak et al, 2010), perhaps type II PKC-δ+ cells represent ‘anxiety off’ neurons in the BNST (Figure 1d). In addition to inhibition via local GABAergic connections, CRF action and CRF neurons themselves are opposed by NPY (Ide et al, 2013; Kash and Winder, 2006; Pleil et al, 2015). In fact, NPY in the BNST has been shown to block CRF-induced place aversion (Ide et al, 2013). More studies on peptides and the local circuitry involved in the regulation of CRF neuron activity are needed to better understand how the BNST modulates anxiety.
Photomicrographs showing PKC-δ (green) and STEP (red) rarely colocalize in the oval BNST. Magnification × 40.
Norepinephrine
The BNST receives dense noradrenergic input from the ventral noradrenergic bundle (VNB) and lighter input from the dorsal noradrenergic bundle (DNB) (Park et al, 2009). The norepinephrine (NE) terminals are densest in the vBNST (Egli et al, 2004; Phelix et al, 1994); however, NE also acts in the dBNST to affect synaptic transmission and behavior (Hott et al, 2012; Leri et al, 2002a; McElligott et al, 2010; Nobis et al, 2011; Silberman et al, 2013). The A1 cell group in the caudal ventrolateral medulla contributes to the VNB and is the strongest source of NE in the vBNST (Banihashemi and Rinaman, 2006; Forray et al, 2000; Park et al, 2009; Shin et al, 2008). The nucleus of the solitary tract (A2 cell group) also provides a strong NE input through the VNB (Banihashemi and Rinaman, 2006; Forray et al, 2000). Finally, there is a potential small NE input from the LC through the DNB, but the support for this connection is weak and, unlike the inputs from the VNB, inputs from the LC are not involved in stress-induced reinstatement of drug seeking (Aston-Jones et al, 1999; Forray et al, 2000; Park et al, 2009; Shaham et al, 2000).
Norepinephrine has both a tonic and phasic control over the BNSTALG. There is a rise in NE release in the vBNST as a result of aversive stimuli including immobilization stress, being exposed to a context that was previously associated with a foot-shock, tail pinch, and an aversive tastant (Cecchi et al, 2002; Onaka and Yagi, 1998; Park et al, 2012, 2015). NE is also released into the BNST when a rewarding stimulus is not received when expected (Park et al, 2013). In addition, there is evidence that NE is released into the BNST in basal conditions to modulate glutamatergic transmission (Forray et al, 1999). Together, these data suggest that NE in the BNST tonically modulates input into the BNST and participates in the response to aversive stimuli, including the lack of an anticipated reward.
NE acts in the BNST to promote fear and anxiety-like behavior as well as stress-induced reinstatement of drug seeking and symptoms of opiate withdrawal (Cecchi et al, 2002, 2007; Fendt et al, 2005; Hott et al, 2012; Leri et al, 2002b; Mantsch et al, 2014; Vranjkovic et al, 2012). Rodents are innately afraid of the odor of predators, such as the fox. Exposure to a component of fox odor, trimethylthiazoline (TMT), increases c-fos expression in the oval BNST, LC, and nucleus of the solitary tract (NTS), indicating that both norepinephrine and the BNST are involved in the fear response (Day et al, 2004). Indeed, NE release increases in the vBNST during TMT exposure (Fendt et al, 2005). Clonidine, an α2-AR agonist, acts on the presynaptic α2-AR autoreceptors to inhibit NE release into the vBNST, thereby blocking the rise in NE caused by exposure to TMT. This treatment also blocks the fear behavior induced by TMT exposure, indicating that NE transmission in the vBNST is critical for the fear behavior (Fendt et al, 2005).
NE can act on four subtypes of adrenoreceptors in the BNST: β1-AR, β2-AR, α1-AR, or α2-AR. Specific agonists and antagonists to these receptors have helped to elucidate the unique role of each receptor variant in the BNST. The behavioral and physiological mechanism of NE action in the BNST is summarized in Table 2. As mentioned previously, the α2-AR primarily acts as a presynaptic autoreceptor to inhibit the release of NE into the BNST (Forray et al, 1999; Park et al, 2009), and is therefore able to block fear behavior toward TMT (Fendt et al, 2005) as well as reduce stress-induced reinstatement of drug seeking (Shaham et al, 2000). The other three adrenoreceptors are believed to act primarily through a postsynaptic mechanism in the BNST.
The β-adrenergic receptors are involved in both anxiety-like behavior and drug withdrawal. A cocktail of β1-AR and β2-AR antagonists (betaxol+ICI118,551) in the BNST blocks anxiety-like behavior after an acute immobilization stress (Cecchi et al, 2002). Similarly, a nonselective β-AR antagonist, phentolamine, in the BNST reduced freezing in a context previously paired with shock (Hott et al, 2012). A selective β1-AR antagonist (CGP20712), but not β2-AR antagonist (ICI118,551), replicated this reduction in anxiety-like behavior. From these data we can conclude that β-adrenergic signaling, primarily β1-adrenergic signaling, in the BNST promotes anxiety-like behavior. The β-adrenergic receptors are also involved in stress-induced drug reinstatement and opiate withdrawal. Hence, β-AR blockade in the BNST dose-dependently attenuates foot-shock-induced reinstatement of cocaine seeking but not cocaine-induced reinstatement of cocaine seeking (Leri et al, 2002b), and a β2-AR antagonist in the BNST by itself was enough to attenuate reinstatement (Mantsch et al, 2014). Another study using swim stress to induce reinstatement of cocaine seeking found a cooperative role of both β1-AR and β2-AR in reinstatement (Vranjkovic et al, 2012). Together, this suggests that both β-ARs in the BNST facilitate stress-induced reinstatement. The β-ARs also play a role in symptoms of opiate withdrawal. Blocking both β1-AR and β2-ARs abolishes withdrawal-induced place aversion (Aston-Jones et al, 1999). A selective β1-AR antagonist in the dBNST blocks withdrawal-induced aversion and attenuates opiate-withdrawal symptoms in rats with high reactivity to novelty (Cecchi et al, 2007). Overall, β-ARs act in the BNST to contribute to opiate-withdrawal symptoms and promote anxiety-like behavior and stress-induced reinstatement of drug seeking.
Like the β-ARs, the α1-AR also promotes anxiety-like behavior. A selective α1-AR antagonist (WB4101), but not α2-AR antagonist (RX821002) in the BNST reduced freezing in a context previously paired with foot-shock (Hott et al, 2012). In addition, the selective α1-AR antagonist, benoxathian, blocked anxiety-like behavior and reduced the plasma levels of adrenocorticotropin hormone (ACTH) after an acute stressor (Cecchi et al, 2002). Intriguingly, this suggests that although both β-ARs and α1-AR promote anxiety-like behavior, only the α1-AR facilitates activation of the hypothalamic–pituitary–adrenal (HPA) axis. Furthermore, the α1-ARs are not involved in stress-induced drug reinstatement (Vranjkovic et al, 2012). The similar yet distinct roles of the α- and β-ARs in the BNST suggest that these receptors work through distinct mechanisms activating both separate and overlapping pathways in the BNST.
Both β-ARs are metabotropic receptors generally linked to the GS protein and act to facilitate synaptic transmission in the BNST. Although the majority of the noradrenergic afferents are found in the vBNST, the β-ARs primarily act in the dBNST; however, the lack of effect seen in the vBNST could be due to desensitization of receptors in this region (Egli et al, 2004). In fact, activation of β-ARs in the vBNST has been shown to mediate the negative affective component of pain in rats (Deyama et al, 2008). Regardless, there is no direct physiological evidence of the action of β-ARs in the vBNST at this time. In the dBNST, the nonspecific β-AR agonist, isoproterenol, increases the frequency of spontaneous EPSCs (Nobis et al, 2011; Silberman et al, 2013). This effect can be blocked by application of a β1-AR- but not β2-AR-specific antagonist, suggesting that the facilitation of glutamatergic transmission occurs through the β1-AR (Nobis et al, 2011). As mentioned previously, this enhancement of excitatory transmission in the BNST acts through a CRFR1-dependent mechanism (Nobis et al, 2011). Isoproterenol directly depolarizes CRF neurons in the dBNST (Silberman et al, 2013), potentially increasing local CRF release that would then act on presynaptic CRFR1 receptors to facilitate glutamatergic transmission. However, it is unknown whether the β-AR is also increasing CRF release in the BNST by acting on CRF-terminals originating in the CeA. It is possible that NE is increasing CRF release into the BNST by acting on CRF neurons in both the dBNST and CeA. In fact, although β-AR blockade in the BNST dose-dependently attenuates foot-shock-induced reinstatement of drug-seeking, β-AR blockade in the CeA completely blocks reinstatement (Leri et al, 2002a). Furthermore, there is evidence for a role in the CRF projection from the CeA to the BNST in stress-induced reinstatement (Erb et al, 2001). If CRF from the CeA is necessary for stress-induced reinstatement, and if NE acts in the CeA to enhance CRF release, then this could explain how β-AR blockade in the CeA completely blocks stress-induced reinstatement. In this model, β-ARs on CRF neurons in both the CeA and BNST would facilitate CRF release in the BNST, but only the CeA projection is necessary to produce stress-induced reinstatement. In contrast, local CRF release from the BNST acts to modulate the magnitude of the increase in glutamatergic transmission. Regardless of the source of the CRF input in the BNST, these studies indicate that NE interacts with CRF to increase the glutamatergic transmission in the BNST, in effect, amplifying the salient inputs into the BNST during times of stress.
In contrast, another study showed a similar effect of isoproterenol in the dBNST; the nonselective β-AR agonist enhanced excitatory transmission, but this was only blocked by the β2-AR-specific antagonist, ICI-118,551, suggesting a β2-AR-dependent mechanism (Egli et al, 2004). It is possible that this study was actually looking at a different form of modulation of glutamatergic transmission in the dBNST that is β2-AR dependent. In fact, there are important differences in the effects seen in this study and the studies done by Nobis et al (2011). Egli et al (2004) showed no significant change in the paired-pulse ratio after isoproterenol, indicating this effect is not presynaptic. In contrast, Nobis et al (2011) did show a significant change in the paired-pulse ratio indicating an increase in presynaptic glutamate release. It is possible the β2-AR-dependent enhancement of excitatory transmission in the dBNST is a different mechanism than the β1-AR- and CRFR1-dependent enhancement.
Whereas β-ARs act primarily in the dBNST to facilitate excitatory transmission, activation of the α2-AR subtype attenuates excitatory transmission in both the dBNST and vBNST (Egli et al, 2004). In the vBNST, application of NE only decreases excitatory transmission. However, in the dBNST, there are two competing effects of NE: the facilitation of glutamatergic transmission through β-ARs and the inhibition of glutamatergic transmission through α2-AR. In field recordings in the dBNST, NE application resulted in a facilitation of glutamatergic transmission 62.2% of the time, but in 37.8% of the recordings, NE resulted in a long-lasting decrease of glutamatergic transmission (Egli et al, 2004). Because these data are from extracellular recordings, it is unclear whether these competing processes are occurring in individual or separate cells. Interestingly, glutamatergic input from the parabrachial nucleus to the dBNST is sensitive to the α2-AR agonist, guanfacine, whereas glutamatergic input from the basolateral amygdala (BLA) is not, suggesting specificity in noradrenergic modulation of inputs into the BNST (Flavin et al, 2014). The modulation of the β- and α2-AR pathways could tip the scale in either direction. For example, administration of α2-AR agonists blocks foot-shock-induced reinstatement of heroin seeking (Erb et al, 2000). In that vein, facilitation of the α2-AR pathway could suppress the NE-induced increase in glutamatergic transmission in the dBNST, thereby decreasing some of the behavioral actions of NE.
In addition to modulating glutamatergic transmission in the BNST, NE has also been shown to enhance GABAergic transmission in the vBNST. Neurons in the vBNST that project to the VTA, labeled with fluorescent microspheres that were injected into the VTA and retrogradely transported to the vBNST, only exhibit a small hyperpolarization in response to NE application, whereas unlabeled vBNST neurons exhibit a large depolarizing response (Dumont and Williams, 2004). However, VTA-projecting neurons show an increase in frequency of spontaneous GABAA IPSCs with application of NE, raising the possibility that the non-VTA-projecting neurons in the vBNST send a GABAergic projection to the VTA-projecting vBNST neurons. This increase in frequency of IPSCs is blocked by the α1-AR antagonist, prazosin, indicating it is an α1-AR-dependent effect. The same increase in GABAA IPSCs occurs in rats after withdrawal from a 5-day treatment with morphine; however, this effect is attenuated by the nonselective β-AR antagonist, propranolol, as well as prazosin (Dumont and Williams, 2004). In addition, the protein kinase A (PKA) inhibitor H89 also attenuates this effect only in animals treated with morphine. Chronic morphine treatment can result in a hyperactive adenylyl cyclase (AC)/PKA pathway, and β-AR GS-signaling cascade results in activation of this pathway. These data suggest that during morphine withdrawal, β-ARs are recruited into facilitating GABAergic transmission onto VTA-projecting vBNST neurons through an overactive AC/PKA pathway (Dumont and Williams, 2004). This increased inhibitory drive could come from the local GABA neurons in the vBNST and/or GABAergic neurons in the dBNST. A nonspecific β-AR agonist is known to depolarize the GABAergic CRF neurons in the dBNST, but we do not know whether activation of α1-ARs would have a similar effect (Silberman et al, 2013). There is a strong inhibitory connection from the dBNST to the vBNST, supporting the idea that some of the inhibitory control of VTA-projecting neurons in the vBNST originates from the dBNST (Turesson et al, 2013).
The α1-ARs are also implicated in modulating glutamatergic transmission in the BNST. In both the dorsal and ventral BNST, activation of α1-ARs causes a Gq receptor-dependent long-term depression (LTD) of glutamatergic transmission in the BNST (McElligott and Winder, 2007; McElligott et al, 2010). This Gq-dependent plasticity is maintained by a loss of functional calcium-permeable AMPA receptors (CP-AMPARs) and is modulated by stress (McElligott et al, 2010). After 10 days of chronic restraint stress, which increases extracellular levels of NE in the BNST, α1-AR LTD was blocked in the vBNST and significantly attenuated in the dBNST (McElligott et al, 2010). This was because of a loss of function of CP-AMPARs. Chronic restraint stress caused an increase of NE release into the BNST that acted on α1-ARs in vivo resulting in LTD, thereby already decreasing the function of the CP-AMPARs by the time of the in vitro recordings. The authors hypothesize that, as a GABAergic nucleus, the BNST acts as a brake on the PVN and the amygdala. After stress, the LTD disengages that brake, resulting in an unregulated stress axis and limbic system (McElligott et al, 2010). However, this interpretation must be reexamined in light of recent evidence that the different nuclei of the BNSTALG have opposing roles in anxiety-like behavior (Kim et al, 2013). Therefore it is unlikely that the BNST as a whole acts as a brake on the PVN and amygdala. However, this LTD does change the set point for the response to future incoming stimuli.
To summarize, NE is released into the BNST during stress and other aversive events. It acts in the dBNST through β-ARs to increase CRF release and facilitate the glutamatergic input into the dBNST (Egli et al, 2004; Nobis et al, 2011; Silberman et al, 2013). In this way, NE tunes the dBNST to possible salient inputs potentially increasing the output of the anxiogenic portion of the dBNST to increase anxiety-like behaviors (Cecchi et al, 2002; Hott et al, 2012). Although there are both anxiolytic and anxiogenic outputs in the dBNST, the predatory odor TMT that causes an increase in NE release into the BNST specifically increases c-fos expression in the oval BNST that is known to be a significant contributor to the anxiogenic pathway (Day et al, 2004; Fendt et al, 2005; Kim et al, 2013). NE also acts in the vBNST on α1-ARs, perhaps more strongly than the actions in the dBNST because of more NE release in this region (Egli et al, 2004; Phelix et al, 1994). Here NE application results in increased local GABAergic transmission potentially inhibiting the anxiolytic projection from the vBNST (Dumont and Williams, 2004). Action on α1-ARs may also cause a feed-forward increase in NE release into the BNST (Forray et al, 1999; Park et al, 2009). The α2-ARs, however, act to control the effects of NE in the BNST. Activation of α2-ARs inhibits NE release and decreases excitatory transmission (Egli et al, 2004; Forray et al, 1999; Park et al, 2009). After chronic stress, the prolonged NE release may cause LTD of glutamatergic transmission in the dBNST and vBNST through the α1-ARs (McElligott et al, 2010). Because chronic stress results in an increase in anxiety-like behavior, it is hypothesized that this LTD inhibits the anxiolytic pathway in the BNST. However, although unlikely, it is also possible that this LTD is a compensatory mechanism for the increase in excitatory transmission into the BNST. More research needs to be done on the effect of chronic stress on NE actions in the BNST. As the literature stands, it seems the β-ARs and α1-ARs act to potentiate the anxiogenic pathway and inhibit the anxiolytic pathway in the BNST, whereas the α2-AR stands alone in its ability to inhibit the anxiogenic effects of NE release. Modulating these opposing noradrenergic pathways may be a potential target in the treatment of drug addiction and anxiety disorders.
Dopamine
The BNST receives dopaminergic input from the PAG, VTA, and, to a lesser extent, the substantia nigra pars compacta (Hasue and Shammah-Lagnado, 2002; Meloni et al, 2006). Injections of the retrograde tracer Fluoro-Gold (FG) into the dBNST combined with tyrosine hydroxylase immunofluorescence revealed that the A10dr and A10dc dopaminergic cell groups in the PAG are the strongest sources of dopaminergic afferents in the dBNST (Meloni et al, 2006). Similar to NE, there is both anatomical and functional evidence that DA interacts with CRF in the BNST to affect stress behaviors (Day et al, 2002; Kash et al, 2008; Meloni et al, 2006; Phelix et al, 1994; Silberman et al, 2013); however, unlike the NE projections, the DA projections are primarily in the dBNST and form synapses with the CRF neurons in the oval BNST (Freedman and Cassell, 1994; Phelix et al, 1994). Both DA and NE cause a direct depolarization in CRF neurons in the BNST of mice (Silberman et al, 2013) and an indirect increase in frequency of sEPSCs in the BNST through CRF signaling, but DA and NE are most likely facilitating distinct populations of glutamatergic synapses (Kash et al, 2008; Nobis et al, 2011; Silberman et al, 2013). On the other hand, there is evidence for some cross-talk between systems. For example, DA has been shown to inhibit glutamatergic input into the BNST by acting on the α2-AR (Krawczyk et al, 2011a). The precise circuitry affected by NE- and DA-induced CRF signaling will need to be elucidated in order to better understand their differing roles.
Interestingly, there is some debate over the nature of DA receptor distribution in the dBNST, with various groups reporting the presence or absence of the D1, D2, and D3 receptors in this region (Eiler et al, 2003; Kim et al, 2013; Krawczyk et al, 2011b; Mengod et al, 1992; Savasta et al, 1986; Scibilia et al, 1992). Using receptor autoradiography and immunohistochemistry, there is little evidence for the presence of the D1 receptor in the dBNST in control animals (Krawczyk et al, 2011a; Savasta et al, 1986). This is intriguing given evidence that the D1-specific antagonist, SCH 23390, in the BNST dose-dependently reduces alcohol-motivated responding, whereas the D2 antagonist, eticlopride, has no effect (Eiler et al, 2003). In addition, the Drd1a::cre transgenic mouse that expresses Cre in cells in which D1 is expressed specifically labels the oval nucleus of the BNST (Kim et al, 2013). As discussed in a previous section of this review, preliminary single-cell RT-PCR results have shown that mRNA for the D1 receptor is specifically expressed in type III CRF neurons of the oval BNST (unpublished observation). It is possible that the mRNA for the D1 receptor is expressed in these neurons without being translated into functional protein under basal conditions. In support of this hypothesis, there is a switch from a D2-mediated response in the dBNST of drug-naive rats to a D1-mediated response in cocaine self-administering rats (Krawczyk et al, 2011b). In control rats, DA was found to suppress evoked GABA transmission in the dBNST through a presynaptic D2 receptor mechanism (Krawczyk et al, 2011a, b). However, after prolonged cocaine self-administration, DA acted on D1 receptors to increase IPSC amplitude. Importantly, this switch was not observed in rats that received cocaine passively, emphasizing the involvement of DA in motivated behaviors. Perhaps self-administration of drugs of abuse triggers translation of D1 mRNA in the dBNST into functional protein to mediate drug-motivated behavior. However, if D1 receptors are not functional in drug-naive animals, it is unclear how DA could depolarize CRF neurons in the BNST (Silberman et al, 2013). Both D2 and D3 receptors are Gi-coupled receptors, the activation of which generally enhances G protein-coupled inwardly-rectifying potassium (GIRK) channel activity thereby hyperpolarizing the neuron and preventing synaptic release (Michaeli and Yaka, 2010). It is more likely that DA acts on the GS-coupled D1 receptor to depolarize the CRF neurons in the BNST. More research needs to be done to clarify the functional expression of DA receptors in the BNST in both naive and drug-exposed animals.
As the similar effects on the local circuit may suggest, DA and NE seem to be involved in similar processes, but the timing of their release indicates that they relay related but separate and sometimes reciprocal signals. In a study looking at the release of DA and NE in response to intracranial self-stimulation (ICSS) in the region of the VTA/substantia nigra, both catecholamines were released into the BNST; however, DA was released into the dBNST in response to cues that predicted reward, whereas NE was not released into the vBNST at these times. Conversely, there was a suppression of extracellular DA during extinction of a lever press being paired with ICSS and an increase in release of NE into the vBNST (Park et al, 2013). Similarly, in another study, DA release in the dBNST increased in response to intraorally-administered sucrose but decreased in response to the aversive tastant, quinine (Park et al, 2012). This indicates that DA relays information about obtaining a reward or pleasurable stimulus, whereas NE relays information about the lack of an anticipated reward.
The involvement of DA in reward suggests it plays an important role in drug abuse. Like in the nucleus accumbens shell, drugs of abuse increase extracellular DA in the BNST (Carboni et al, 2000). Cocaine, nicotine, morphine, and tetrahydrocannabinol (THC; the psychoactive component of cannabis) all increase extracellular signal-related kinase (ERK) activation in the BNSTALG (Valjent et al, 2004). The ERK pathway plays an important role in synaptic plasticity, learning, and memory, indicating it could be a potential molecular mechanism for the long-lasting effects of drugs of abuse. Importantly, this increase in ERK activation can be blocked with an injection of the D1 receptor antagonist SCH 23390 15 min before drug administration, suggesting DA acts on D1 receptors in the BNST to increase ERK activation (Valjent et al, 2004). In the striatum, the ERK signaling cascade is activated with simultaneous activation of NMDA and D1 receptors (Valjent et al, 2005). In this way, the ERK cascade acts as a coincidence detector and is activated during times of high glutamatergic input and DA release. Perhaps the same process is occurring in the BNST. Interestingly, STEP inhibits ERK activity and thereby regulates the duration of ERK signaling (Valjent et al, 2005; Yang et al, 2012). STEP is specifically expressed in the CRF neurons of the oval BNST, whereas ERK1/2 is found in both cells coexpressing STEP and those not expressing STEP (Dabrowska et al, 2013b). NMDA and DA have the potential to activate the ERK cascade in all of these neurons, but only the CRF cells contain STEP, the molecular brake for the ERK signal. However, STEP expression in the oval BNST is reduced after chronic stress causing a potential increase in ERK activation with DA present in the BNST (Dabrowska et al, 2013b). This may be relevant for stress-induced drug seeking, where the loss of the molecular brake on ERK signaling in CRF neurons in the dBNST could contribute to drug craving or the motivation to seek out drugs.
DA interacts with CRF signaling in the BNST to play an important role in stress behavior in other ways as well. For example, a unilateral 6-hydroxydopamine (6-OHDA) lesion causes a hemispheric asymmetry in CRF mRNA expression in the CeA and oval nucleus of the BNST (Day et al, 2002). This lesion selectively removes the mesostriatal dopaminergic inputs to the brain through use of the neurotoxin 6-OHDA injected into the medial forebrain bundle. The hemisphere with the lesion exhibited reduced CRF mRNA in the oval BNST compared with the hemisphere with the mesostriatal DA system still intact (Day et al, 2002). Interestingly, there was no effect of the 6-OHDA lesion on ENK mRNA expression in the BNST. As CRF and ENK are expressed in separate cell populations in the BNST, this suggests that the DA system effects mRNA expression in a specific subset of cells, namely the CRF neurons. In addition to promoting CRF expression, DA is involved in facilitating CRF-enhanced startle. The peripheral administration of the D1 antagonist, SCH 23390, attenuates CRF-enhanced startle, a behavior in which the BNST is critically involved (Lee and Davis, 1997; Meloni et al, 2006). This raises the possibility that CRF kicks off a feed-forward circuit between the BNST and a major source of DA input such as the PAG. In this model put forward by Meloni et al (2006), CRF acts on CRFR1 to activate CRF neurons in the BNST that then send projections to the PAG. Indeed, the BNST sends strong projections to the PAG including a CRF projection from the oval nucleus (Dong and Swanson, 2004; Dong et al, 2001a, 2001b; Gray and Magnuson, 1992). CRF depolarizes neurons in the PAG, thereby activating the dopaminergic cells to release DA in the dBNST (Bowers et al, 2003). DA then acts on D1 receptors in the BNST, particularly on CRF neurons, to further increase CRF release into the BNST (Silberman et al, 2013). CRF then facilitates glutamatergic transmission into the BNST through its action on presynaptic CRFR1 (Kash et al, 2008; Silberman et al, 2013). In this way, DA and CRF create a feed-forward circuit that acts to increase CRF signaling and activation of the BNST pathway involved in the startle reflex. The role of DA in appetitive signaling and its interaction with CRF place DA at the intersection of stress and reward.
There is little direct evidence that DA is released into the dBNST after a stressor. Activity of DA neurons in the dorsal VTA is primarily decreased by an acute stressor such as a foot-shock (Brischoux et al, 2009). However, a smaller group of DA neurons in the ventral VTA is activated by foot-shock, yet it is unknown whether these DA neurons project to the BNST (Brischoux et al, 2009). Extracellular DA levels increase in the dorsal striatum and nucleus accumbens core during tail pinch and increase in the nucleus accumbens shell only at the termination of tail pinch (Budygin et al, 2012). This indicates DA is released during both aversive and rewarding stimuli; however, it is still unclear where and when DA is released into the BNST. Social defeat stress and exposure to TMT, a component of fox odor, produces increased c-fos activation in the PAG, possibly indicating an increase in activity of PAG DA neurons that project to the BNST (Janitzky et al, 2014; Miczek et al, 1999). It is clear that DA cells throughout the brain are activated by different stimuli at different times, and hence the role of DA in the BNST is complex. DA in the BNST seems to both signal reward and facilitate the stress response. Rather than acting to suppress or enhance the anxiolytic or anxiogenic circuit within the BNST, DA seems to facilitate both pathways to modulate motivated behavior.
The BNST not only receives dopaminergic projections, but also sends reciprocal connections to the main sources of dopaminergic input including the PAG and VTA (Dong and Swanson, 2004; Georges and Aston-Jones, 2001, 2002; Gray and Magnuson, 1992; Jalabert et al, 2009; Kudo et al, 2012; Jennings et al, 2013; Kim et al, 2013; Silberman et al, 2013). In addition, the LC and VTA are reciprocally connected, indicating that the release of each of the catecholamines can influence that of the other (Mansari et al, 2010). Both NE and DA cause an increase in CRF signaling that results in enhancement of glutamatergic signaling into the BNST, including onto neurons that project to the VTA (Silberman et al, 2013). There is evidence that VTA-projecting neurons in the BNST activate DA neurons in the VTA via an excitatory amino acid pathway (Georges and Aston-Jones, 2001, 2002); however, both glutamatergic and GABAergic neurons in the vBNST project to the VTA and form connections with medial DA neurons as well as non-DA neurons (Kudo et al, 2012; Jennings et al, 2013). Hence, there is a complex circuit controlling the activity of dopaminergic VTA neurons by the vBNST through both direct and indirect excitatory and inhibitory projections (Jennings et al, 2013). Activation of the glutamatergic projection from the vBNST to the VTA resulted in aversive behaviors including avoidance of the chamber paired with a rewarding brain stimulation, a reduction in active reward seeking, and an increase in anxiety-like behavior in an open field test. In contrast, activation of the GABAergic projection from the vBNST to the VTA resulted in a combination of behaviors signaling a pleasurable state including preference for the chamber in which the stimulation occurred, active reward seeking, and anxiolytic behavior in an elevated plus maze task (Jennings et al, 2013). Similarly, stimulation of the projections from the AD BNST to the VTA, also a GABAergic projection, produced a conditioned place preference, indicating activation of this pathway is rewarding (Kim et al, 2013). GABAergic CRF neurons in the oval nucleus also project to the VTA (Rodaros et al, 2007; for a review on the control of VTA-DA neurons by the BNST see Jalabert et al, 2009). It is still unclear whether the GABAergic and glutamatergic projections from the BNST to the VTA result in an increase or decrease in DA release to the BNST and other regions. With both excitatory and inhibitory projections synapsing on dopaminergic cells, the circuit is not made obvious. In addition, excitatory and inhibitory projections synapse onto non-DA inhibitory interneurons in the VTA (for a review on the heterogeneity of the VTA, see Walsh and Han, 2014). Indeed, not all VTA DA neurons respond the same way to reward and stress, and therefore it is possible that one pathway from the BNST will increase activity of a subpopulation of DA neurons and inhibit others (Brischoux et al, 2009). Future experiments will need to isolate the effect of the different projections from the BNST to the VTA on DA cell firing. Of equal importance, new research will need to elucidate the role of DA in the BNST on motivated behavior. As the literature stands, DA is intricately involved in both reward and stress, but the precise mechanism of action is unknown.
Serotonin
The serotonin system is an important target for treatment of affective and anxiety disorders. The most commonly prescribed pharmacological treatments for depression and anxiety disorders are selective serotonin reuptake inhibitors (SSRIs) (Kent et al, 1998; Stokes and Holtz, 1997). Although SSRIs effectively treat depression in the long term, the therapeutic improvement only occurs after several weeks, and there is an acute effect of SSRI treatment associated with an exacerbation of the expression of fear and anxiety behavior in animals and humans (Burghardt et al, 2004; Grillon et al, 2007; for a review on the effect of SSRIs on fear conditioning in rodents, see Burghardt and Bauer, 2013). Intriguingly, a drug that enhances serotonin reuptake, tianeptine, has also been shown to be an effective antidepressant, specifically in patients with coexisting depression and anxiety (Wilde and Benfield, 1995). There is evidence that serotonin acts in the BNST to affect anxiety behavior in humans, non-human primates, and rodents alike. Acute tryptophan depletion, causing a reduction in serotonin levels in the brain, significantly increases long-duration anxiety-potentiated startle in humans while having no effect on short-duration fear-potentiated startle (Robinson et al, 2012). The possible role for serotonin in long-duration anxiety and not the phasic fear response implicates the BNST, as it is specifically involved in long- but not short-duration responses (Walker et al, 2009). In addition, serotonin transporter (5-HTT) availability in the BNST positively correlates with individual differences in anxious temperament in rhesus monkeys (Oler et al, 2009). This could imply that SSRIs affect anxiety in highly anxious individuals by inhibiting excess 5-HTT activity in the BNST, thereby increasing the amount of serotonin in the synapse. Indeed, serotonin fibers innervate both the dBNST and vBNST, with a denser innervation in the dBNST, and appear to make connections with the CRF cells in both of these regions (Commons et al, 2003; Phelix et al, 1992). The serotonin fibers originate in the dorsal raphe nucleus (DRN), specifically the medial to caudal aspect of the dorsal DRN (DRD) (Petit et al, 1995; Weller and Smith, 1982). In this region of the DRD, there is a cluster of CRF-containing cell bodies, most of which show dual labeling for serotonin, indicating that both CRF and serotonin could be coreleased into the BNST in some conditions (Commons et al, 2003). In order to understand the mechanisms behind the actions of SSRIs and to improve pharmacological therapy, a better knowledge of serotonin’s actions in the BNST and interaction with CRF is necessary.
Serotonergic neurons of the dorsal raphe are activated in response to uncontrollable stressors such as inescapable foot-shock, anxiogenic drugs, and social defeat, and CRF mediates this response (Abrams et al, 2005; Amat et al, 2005; Gardner et al, 2005; Hammack et al, 2002; Grahn et al, 1999; for a review on the functional neuroanatomy of defined serotonergic systems, see Lowry, 2002). CRF acts on both CRFR1 and CRFR2 in the DRD to affect serotonin release (Amat et al, 2004; Hale et al, 2010; Kirby et al, 2000; for a review on the interactions between CRF and sertonergic systems, see Fox and Lowry, 2013). At low doses, CRF inhibits firing in the DRN, but at higher doses, CRF becomes excitatory. The CRFR1 antagonist, antalarmin, attenuates the inhibitory effect of CRF at low doses. In contrast, the CRFR2 agonist, urocortin 2 (UCN 2), increases c-fos expression in serotonergic neurons of the DRD that project to limbic regions, including the BNST, and increases serotonin release (Amat et al, 2004; Hale et al, 2010; Staub et al, 2005). Because CRF has a higher binding affinity for CRFR1 than CRFR2, these data suggest that low levels of CRF inhibit the DRD through the CRFR1 receptor and high levels of CRF activate the serotonergic neurons of the DRD through the CRFR2 receptor. Interestingly, a selective CRFR2, but not CRFR1, antagonist in the DRD blocks the behavioral consequences of uncontrollable stress, indicating CRF acts on CRFR2 in the DRD to facilitate the prolonged activation of serotonergic neurons of the DRD in uncontrollable stress (Hammack et al, 2003). Importantly, the dBNST and vBNST provide input into the DRD, potentially contributing to the CRF projections there (Peyron et al, 1998).
The effects of serotonin on BNST circuitry are complex (for a summary, see Table 3). Serotonin acts presynaptically in the BNST to modulate glutamatergic transmission (Guo and Rainnie, 2010). In whole-cell patch-clamp recording experiments, serotonin application reduced the amplitude of evoked EPSCs (eEPSCs), which was accompanied by an increase in paired-pulse ratio. The nonselective 5-HT1B/D agonist sumatriptan and the selective 5-HT1B agonist CP93129 both mimicked the effect, whereas the 5-HT1B antagonist GR55562 attenuated the inhibitory effect of serotonin on eEPSC amplitude (Guo and Rainnie, 2010; however see Krawczyk et al, 2011a). In this way, serotonin release in the BNST after stress may counteract the facilitation of glutamatergic transmission into the BNST caused by CRF. Furthermore, it is possible that 5-HT1B receptor activation in the BNST limits other transmitter release into the BNST, such as CRF from the CeA, thereby providing an inhibitory control over the anxiety response after a stressor. More research on how serotonin and CRF interact to affect input into the BNST needs to be done to clarify this circuit.
The postsynaptic modulation of neurons in the BNST by serotonin is determined by the specific combination of serotonin receptor subtypes expressed in each individual neuron. The anterolateral BNST expresses mRNA transcripts for the 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1F, 5-HT2A, 5-HT2C, 5-HT3, 5-HT4, 5-HT5A, 5-HT6, and 5-HT7 receptors, and the complex response to serotonin mirrors the heterogeneous expression of serotonin receptor subtypes (Guo et al, 2009). Bath application of serotonin in the dBNST results in one of four responses: ∼16% of neurons exhibit a pure hyperpolarization response (5-HTHyp), ∼28% show hyperpolarization followed by a delayed depolarization response (5-HTHyp-Dep), and ∼34% show only depolarization (5-HTDep). Finally, ∼22% of cells show no postsynaptic response to serotonin application (5-HTNR). Because the depolarizing portion of the 5-HTHyp-Dep response was rarely large enough to overcome the initial hyperpolarization, the most common response to serotonin application in the dBNST is hyperpolarization (Guo et al, 2009; Levita et al, 2004). All of the postsynaptic responses to serotonin are associated with a decrease in membrane resistance and an increase in conductance, suggesting serotonin application results in a facilitation or opening of ion channels (Levita et al, 2004). In ∼25% of the cells that respond to serotonin with a 5-HTHyp response, the serotonin current reverses direction at −89 mV, close to the potassium equilibrium potential predicted by the Nernst equation. In fact, serotonin receptors can act to open GIRK channels. The hyperpolarizing response in these neurons could be blocked with a GIRK channel inhibitor, tertiapin-Q, confirming involvement of GIRK channels in the 5-HTHyp response. The other 75% of cells with a 5-HTHyp response exhibited a reversal potential ∼−74 mV that most likely reflects a combined reversal of a few different serotonin receptor subtypes including those that act on GIRK channels. The hyperpolarizing response to serotonin is most likely because of activation of the 5-HT1A receptor. Indeed, the hyperpolarizing response could be blocked by the 5-HT1A-specific antagonist WAY 100635 (Levita et al, 2004). The 5-HTDep response and the depolarizing component of the 5-HTHyp-Dep response are mediated by the 5-HT2A, 5-HT2C, and/or the 5-HT7 receptors. Neurons that exhibit a 5-HTHyp-Dep response only show a monophasic inward current in the presence of WAY 100635. This current can be attenuated by the 5-HT2A antagonist MDL 100907, the 5-HT2C antagonist RS 102221, and/or the 5-HT7 antagonist SB 269970, indicating that any combination of these receptors can contribute to the depolarization response to serotonin (Guo et al, 2009).
The complicated pharmacological profile of BNST neurons to serotonin suggests an equally complicated receptor expression pattern. Single-cell RT-PCR was used to screen mRNAs corresponding to the different serotonin receptor subtypes in individual dBNST neurons. Indeed, dBNST neurons exhibited a composite pattern of serotonin receptor gene expression, with some neurons expressing mRNA for one subtype and some expressing mRNA for two or three subtypes. As predicted by the primarily inhibitory nature of serotonin in the BNST, one of the most prominent receptor subtypes is 5-HT1A, expressed in 41% of neurons tested. The other common receptor subtype is 5-HT7, expressed in 46% of neurons, but 23% of those neurons that express 5-HT7 also coexpress 5-HT1A, predicting a combined hyperpolarizing and depolarizing response to serotonin. Indeed, the serotonin response profile predicted by the 5-HT receptor mRNA expression was not statistically different from the observed serotonin response profile of the BNST neurons (Guo et al, 2009). Interestingly, the three different cell types in the BNST (types I–III) express different distributions of serotonin receptor subtypes. In short, the type I cells express high levels of 5-HT1A mRNA, the type II cells express high levels of 5-HT7 and 5-HT1A mRNA, and the type III cells express high levels of 5-HT1A and 5-HT2C mRNA. As expected, the three cell types respond to serotonin application differently. The type III cells also express mRNA for the 5-HT1B receptor. As mentioned previously, this receptor is often expressed on axon terminals, indicating that the type III neurons express the 5-HT1B receptor on terminals in target areas to modulate neurotransmitter release (Guo et al, 2009). The type III neurons may also express the 5-HT1B receptor on local axon terminals, allowing serotonin to modulate local synaptic transmission as well. Interestingly, serotonin has been reported to bidirectionally modulate evoked IPSC (eIPSC) amplitude in the BNST (Krawczyk et al 2011a). The inhibitory effects of serotonin on eIPSCs can be mimicked by 5-HT1B agonists and blocked with 5-HT1B antagonists, indicating that local 5-HT1B expression in the BNST inhibits GABAergic transmission within the nucleus (unpublished observation). Importantly, the different serotonin receptor expression among the three cell types offers an opportunity for specific modulation of BNST neurons by serotonin ligands. As mentioned above, the type III neurons are mainly CRF neurons, hence drugs targeting the 5-HT2C or 5-HT1B receptor may specifically modulate CRF neurons.
The primary action of serotonin in the BNST is to inhibit neurons through both postsynaptic and presynaptic mechanisms. However, the BNST may also play a role in activating the serotonergic DRD neurons themselves. In a review on serotonin’s actions in the BNST, Hammack et al (2009) proposed a model in which the activation of the serotonin neurons of the DRD by the BNST forms a negative feedback loop to attenuate anxiety levels in the presence of a stressor. An acute stressor activates the BNST, causing an increase in CRF release in target areas including the DRD. With enough CRF, CRF acts on CRFR2 to increase serotonergic release in limbic regions including the BNST (Amat et al, 2004; Hale et al, 2010; Staub et al, 2005). Serotonin acts on the BNST in a primarily inhibitory manner, thereby inhibiting further CRF release and attenuating the anxiety response. However, maintaining this negative feedback loop requires the balance of serotonin receptor subtypes in the BNST to remain in favor of inhibition.
In support of this hypothesis, all cell types in the BNST express the mRNA for the 5-HT1A receptor that causes a hyperpolarizing inhibitory response (Guo et al, 2009; Hazra et al, 2012). Single-cell RT-PCR analysis showed that 5-HT1A is expressed in 63% of type I, 32% of type II, and 41% of type III neurons in the dBNST (Hazra et al, 2012). In support of the negative feedback hypothesis described above, there is evidence that 5-HT1A activation in the BNST results in a reduction of anxiety-like behavior. The 5-HT1 agonist 5-CT infused into the BNST significantly reduced the acoustic startle response in rats, indicating an anxiolytic-like effect (Levita et al, 2004). Consistent with this observation, cannabidiol (CBD) in the BNST attenuates expression of context fear conditioning and anxiety-like behavior (Gomes et al, 2011, 2012). CBD is a component of cannabis that has been shown to have antipsychotic, antidepressive, and anxiolytic effects, but does not have the psychotomimetic effects of cannabis. CBD can act as a 5-HT1A agonist as well as block reuptake and degradation of the endogenous cannabinoid anandamide. CBD in the BNST attenuates freezing and fear-induced increase in heart rate and mean arterial pressure (MAP) in a context previously paired with foot-shock. Pretreatment with the 5-HT1A antagonist, WAY 100635, reduced the behavioral and cardiovascular effects of CBD, indicating that CBD acts through the 5-HT1A receptor to affect the expression of contextual fear conditioning (Gomes et al, 2012). CBD in the BNST also decreases anxiety-like behavior in the elevated plus maze and Vogel conflict test through actions on 5-HT1A receptors. Similarly, the 5-HT1A receptor agonist, 8-OH-DPAT, also decreased anxiety-like behavior in these tests (Gomes et al, 2011). CBD also acts through 5-HT1A receptors in the BNST to modulate the BNST control of the parasympathetic cardiac response (Alves et al, 2010; Gomes et al, 2013). The BNST has a tonic inhibitory influence on the parasympathetic component of the baroreflex, but during acute restraint stress, the BNST activates the parasympathetic system to modulate the heart rate increase associated with acute stress (Crestani et al, 2006, 2009). This suggests that the two opposing parallel circuits in the BNST modulate the parasympathetic system in opposing ways during basal and stress states. CBD and 8-OH-DPAT facilitate baroreceptor reflex bradycardia in basal conditions, and this effect is blocked by WAY 100635 (Alves et al, 2010). This is in agreement with 5-HT1A activation inhibiting the inhibitory influence of the BNST on parasympathetic response. Similarly, CBD acts through 5-HT1A receptors in the BNST to enhance the increase in heart rate during restraint stress, consistent with 5-HT1A activation inhibiting the role of BNST in activating the parasympathetic system to modulate heart rate during acute stress (Gomes et al, 2013; for a review on the role of BNST in modulating autonomic functions, see Crestani et al, 2013). Although 5-HT1A activation facilitating the increase in heart rate seems in contrast to its role in reducing anxiety, it suggests that individual aspects of the response to acute stress are modulated by different circuits within the BNST, and serotonin affects multiple aspects of the circuit.
In contrast to 5-HT1A, 5-HT7 contributes to the depolarization response to serotonin in the BNST. 5-HT7 is the most commonly expressed serotonin receptor subtype in the BNST; however, it is not expressed in the type III neurons, whereas it is expressed in the majority of type I and type II cells (Guo et al, 2009; Hazra et al, 2012). This raises an intriguing question about the functional and behavioral role of the 5-HT7 receptors in the BNST. If the type III CRF neurons are the anxiogenic projection neurons of the dBNST, then it is likely that type I and/or type II cells provide an inhibitory control over the output of the CRF neurons as described previously. In this case, 5-HT7 activation could potentially facilitate the inhibition of CRF neurons by activating the local inhibitory circuit. In fact, 5-CT is a mixed 5-HT1/7 agonist but acts to reduce anxiety-like behavior in the BNST (Hammack et al, 2009). As type III neurons do not express 5-HT7, 5-CT would only act on 5-HT1A receptors, resulting in a hyperpolarizing response. In contrast, perhaps 5-CT has more of a mixed response in type I/II cells that could potentially result in depolarization because of the high prevalence of 5-HT7 receptors. This could further facilitate the anxiolytic effect of 5-CT by activating the inhibitory control over the type III cells. In this model, 5-HT7 receptor activation in the BNST would be hypothesized to be anxiolytic, but elsewhere in the brain, blockade of 5-HT7 receptors produces a fast antidepressive effect (Mnie-Filali et al, 2011; for a review on 5-HT7 and its role in nervous system disorders, see Hedlund, 2009). In fact, pharmacological blockade of the 5-HT7 receptor has been investigated as a potential antidepressant strategy. Unlike the SSRI fluoxetine, acute administration of the 5-HT7 antagonist, SB 269970, does not increase anxiety-like behavior in the open field test. In fact, coadministration of SB 269970 with fluoxetine counteracted the anxiogenic-like effect of fluoxetine alone. Furthermore, treatment with SB 269970 significantly reduced immobility time in the forced swim test, an important predictor of a successful antidepressant (Mnie-Filali et al, 2011). This suggests 5-HT7 activation in the BNST could potentially act to increase anxiety-like behavior. However, it is important to note that an anxiogenic role of a receptor in other parts of the brain does not mean it cannot play a different role in the BNST. This is a prime example of why it is crucial to better understand the local circuitry of the BNST and the role of specific receptor subtypes.
Along with the 5-HT7 receptor, the 5-HT2C receptor also acts in the BNST to depolarize neurons and affect anxiety-like behavior. 5-HT2C knockout mice are deficient in stress-induced activation of dBNST CRF neurons and show lower anxiety-like behavior relative to wild-type mice, indicating 5-HT2C is involved in facilitating the anxiety response through activation of CRF neurons in the BNST (Heisler et al, 2007). Unlike the 5-HT7 receptor, the 5-HT2C receptor is expressed almost exclusively in the type III putative CRF neurons in the dBNST (Guo et al, 2009; Hazra et al, 2012). There is also evidence that serotonin acts on 5-HT2C receptors in the vBNST to facilitate stress-induced anxiety-like behaviors. The potent stressor, CIE exposure, is known to increase general and social anxiety-like behavior in rodents and c-Fos expression in the vBNST. Peripheral injections of the selective 5-HT2C antagonist, SB 242,084, mitigate the CIE-induced increase in social anxiety-like behavior as well as the increase in c-fos expression in the vBNST (Marcinkiewcz et al, 2015). As mentioned previously, there is a CRF-dependent increase in glutamatergic input into the BNST after CIE (Silberman et al, 2013). Perhaps the increase in BNST activation due to CIE causes CRF to be released into the DRD, thereby increasing serotonin activity in the BNST. Serotonin can then act on 5-HT2C receptors in the BNST (potentially specifically on CRF neurons) to further increase their activity and facilitate anxiety-like behavior. In whole-cell patch-clamp recordings in the vBNST, CIE treatment increased neuronal excitability. These cells were induced to fire significantly more action potentials than neurons from animals not given CIE. This increase in firing rate was blocked with application of the 5-HT2C antagonist, RS 102221. In addition, bath application of mCPP, a 5-HT2C agonist, depolarized cells more in the CIE-treated group than control group, indicating enhancement of 5-HT2C signaling after withdrawal (Marcinkiewcz et al, 2015). This suggests that serotonin’s actions on 5-HT2C receptors in the BNST can actually create a feed-forward loop to facilitate the anxiety response. However, this feed-forward increase in activity would be tempered by serotonin’s ability to inhibit BNST activity through actions on 5-HT1A and 5-HT1B receptors. Therefore, serotonin’s effect on anxiety-like behavior may be critically dependent on the balance of excitatory and inhibitory serotonin receptors in the BNST (Hammack et al, 2009).
Importantly, chronic stress can alter the serotonin receptor subtype expression in the BNST, thereby potentially drastically altering its effects on the circuitry of the BNST and resulting anxiety-like behavior (Hazra et al, 2012). After 4 days of unpredictable shock stress (USS), there was a 2.8-fold decrease in 5-HT1A mRNA, 2-fold increase in 5-HT1B mRNA, and 3.5-fold increase in 5-HT7 mRNA in the BNST. Single-cell RT-PCR was also used to characterize the effects of stress on serotonin receptor expression in the different cell types. There was a reduction in the number of neurons expressing 5-HT1A mRNA across all cell types. As this is the primary inhibitory serotonin receptor, this reduction in expression may impair serotonin’s ability to complete the negative feedback loop required to dampen anxiety-like behavior. However, there was also an increase in the number of type III neurons that express the mRNA for the 5-HT1B receptor after chronic USS (Hazra et al, 2012). The increase in 5-HT1B expression in type III neurons could potentially act to compensate for the increase in the excitability of type III neurons after stress by inhibiting neurotransmitter release. Finally, more type I and type II cells expressed mRNA for the 5-HT7 receptor after chronic USS. If 5-HT7 receptor activation facilitates local inhibitory connections onto the anxiogenic output of the BNST, then this increase in expression could be counteracting the loss of 5-HT1A expression. On the other hand, if 5-HT7 activation facilitates the anxiety response, then the increase in 5-HT7 receptor expression combined with the decrease in 5-HT1A expression could result in a loss of the negative feedback loop between the BNST and DRD and facilitate serotonin’s ability to create a feed-forward increase in BNST activity. Overall, chronic USS seems to cause a shift from inhibitory to excitatory serotonergic control in the BNST after stress, but more research needs to be done to understand the effects of this change on BNST circuitry and the impact on anxiety-like behavior. The behavioral roles and physiological actions of the neuromodulators discussed above are summarized in Table 4.
STRESS MODULATION OF SYNAPTIC PLASTICITY
Synaptic plasticity is a mechanism by which brain circuits can use prior experience to restructure future responses. Stress is known to cause a long-lasting increase in anxiety-like behavior, and as BNST is a crucial structure in modulating both the stress response and anxiety behavior, it is reasonable to predict that stress would cause a long-lasting change in BNST synaptic plasticity. In fact, multiple studies have shown that stress can affect synaptic plasticity in the BNST (Conrad et al, 2011; Dabrowska et al, 2013b; Francesconi et al, 2009; Glangetas et al, 2013; McElligott and Winder, 2007), but the results of these studies are seemingly inconsistent. In this section, we will reexamine these studies in light of how stress interacts with neuromodulators as discussed above and new research on the distinct roles of different BNST subnuclei in modulating anxiety-like behavior.
Stress can result in structural and morphological changes in the BNST that are associated with changes in synaptic strength. For example, chronic unpredictable stress causes an increase in BNST but not amygdala volume (Pêgo et al, 2008). Similarly, there is a significant increase in dendritic branching in the BNST but not the CeA after chronic immobilization stress (Vyas et al, 2003). These studies suggest that the BNST is uniquely sensitive to significant neuronal plasticity after stress. In addition to gross morphological changes, alterations in glutamatergic receptor content and localization can occur with synaptic plasticity. A recent study looked at the effect of chronic stress on AMPA receptor distribution in the BNST. After 4 days of USS, no significant change was found in the labeling of the AMPA receptor subunit GluR1 in the dBNST. However, there was a trend toward an increase in the ratio of GluR1-labeled spines to GluR1-labeled dendrites after stress (Hubert and Muly, 2014). This indicates that there may be more AMPA receptors in the spines than other parts of the dendrites after stress. The authors speculate that looking at a specific cell type in the BNST may reveal a significant change in AMPA receptor expression that is being washed out in the average of all cell types in the dBNST. In fact, there is evidence that the type III neurons are more susceptible to stress-induced alterations in plasticity than type I and type II neurons as discussed shortly (Dabrowska et al, 2013b). Understanding how stress differentially affects the unique components of the BNST circuit will be critical to determining how the BNST contributes to the long-lasting increase in anxiety after chronic stress. It is also possible that stress does not affect AMPA receptor distribution, but rather changes NMDA receptor distribution (Hubert and Muly, 2014). Indeed, after 4 days of repeated restraint stress, there is a significant increase in protein expression of the NMDA subunit GluN1 (also known as GRIN1) in the synaptic membrane fraction of the dBNST (Dabrowska et al, 2013b). These changes in receptor distribution and dendritic morphology reflect long-lasting changes in the way the BNST responds to input after stress.
There are multiple studies that have begun to explore how acute or chronic stress modulates the response of the BNST to upstream inputs. Acute stress can cause a 10-Hz stimulation of the medial prefrontal cortex (mPFC) input into the BNST to switch from resulting in LTD to LTP. The functional consequence of this switch from LTD to LTP is unknown; perhaps, it acts to boost the signal of salient events after acute stress (Glangetas et al, 2013). The BNST serves as a relay between the mPFC and VTA DA neurons. This pathway is under the control of the CB1R that decreases mPFC glutamate inputs in the BNST (Massi et al, 2008). Interestingly, cannabinoid receptor 1 (CB1) knockout mice did not exhibit stable LTD in baseline conditions or stable LTP after acute stress. In fact, infusion of the CB1 antagonist into the BNST blocked the LTP elicited by stimulation of the mPFC in stressed wild-type mice (Glangetas et al, 2013). Besides the VTA, the BNST relays to multiple nuclei critically involved in the stress response, and therefore the plastic change of the mPFC to BNST glutamate transmission after acute stress will undoubtedly affect more than the VTA DA system.
Whereas acute stress can cause a switch from LTD to LTP, chronic stress has been shown to cause a LTD in the BNST. As mentioned previously, chronic restraint stress can cause LTD of the eEPSC in the BNST that is dependent on α1-ARs (McElligott et al, 2010). This LTD changes the set point for the response to future incoming stimuli. Because this LTD is maintained by a postsynaptic loss of function of CP-AMPARs, it is possible that different neurons in the BNST could experience different relative levels of LTD. The neurons that are incorporated into the circuit to reduce anxiety could experience a more significant depression than those in the anxiogenic pathway, thereby shifting the balance of the opposing circuits. On the other hand, the LTD could reduce the input that activates the anxiogenic circuit. In addition, it is possible some synapses will be depressed more than others in the same neuron, causing the cell to respond more or less to different inputs. Isolating the different inputs into the BNST and the different cell types within the BNST through optogenetic and other molecular techniques will help to decipher how chronic stress affects the multiple circuits within the nucleus.
A few studies have examined how LTP in the BNST changes in response to stress. In unstressed animals, type III cells in the dBNST achieve a significantly lower magnitude of LTP in response to high-frequency stimulation than both type I and type II cells; however, after 4 days of repeated restraint stress, type III cells achieve a significantly higher magnitude of LTP than type III cells of nonstressed animals. In contrast, there is no significant change in the magnitude in type I and type II cells after stress. This cell type-specific change in LTP is at least partially because of STEP downregulation after chronic stress (Dabrowska et al, 2013b). STEP is a known modulator of synaptic plasticity by dephosphorylating subunits of the NMDA receptor promoting their internalization (Goebel-Goody et al, 2012). In fact, STEP has been reported to regulate LTP in the amygdala, and the downregulation of STEP is thought to play a role in the etiology of stress-induced anxiety disorders (Paul et al, 2007; Yang et al, 2012). As mentioned previously, STEP is specifically expressed in CRF neurons in the dBNST. Importantly, rats that underwent repeated restraint stress showed less STEP mRNA and protein expression than controls, and there was a reduction in the number of type III cells that expressed the mRNA for STEP (Dabrowska et al, 2013b). Consequently, NMDA receptor dephosphorylation and internalization by STEP is attenuated. This evidence supports the idea that type III cells are buffered against LTP by STEP in control conditions; however, the loss of STEP after chronic stress makes them more susceptible to LTP induction. In support of this theory, intracellular administration of STEP abolished the stress-induced increase in LTP magnitude but had no effect in control animals (Dabrowska et al, 2013b). These data suggest that type III CRF neurons of the oval BNST are protected against overactivation during an acute stressor. Interestingly, a systemic injection of interleukin-1β, an immune challenge that activates the BNST, results in c-fos activation of ENK but not CRF-containing neurons (Day et al, 1999). This could be a result of the inhibitory influence of STEP buffering the CRF cells against activation due to a single stressor. Loss of this buffer would cause ectopic CRF cell activation resulting in overactivation of the anxiogenic pathway in the BNST.
Not all studies have shown an increase in LTP magnitude in the BNST after stress. In mice, both chronic treatment with cortisol and chronic social isolation caused an increase in anxiety-like behavior as measured in the elevated zero maze and open field test; however, there was a corresponding blunting of LTP in both groups (Conrad et al, 2011). In addition, there was significant blunting of LTP in animals that underwent an acute social isolation stressor (24 h instead of 6 to 8 weeks), although there was no effect of the acute social isolation on anxiety-like behavior. This suggests that the physiological changes because of stress precede the behavioral outcome. It is unknown why chronic stress in one case causes an increase in LTP magnitude in a population of cells in the dBNST, whereas another shows that chronic stress results in a decrease in LTP magnitude in the dBNST (Conrad et al, 2011; Dabrowska et al, 2013b), but there are many differences in the experiments that could contribute to this discrepancy. For example, although both studies were performed ex vivo, Dabrowska et al (2013b) used single-cell patch-clamp recordings, whereas Conrad et al (2011) used extracellular field potential recordings. In addition, the nature of the stressor was different; in the experiment performed by Dabrowska et al (2013b), the rats underwent 1 h of restraint stress for 4 consecutive days, whereas the mice in this experiment were either given 10 days of cortisol treatment or 6 to 8 weeks of social isolation. Other studies have shown different effects on the BNST from different types of stressors; for example, dorsal and ventral CRF mRNA both increase after an intermittent foot-shock stressor but are differentially affected by social defeat and yohimbine (Funk et al, 2006). Not all stressors have the same behavioral or physiological effect. Another potentially crucial difference between these experiments is the delay after the end of the stressor until the collection of the data. In the paper by Dabrowska et al (2013b), there were 6 days between the last day of restraint stress and recording, whereas in the paper by Conrad et al (2011), there were only 24 h between the end of the stressor and data collection. It is possible that the 6-day-delay allowed for a necessary incubation period during which there are long-term changes to the circuit. The time course and mechanism of these different changes in synaptic plasticity after stress need to be studied more to better understand the effect stress has on the circuit.
Another study showed withdrawal from alcohol and other drugs of abuse caused a long-lasting impairment in a different form of LTP in the juxtacapsular BNST, long-term potentiation of intrinsic excitability (LTP-IE) (Francesconi et al, 2009). Importantly, this study only included neurons in the juxtacapsular portion of the BNST (see Figure 1b). However, there is not a well-defined line between the juxtacapsular and oval portion of the BNST. Therefore, it is likely that the population sampled in the study performed by Francesconi et al (2009) was not entirely separate from the population sampled in Dabrowska et al (2013b), but rather overlapped to some unknown degree. Like stress, withdrawal from drugs of abuse is characterized by an increase in anxiety-like behavior, and drug withdrawal is known to be a potent stressor. In this study, a high-frequency stimulation of the stria (100 Hz for 1 s repeated five times with 10 s intervals) does not result in a long-lasting increase in the excitatory postsynaptic potential, but rather a long-lasting decrease in the threshold for action potential and corresponding increase in temporal fidelity of spiking. Protracted withdrawal from alcohol in alcohol-dependent rats leads to a significant reduction in LTP-IE in the BNST (Francesconi et al, 2009). This was replicated in withdrawal from cocaine and heroin, as well as with repeated ICV administration of CRF. In addition, treatment with the selective CRFR1 antagonist R121919 during withdrawal restored LTP-IE in alcohol-dependent rats. The authors conclude with a model in which the BNST acts as a brake on the CeA. When the BLA is active during times of stress or drug craving, the BNST can undergo LTP-IE, and with that an increase in temporal fidelity of firing, thus providing a bigger inhibitory control on the CeA, the output of the amygdala. But with drug dependence, or with chronic CRF and potentially chronic stress, there is a reduced capacity for LTP-IE, as well as reduced temporal fidelity in firing, making the BNST a less efficient brake on the CeA, resulting in increased emotional arousal (Francesconi et al, 2009). However, this conclusion needs to be reexamined in light of new evidence for opposing circuits of the BNST—one that promotes and another that inhibits anxiety (Kim et al, 2013; Jennings et al, 2013). Although there was no reported difference in the effect of protracted withdrawal on the different cell types, the loss of LTP-IE could have a relatively different effect on type III, putative CRF cells than type I and II cells. Type II cells in the oval nucleus of the BNST have a significantly lower threshold for action potential than type III cells in the oval and no significant difference in threshold than the type I cells (unpublished observation). In addition, type III cells have a lower resting membrane potential than both type I and type II cells (Hammack et al, 2007). If all of the cells in the BNST have a similar decrease in threshold after LTP-IE, type III cells will still be harder to activate than the type I and II cells. In fact, the authors report that there was a significant reduction in threshold for all three cell types in the BNST (type I, 5.63±1.4 mV(t=−4.04, P<0.01, n=6); type II, 5.22 ±1.0 mV (t=−5.49, P<0.01, n=6), and type III, 3.52±1.0 mV (t= −3.44, P<0.05, n 4)), but they do not directly compare the reduction in threshold between cell types. A one-way ANOVA using the average reduction in threshold, SD, and sample size for the three cell types provided shows a significant difference between cell types (P<0.05, F=4.160). Furthermore, Tukey’s multiple comparison test showed type III cells had a significantly lower reduction in threshold than type I cells (P<0.05). This might be consistent with a role for STEP in buffering type III cells against both forms of potentiation, classic LTP and LTP-IE, and move the threshold for type III cells further away from that of type I and type II. Perhaps, only the type I and type II cells are the ‘brake’ on the CeA. A more detailed knowledge of the circuit between the BNST and CeA as defined by electrophysiological cell type would help clarify this possibility. If the type I and type II cells are the ‘brake’ on the CeA and the type III cells promote an anxiety response, the lower threshold for action potential in the type I and type II cells could increase their ability to inhibit anxiety. But after withdrawal, or potentially chronic stress, this reduction in threshold is impaired, but the relative action potential thresholds between the cell types remain intact. However, as Dabrowska et al (2013b) suggests, the type III neurons may have lost STEP, an inhibitor of LTP. In this scenario, type I and type II cells will not be able to act as the proper brake on the CeA, and the type III CRF cells of the dBNST would have lost their intrinsic brake on plasticity, resulting in a shift in the balance of the opposing circuits in the BNST from anxiolytic to anxiogenic.
CLINICAL IMPLICATIONS
Research across species, from mice and rats to non-human primates and humans, is now highlighting the importance of the BNST in anxiety and addiction. For example, Pleil et al (2015) found an effect of chronic alcohol drinking on the BNST that was conserved between mice and monkeys. Importantly, recent neuroimaging studies have shown that the connectivity of the BNST in humans is in large part similar to that of rodents and non-human primates, with the addition of connections between the BNST and more rostral cortical areas such as the orbitofrontal cortex (Avery et al, 2014; Krüger et al, 2015). Functional imaging studies have shown that the BNST is hyperactive in patients with generalized anxiety disorder (Yassa et al, 2012) and in patients with specific phobias (Straube et al, 2007), consistent with a role for the BNST in pathological anxiety in humans. As we increase our understanding of the computing power of the BNST based on knowledge about discrete microcircuits and distinct cell types and how they are affected by stress, we hope to identify novel targets to pharmacologically manipulate portions of the circuit for clinical intervention. For example, targeted manipulations aimed to enhance the activity of STEP may lead to a novel treatment strategy for anxiety disorders as it has been shown to play an important role in the modulation of CRF cell plasticity (Dabrowska et al, 2013b). In addition, learning more about the role of specific serotonin receptor subtypes and how they change after stress may help to find pharmacological agents that could act to enhance the antidepressive and anxiolytic effects of SSRIs by blocking 5-HT receptors that may facilitate anxiogenic circuits. This review only begins to scratch the surface of the complex effects neuromodulators have on the BNST (for a brief summary see Table 4). Defining models of microcircuits within the BNST, like that depicted in Figure 1d, will allow clinical research to hone in on therapies that can act to maintain the critical balance between opposing pathways.
Beyond treatments for anxiety and depression, modulating the circuitry of the BNST has the potential to reveal possible treatments for drug addiction. With both aversive and rewarding pathways that are sensitive to stress modulation, the BNST is a prime target for intervention to prevent stress-induced drug recidivism. Learning more about how DA and norepinephrine affect different portions of the BNST circuit during drug use, withdrawal, and stress may help to find pharmacological agents that could buffer the detrimental effects of stress in recovering drug addicts thereby preventing relapse. As we learn more about these separate cell populations and their particular role in the circuitry of the BNST, future clinical studies will be able to better select drugs that can target the appropriate circuit for modulation.
FUTURE RESEARCH DIRECTIONS
With more precise molecular tools now available to dissect circuits in the BNST on the cellular level, the field has begun to move beyond the notion that the BNST has a univalent effect on anxiety-like behavior. Consequently, we must now extend these observations to examine how stress and drugs of abuse may affect the opposing portions of the circuit to modulate behavior. The use of optogenetic strategies, like those used in experiments by Kim et al (2013) and Jennings et al (2013) will be crucial in furthering our understanding of the role of specific inputs into the BNST as well as the local circuitry of the nucleus. These tools are made even more powerful by transgenic animals expressing Cre in specific cell populations, allowing for targeted expression of viral vectors (for a review on the use of optogenetic strategies in the BNST, see Sparta et al, 2013). The DREADDs will also be useful in teasing apart the behavioral role of specific cell types within the BNST as done in Pleil et al (2015). Moreover, our increasing knowledge of distinct cell types within the BNST and how they are affected by stress may be used to pharmacologically isolate portions of the circuit for clinical intervention.
Finally, future research will need to investigate how individual differences within the BNST circuit contribute to resiliency or sensitivity to chronic stressors. Clearly, not all people who experience chronic stress develop an anxiety disorder. Evidence suggests that the BNST mediates interindividual variation in anxiety-like behavior and generalization of fear in rats (Duvarci et al, 2009) and primates (Fox et al, 2010; Kalin et al, 2005; Oler et al, 2009); however, little is known about how this variation is coded in the circuit. Future research should investigate individual variation in anxiety behavior, stress response, and drug addiction, and aim to define electrophysiological and molecular correlates of these characteristics in the BNST. This will help to further narrow down potential targets for pharmacological intervention in people suffering from affective and anxiety disorders.
FUNDING AND DISCLOSURE
This work was funded by the following grants from the National Institutes of Health: MH-072908 to DGR, base grant ORIP/OD P51OD011132 (formerly NCRR P51RR000165) to the Yerkes National Primate Research Center, and 5F31MH-097331 to SED. The authors declare no conflict of interest.
References
Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA (2005). Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience 133: 983–997.
Albert PR, Vahid-Ansari F, Luckhart C (2014). Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Front Behav Neurosci 8: 199.
Alheid GF, Heimer L (1988). New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience 27: 1–39.
Alves FHF, Crestani CC, Gomes FV, Guimarães FS, Correa FMA, Resstel LBM (2010). Cannabidiol injected into the bed nucleus of the stria terminalis modulates baroreflex activity through 5-HT1A receptors. Pharmacol Res 62: 228–236.
Amat J, Baratta MV, Paul ED, Bland ST, Watkins LR, Maier SF (2005). Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci 8: 365–371.
Amat J, Tamblyn JP, Bland ST, Amat P, Foster AC, Watkins LR et al (2004). Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neurosci 129: 509–519.
Aston-Jones G, Delfs JM, Druhan J, Zhu Y (1999). The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann NY Acad Sci 877: 486–498.
Avery SN, Clauss JA, Winder DG, Woodward N, Heckers S, Blackford JU (2014). BNST neurocircuitry in humans. Neuroimage 91: 311–323.
Bale TL, Vale WW (2004). CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44: 525–557.
Banihashemi L, Rinaman L (2006). Noradrenergic inputs to the bed nucleus of the stria terminalis and paraventricular nucleus of the hypothalamus underlie hypothalamic-pituitary-adrenal axis but not hypophagic or conditioned avoidance responses to systemic yohimbine. J Neurosci 26: 11442–11453.
Bota M, Sporns O, Swanson LW (2012). Neuroinformatics analysis of molecular expression patterns and neuron populations in gray matter regions: the rat BST as a rich exemplar. Brain Res 1450: 174–193.
Bowers LK, Swisher CB, Behbehani MM (2003). Membrane and synaptic effects of corticotropin-releasing factor on periaqueductal gray neurons of the rat. Brain Res 981: 52–57.
Brischoux F, Chakraborty S, Brierley DI, Ungless MA (2009). Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci USA 106: 4894–4899.
Budygin EA, Park J, Bass CE, Grinevich VP, Bonin KD, Wightman RM (2012). Aversive stimulus differentially triggers subsecond dopamine release in reward regions. Neuroscience 201: 331–337.
Burghardt NS, Bauer EP (2013). Acute and chronic effects of selective serotonin reuptake inhibitor treatment on fear conditioning: implications for underlying fear circuits. Neuroscience 247: 253–272.
Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE (2004). The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry 55: 1171–1178.
Carboni E, Silvagni A, Rolando MT, Di Chiara G (2000). Stimulation of in vivo dopamine transmission in the bed nucleus of stria terminalis by reinforcing drugs. J Neurosci 20: RC102.
Cecchi M, Capriles N, Watson SJ, Akil H (2007). Beta1 adrenergic receptors in the bed nucleus of stria terminalis mediate differential responses to opiate withdrawal. Neuropsychopharmacology 32: 589–599.
Cecchi M, Khoshbouei H, Javors M, Morilak DA (2002). Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience 112: 13–21.
Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP (2007). Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci 27: 2025–2034.
Commons KG, Connolley KR, Valentino RJ (2003). A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology 28: 206–215.
Conrad KL, Louderback KM, Gessner CP, Winder DG (2011). Stress-induced alterations in anxiety-like behavior and adaptations in plasticity in the bed nucleus of the stria terminalis. Physiol Behav 104: 248–256.
Crestani CC, Alves FHF, Gomes FV, Resstel LB, Correa FM, Herman JP (2013). Mechanisms in the bed nucleus of the stria terminalis involved in control of autonomic and neuroendocrine functions: a review. Curr Neuropharmacol 11: 141–159.
Crestani CC, Alves FH, Resstel LB, Corrêa FM (2006). The bed nucleus of the stria terminalis modulates baroreflex in rats. Neuroreport 17: 1531–1535.
Crestani CC, Alves FHF, Tavares RF, Corrêa FMA (2009). Role of the bed nucleus of the stria terminalis in the cardiovascular responses to acute restraint stress in rats. Stress 12: 268–278.
Csáki Á, Kocsis K, Halász B, Kiss J (2000). Localization of glutamatergic/aspartatergic neurons projecting to the hypothalamic paraventricular nucleus studied by retrograde transport of [3H]d-aspartate autoradiography. Neuroscience 101: 637–655.
Cummings S, Elde R, Ells J, Lindall A (1983). Corticotropin-releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study. J Neurosci 3: 1355–1368.
Dabrowska J, Hazra R, Ahern TH, Guo J-D, McDonald AJ, Mascagni F et al (2011). Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: implications for balancing stress and affect. Psychoneuroendocrinology 36: 1312–1326.
Dabrowska J, Hazra R, Guo J-D, Dewitt S, Rainnie DG (2013a). Central CRF neurons are not created equal: phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Front Neurosci 7: 156.
Dabrowska J, Hazra R, Guo J-D, Li CC, DeWitt S, Xu J et al (2013b). Striatal-enriched protein tyrosine phosphatase-STEPs toward understanding chronic stress-induced activation of corticotrophin releasing factor neurons in the rat bed nucleus of the stria terminalis. Biol Psychiatry 74: 817–826. Evidence that the type III neurons of the BNST are more susceptible than type I and type II cells to stress-induced increases in neuronal plasticity.
Dabrowska J, Rainnie DG (2014). Targeting corticotropin releasing factor (CRF) projections from the bed nucleus of the stria terminalis (BNST) using cell-type specific neuronal tracing studies in mice and rats. Neuropsychopharmacology 39: S315–S316.
Davis M, Walker DL (2014). Role of bed nucleus of the stria terminalis and amygdala AMPA receptors in the development and expression of context conditioning and sensitization of startle by prior shock. Brain Struct Funct 219: 1969–1982.
Day HEW, Curran EJ, Watson SJ, Akil H (1999). Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1beta. J Comp Neurol 413: 113–128.
Day HEW, Masini CV, Campeau S (2004). The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res 1025: 139–151.
Day HEW, Vittoz NM, Oates MM, Badiani A, Watson SJ, Robinson TE et al (2002). A 6-hydroxydopamine lesion of the mesostriatal dopamine system decreases the expression of corticotropin releasing hormone and neurotensin mRNAs in the amygdala and bed nucleus of the stria terminalis. Brain Res 945: 151–159.
Deyama S, Katayama T, Ohno A, Nakagawa T, Kaneko S, Yamaguchi T et al (2008). Activation of the beta-adrenoceptor-protein kinase A signaling pathway within the ventral bed nucleus of the stria terminalis mediates the negative affective component of pain in rats. J Neurosci 28: 7728–7736.
Dong HW, Petrovich GD, Swanson LW (2000). Organization of projections from the juxtacapsular nucleus of the BST: a PHAL study in the rat. Brain Res 859: 1–14.
Dong HW, Petrovich GD, Swanson LW (2001a). Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev 38: 192–246.
Dong HW, Petrovich GD, Watts AG, Swanson LW (2001b). Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol 436: 430–455.
Dong HW, Swanson LW (2004). Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol 468: 277–298.
Dumont EC, Williams JT (2004). Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci 24: 8198–8204.
Duvarci S, Bauer EP, Pare D (2009). The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. J Neurosci 29: 10357–10361.
Egli RE, Kash TL, Choo K, Savchenko V, Matthews RT, Blakely RD et al (2004). Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology 30: 657–668. Norepinephrine acts on α 2-ARs to decrease excitatory transmission in the dorsal and ventral BNST; however, β -AR activation increases excitatory transmission in the dorsal but not ventral BNST.
Eiler WJA, Seyoum R, Foster KL, Mailey C, June HL (2003). D1 dopamine receptor regulates alcohol-motivated behaviors in the bed nucleus of the stria terminalis in alcohol-preferring (P) rats. Synapse 48: 45–56.
Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J (2000). Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology 23: 138–150.
Erb S, Salmaso N, Salmaso N, Rodaros D, Stewart J (2001). A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology 158: 360–365.
Erb S, Stewart J (1999). A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci 19: RC35.
Fendt M, Siegl S, Steiniger-Brach B (2005). Noradrenaline transmission within the ventral bed nucleus of the stria terminalis is critical for fear behavior induced by trimethylthiazoline, a component of fox odor. J Neurosci 25: 5998–6004.
Flavin SA, Matthews RT, Wang Q, Muly EC, Winder DG (2014). α(2A)-adrenergic receptors filter parabrachial inputs to the bed nucleus of the stria terminalis. J Neurosci 34: 9319–9331.
Forray MI, Bustos G, Gysling K (1999). Noradrenaline inhibits glutamate release in the rat bed nucleus of the stria terminalis: in vivo microdialysis studies. J Neurosci Res 55: 311–320.
Forray MI, Gysling K, Andrés ME, Bustos G, Araneda S (2000). Medullary noradrenergic neurons projecting to the bed nucleus of the stria terminalis express mRNA for the NMDA-NR1 receptor. Brain Res Bull 52: 163–169.
Fox AS, Shelton SE, Oakes TR, Converse AK, Davidson RJ, Kalin NH (2010). Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. J Neurosci 30: 7023–7027.
Fox JH, Lowry CA (2013). Corticotropin-releasing factor-related peptides, serotonergic systems, and emotional behavior. Front Neurosci 7: 169.
Francesconi W, Berton F, Repunte-Canonigo V, Hagihara K, Thurbon D, Lekic D et al (2009). Protracted withdrawal from alcohol and drugs of abuse impairs long-term potentiation of intrinsic excitability in the juxtacapsular bed nucleus of the stria terminalis. J Neurosci 29: 5389–5401. There is a CRF-dependent change in the plasticity of neurons in the BNST after withdrawal from drugs of abuse.
Freedman LJ, Cassell MD (1994). Distribution of dopaminergic fibers in the central division of the extended amygdala of the rat. Brain Res 633: 243–252.
Funk D, Li Z, Lê AD (2006). Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: relationship to the reinstatement of alcohol seeking. Neuroscience 138: 235–243.
Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA (2005). Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience 136: 181–191.
Georges F, Aston-Jones G (2001). Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci 21: RC160.
Georges F, Aston-Jones G (2002). Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci 22: 5173–5187.
Glangetas C, Girard D, Groc L, Marsicano G, Chaouloff F, Georges F (2013). Stress switches cannabinoid type-1 (CB1) receptor-dependent plasticity from LTD to LTP in the bed nucleus of the stria terminalis. J Neurosci 33: 19657–19663.
Goebel-Goody SM, Baum M, Paspalas CD, Fernandez SM, Carty NC, Kurup P (2012). Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders. Pharmacol Rev 64: 65–87.
Gomes FV, Alves FHF, Guimarães FS, Correa FMA, Resstel LBM, Crestani CC (2013). Cannabidiol administration into the bed nucleus of the stria terminalis alters cardiovascular responses induced by acute restraint stress through 5-HT1A receptor. Eur Neuropsychopharmacol 23: 1096–1104.
Gomes FV, Reis DG, Alves FHF, Correa FM, Guimaraes FS, Resstel LB (2012). Cannabidiol injected into the bed nucleus of the stria terminalis reduces the expression of contextual fear conditioning via 5-HT1A receptors. J Psychopharmacol 26: 104–113.
Gomes FV, Resstel LBM, Guimarães FS (2011). The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors. Psychopharmacology 213: 465–473.
Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR et al (1999). Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Research 826: 35–43.
Gray TS, Magnuson DJ (1992). Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides 13: 451–460.
Grillon C, Levenson J, Pine DS (2007). A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans: a fear-potentiated startle study. Neuropsychopharmacology 32: 225–231.
Guo J-D, Hammack SE, Hazra R, Levita L, Rainnie DG (2009). Bi-directional modulation of bed nucleus of stria terminalis neurons by 5-HT: molecular expression and functional properties of excitatory 5-HT receptor subtypes. Neuroscience 164: 1776–1793.
Guo J-D, Rainnie DG (2010). Presynaptic 5-HT(1B) receptor-mediated serotonergic inhibition of glutamate transmission in the bed nucleus of the stria terminalis. Neuroscience 165: 1390–1401. Serotonin acts on presynaptic 5-HT1B receptors in the BNST to inhibit glutamate transmission.
Hale MW, Stamper CE, Staub DR, Lowry CA (2010). Urocortin 2 increases c-Fos expression in serotonergic neurons projecting to the ventricular/periventricular system. Exp Neurol 224: 271–281.
Hammack SE, Guo J-D, Hazra R, Dabrowska J, Myers KM, Rainnie DG (2009). The response of neurons in the bed nucleus of the stria terminalis to serotonin: implications for anxiety. Prog in Neuropsychopharmacol Biol Psychiatry 33: 1309–1320. A review on how serotonin acts on different serotonin receptor subtypes to affect the output of the BNST and how chronic stress may alter serotonin receptor expression and therefore the actions of serotonin in the BNST.
Hammack SE, Mania I, Rainnie DG (2007). Differential expression of intrinsic membrane currents in defined cell types of the anterolateral bed nucleus of the stria terminalis. J Neurophysiol 98: 638–656. The neurons of the BNST ALG can be divided into three defined cell types based on intrinsic membrane currents.
Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF (2002). The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. J Neurosci 22: 1020–1026.
Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC et al (2003). Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci 23: 1019–1025.
Hasue RH, Shammah-Lagnado SJ (2002). Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol 454: 15–33.
Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R et al (2010). Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468: 270–276.
Hazra R, Guo J-D, Dabrowska J, Rainnie DG (2012). Differential distribution of serotonin receptor subtypes in BNST(ALG) neurons: modulation by unpredictable shock stress. Neuroscience 225: 9–21. Chronic stress alters the expression of serotonin receptor subtypes in the BNST, potentially shifting serotonin's action in the BNST from primarily inhibitory to more excitatory.
Hazra R, Guo J-D, Ryan SJ, Jasnow AM, Dabrowska J, Rainnie DG (2011). A transcriptomic analysis of type I-III neurons in the bed nucleus of the stria terminalis. Mol Cell Neurosci 46: 699–709.
Hedlund PB (2009). The 5-HT7 receptor and disorders of the nervous system: an overview. Psychopharmacology 206: 345–354.
Heisler LK, Zhou L, Bajwa P, Hsu J, Tecott LH (2007). Serotonin 5-HT(2C) receptors regulate anxiety-like behavior. Genes Brain Behav 6: 491–496.
Hott SC, Gomes FV, Fabri DRS, Reis DG, Crestani CC, Correa FMA et al (2012). Both α1- and β1-adrenoceptors in the bed nucleus of the stria terminalis are involved in the expression of conditioned contextual fear. Br J Pharmacol 167: 207–221.
Hubert GW, Muly EC (2014). Distribution of AMPA receptor subunit glur1 in the bed nucleus of the stria terminalis and effect of stress. Synapse 68: 194–201.
Ide S, Hara T, Ohno A, Tamano R, Koseki K, Naka T et al (2013). Opposing roles of corticotropin-releasing factor and neuropeptide Y within the dorsolateral bed nucleus of the stria terminalis in the negative affective component of pain in rats. J Neurosci 33: 5881–5894.
Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W (1991). Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci 11: 585–599.
Jalabert M, Aston-Jones G, Herzog E, Manzoni O, Georges F (2009). Role of the bed nucleus of the stria terminalis in the control of ventral tegmental area dopamine neurons. Prog Neuropsychopharmacol Biol Psychiatry 33: 1336–1346.
Janitzky K, D Hanis W, Kröber A, Schwegler H (2014). TMT predator odor activated neural circuit in C57BL/6J mice indicates TMT-stress as a suitable model for uncontrollable intense stress. Brain Res 1599: 1–8.
Jasnow AM, Davis M, Huhman KL (2004). Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropin-releasing factor in behavioral responses to social defeat. Behav Neurosci 118: 1052–1061.
Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL et al (2013). Distinct extended amygdala circuits for divergent motivational states. Nature 496: 224–228. The glutamatergic and GABAergic projections from the BNST to the VTA result in anxiogenic or anxiolytic behavior respectively.
Ju G, Swanson LW, Simerly RB (1989). Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: II. Chemoarchitecture. J Comp Neurol 280: 603–621.
Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ (2005). Brain regions associated with the expression and contextual regulation of anxiety in primates. Biol Psychiatry 58: 796–804.
Kash TL, Nobis WP, Matthews RT, Winder DG (2008). Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J Neurosci 28: 13856–13865. Dopamine increases CRF activity in the BNST thereby enhancing glutamatergic input into the nucleus through actions on CRFR1.
Kash TL, Winder DG (2006). Neuropeptide Y and corticotropin-releasing factor bi-directionally modulate inhibitory synaptic transmission in the bed nucleus of the stria terminalis. Neuropharmacology 51: 1013–1022.
Kent JM, Coplan JD, Gorman JM (1998). Clinical utility of the selective serotonin reuptake inhibitors in the spectrum of anxiety. Biol Psychiatry 44: 812–824.
Kim S-J, Park S-H, Choi S-H, Moon B-H, Lee K-J, Kang SW et al (2006). Effects of repeated tianeptine treatment on CRF mRNA expression in non-stressed and chronic mild stress-exposed rats. Neuropharmacology 50: 824–833.
Kim S-Y, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS et al (2013). Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496: 219–223. Cells in the oval nucleus promote anxiety-like behavior whereas the rest of the lateral dBNST promotes anxiolytic-like behavior and physiology.
Kimura A, Stevenson PL, Carter RN, MacColl G, French KL, Simons JP et al (2009). Overexpression of 5-HT2C receptors in forebrain leads to elevated anxiety and hypoactivity. Eur J Neurosci 30: 299–306.
Kirby LG, Rice KC, Valentino RJ (2000). Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology 22: 148–162.
Koob GF (2010). The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res 1314: 3–14.
Kormos V, Gaszner B (2013). Role of neuropeptides in anxiety, stress, and depression: from animals to humans. Neuropeptides 47: 401–419.
Krawczyk M, Georges F, Sharma R, Mason X, Berthet A, Bézard E et al (2011a). Double-dissociation of the catecholaminergic modulation of synaptic transmission in the oval bed nucleus of the stria terminalis. J Neurophysiol 105: 145–153.
Krawczyk M, Sharma R, Mason X, Debacker J, Jones AA, Dumont EC (2011b). A switch in the neuromodulatory effects of dopamine in the oval bed nucleus of the stria terminalis associated with cocaine self-administration in rats. J Neurosci 31: 8928–8935.
Krüger O, Shiozawa T, Kreifelts B, Scheffler K, Ethofer T (2015). Three distinct fiber pathways of the bed nucleus of the stria terminalis to the amygdala and prefrontal cortex. Cortex 66: 60–68.
Kudo T, Uchigashima M, Miyazaki T, Konno K, Yamasaki M, Yanagawa Y et al (2012). Three types of neurochemical projection from the bed nucleus of the stria terminalis to the ventral tegmental area in adult mice. J Neurosci 32: 18035–18046.
Larriva-Sahd J (2006). Histological and cytological study of the bed nuclei of the stria terminalis in adult rat. II. Oval nucleus: extrinsic inputs, cell types, neuropil, and neuronal modules. J Comp Neurol 497: 772–807.
Lee Y, Davis M (1997). Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci 17: 6434–6446.
Leri F, Flores J, Rodaros D, Stewart J (2002a). Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci 22: 5713–5718.
Leri F, Flores J, Rodaros D, Stewart J (2002b). Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci 22: 5713–5718.
Levita L, Hammack SE, Mania I, Li XY, Davis M, Rainnie DG (2004). 5-hydroxytryptamine1A-like receptor activation in the bed nucleus of the stria terminalis: electrophysiological and behavioral studies. Neuroscience 128: 583–596.
Liang KC, Chen HC, Chen DY (2001). Posttraining infusion of norepinephrine and corticotropin releasing factor into the bed nucleus of the stria terminalis enhanced retention in an inhibitory avoidance task. Chin J Physiol 44: 33–43.
Lowry CA (2002). Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol 14: 911–923.
Makino S, Gold PW, Schulkin J (1994). Effects of corticosterone on CRH mRNA and content in the bed nucleus of the stria terminalis; comparison with the effects in the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus. Brain Res 657: 141–149.
Mansari, El M, Guiard BP, Chernoloz O, Ghanbari R, Katz N, Blier P (2010). Relevance of norepinephrine-dopamine interactions in the treatment of major depressive disorder. CNS Neurosci Ther 16: e1–17.
Mantsch JR, Vranjkovic O, Twining RC, Gasser PJ, McReynolds JR, Blacktop JM (2014). Neurobiological mechanisms that contribute to stress-related cocaine use. Neuropharmacology 76 Pt B: 383–394.
Marcinkiewcz CA, Dorrier CE, Lopez AJ, Kash TL (2015). Ethanol induced adaptations in 5-HT2c receptor signaling in the bed nucleus of the stria terminalis: implications for anxiety during ethanol withdrawal. Neuropharmacology 89: 157–167.
Martin EI, Ressler KJ, Jasnow AM, Dabrowska J, Hazra R, Rainnie DG et al (2010). A novel transgenic mouse for gene-targeting within cells that express corticotropin-releasing factor. Biol Psychiatry 67: 1212–1216.
Massi L, Elezgarai I, Puente N, Reguero L, Grandes P, Manzoni OJ, Georges F (2008). Cannabinoid receptors in the bed nucleus of the stria terminalis control cortical excitation of midbrain dopamine cells in vivo. J Neurosci 28: 10496–10508.
McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M (1999). Cortical afferents to the extended amygdala. Ann NY Acad Sci 877: 309–338.
McElligott ZA, Klug JR, Nobis WP, Patel S, Grueter BA, Kash TL et al (2010). Distinct forms of Gq-receptor-dependent plasticity of excitatory transmission in the BNST are differentially affected by stress. Proc Natl Acad Sci USA 107: 2271–2276. With chronic stress, norepinephrine acts on α 1-ARs to cause a long-term depression in the response to glutmatergic input into the BNST.
McElligott ZA, Winder DG (2007). α1-adrenergic receptor-induced heterosynaptic long-term depression in the bed nucleus of the stria terminalis is disrupted in mouse models of affective disorders. Neuropsychopharmacology 33: 2313–2323.
Meloni EG, Gerety LP, Knoll AT, Cohen BM, Carlezon WA (2006). Behavioral and anatomical interactions between dopamine and corticotropin-releasing factor in the rat. J Neurosci 26: 3855–3863.
Mengod G, Villaró MT, Landwehrmeyer GB, Martinez-Mir MI, Niznik HB, Sunahara RK et al (1992). Visualization of dopamine D1, D2 and D3 receptor mRNAs in human and rat brain. Neurochem Int 20: 33S–43S.
Michaeli A, Yaka R (2010). Dopamine inhibits GABA(A) currents in ventral tegmental area dopamine neurons via activation of presynaptic G-protein coupled inwardly-rectifying potassium channels. J Neurosci 165: 1159–1169.
Miczek KA, Nikulina E, Kream RM, Carter G, Espejo EF (1999). Behavioral sensitization to cocaine after a brief social defeat stress: c-fos expression in the PAG. Psychopharmacology 141: 225–234.
Mnie-Filali O, Faure C, Lambás-Señas L, Mansari El M, Belblidia H, Gondard E et al (2011). Pharmacological blockade of 5-HT7 receptors as a putative fast acting antidepressant strategy. Neuropsychopharmacology 36: 1275–1288.
Nautiyal KM, Tanaka KF, Barr MM, Tritschler L, Le Dantec Y, David DJ et al (2015). Distinct circuits underlie the effects of 5-HT1B receptors on aggression and impulsivity. Neuron 86: 813–826.
Nakagawa T, Yamamoto R, Fujio M, Suzuki Y, Minami M, Satoh M et al (2005). Involvement of the bed nucleus of the stria terminalis activated by the central nucleus of the amygdala in the negative affective component of morphine withdrawal in rats. Neuroscience 134: 9–19.
Nobis WP, Kash TL, Silberman Y, Winder DG (2011). β-Adrenergic receptors enhance excitatory transmission in the bed nucleus of the stria terminalis through a corticotrophin-releasing factor receptor-dependent and cocaine-regulated mechanism. Biol Psychiatry 69: 1083–1090. Norepinephrine acts on β-ARs to increase CRF activity in the BNST thereby enhancing glutamatergic input into the nucleus through actions on CRFR1.
Oler JA, Fox AS, Shelton SE, Christian BT, Murali D, Oakes TR et al (2009). Serotonin transporter availability in the amygdala and bed nucleus of the stria terminalis predicts anxious temperament and brain glucose metabolic activity. J Neurosci 29: 9961–9966.
Onaka T, Yagi K (1998). Role of noradrenergic projections to the bed nucleus of the stria terminalis in neuroendocrine and behavioral responses to fear-related stimuli in rats. Brain Res 788: 287–293.
Park J, Bucher ES, Budygin EA, Wightman RM (2015). Norepinephrine and dopamine transmission in 2 limbic regions differentially respond to acute noxious stimulation. Pain 156: 318–327.
Park J, Bucher ES, Fontillas K, Owesson-White C, Ariansen JL, Carelli RM et al (2013). Opposing catecholamine changes in the bed nucleus of the stria terminalis during intracranial self-stimulation and its extinction. Biol Psychiatry 74: 69–76.
Park J, Kile BM, Wightman RM (2009). In vivo voltammetric monitoring of norepinephrine release in the rat ventral bed nucleus of the stria terminalis and anteroventral thalamic nucleus. Eur J Neurosci 30: 2121–2133.
Park J, Wheeler RA, Fontillas K, Keithley RB, Carelli RM, Wightman RM (2012). Catecholamines in the bed nucleus of the stria terminalis reciprocally respond to reward and aversion. Biol Psychiatry 71: 327–334.
Paul S, Olausson P, Venkitaramani DV, Ruchkina I, Moran TD, Tronson N et al (2007). The striatal-enriched protein tyrosine phosphatase gates long-term potentiation and fear memory in the lateral amygdala. Biol Psychiatry 61: 1049–1061.
Petit JM, Luppi PH, Peyron C, Rampon C, Jouvet M (1995). VIP-like immunoreactive projections from the dorsal raphe and caudal linear raphe nuclei to the bed nucleus of the stria terminalis demonstrated by a double immunohistochemical method in the rat. Neurosci Lett 193: 77–80.
Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH (1998). Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience 82: 443–468.
Pêgo JM, Morgado P, Pinto LG, Cerqueira JJ, Almeida OFX, Sousa N (2008). Dissociation of the morphological correlates of stress-induced anxiety and fear. Eur J Neurosci 27: 1503–1516.
Phelix CF, Liposits Z, Paull WK (1992). Serotonin-CRF interaction in the bed nucleus of the stria terminalis: a light microscopic double-label immunocytochemical analysis. Brain Res Bull 28: 943–948.
Phelix CF, Liposits Z, Paull WK (1994). Catecholamine-CRF synaptic interaction in a septal bed nucleus: afferents of neurons in the bed nucleus of the stria terminalis. Brain Res Bull 33: 109–119. Dopamine terminals make synaptic connections with the CRF neurons in the dBNST whereas norepinephrine terminals make synaptic connections with CRF neurons in the vBNST.
Pleil KE, Rinker JA, Lowery-Gionta EG, Mazzone CM, McCall NM, Kendra AM et al (2015). NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat Neurosci 18: 545–552.
Radley JJ, Gosselink KL, Sawchenko PE (2009). A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci 29: 7330–7340.
Robinson OJ, Overstreet C, Allen PS, Pine DS, Grillon C (2012). Acute tryptophan depletion increases translational indices of anxiety but not fear: serotonergic modulation of the bed nucleus of the stria terminalis? Neuropsychopharmacology 37: 1963–1971.
Rodaros D, Caruana DA, Amir S, Stewart J (2007). Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neurosci 150: 8–13.
Rodríguez-Sierra OE, Turesson HK, Pare D (2013). Contrasting distribution of physiological cell types in different regions of the bed nucleus of the stria terminalis. J Neurophysiol 110: 2037–2049.
Sahuque LL, Kullberg EF, Mcgeehan AJ, Kinder JR, Hicks MP, Blanton MG et al (2006). Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: role of CRF receptor subtypes. Psychopharmacology 186: 122–132.
Sakanaka M, Shibasaki T, Lederis K (1986). Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res 382: 213–238.
Saudou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L et al (1994). Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science 265: 1875–1878.
Savasta M, Dubois A, Scatton B (1986). Autoradiographic localization of D1 dopamine receptors in the rat brain with [3H]SCH 23390. Brain Res 375: 291–301.
Sawchenko PE, Swanson LW (1985). Localization, colocalization, and plasticity of corticotropin-releasing factor immunoreactivity in rat brain. Fed Proc 44: 221–227.
Scibilia RJ, Lachowicz JE, Kilts CD (1992). Topographic nonoverlapping distribution of D1 and D2 dopamine receptors in the amygdaloid nuclear complex of the rat brain. Synapse 11: 146–154.
Shaham Y, Highfield D, Delfs J, Leung S, Stewart J (2000). Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci 12: 292–302.
Shalev U, Morales M, Hope B, Yap J, Shaham Y (2001). Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology 156: 98–107.
Shin J-W, Geerling JC, Loewy AD (2008). Inputs to the ventrolateral bed nucleus of the stria terminalis. J Comp Neurol 511: 628–657.
Silberman Y, Matthews RT, Winder DG (2013). A corticotropin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of the stria terminalis. J Neurosci 33: 950–960. Norepinephrine and dopamine depolarize CRF neurons in the BNST, potentially increasing CRF release that acts to enhance glutamatergic transmission in the BNST.
Sink KS, Davis M, Walker DL (2013). CGRP antagonist infused into the bed nucleus of the stria terminalis impairs the acquisition and expression of context but not discretely cued fear. Learn Mem 20: 730–739.
Somerville LH, Whalen PJ, Kelley WM (2010). Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry 68: 416–424.
Sparta DR, Jennings JH, Ung RL, Stuber GD (2013). Optogenetic strategies to investigate neural circuitry engaged by stress. Behav Brain Res 255: 19–25.
Staub DR, Spiga F, Lowry CA (2005). Urocortin 2 increases c-Fos expression in topographically organized subpopulations of serotonergic neurons in the rat dorsal raphe nucleus. Brain Res 1044: 176–189.
Stokes PE, Holtz A (1997). Fluoxetine tenth anniversary update: the progress continues. Clin Ther 19: 1135–1250.
Straube T, Mentzel H-J, Miltner WHR (2007). Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage 37: 1427–1436.
Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, Ledoux JE (2004). Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience 128: 7–14.
Swanson LW, Sawchenko PE, Rivier J, Vale WW (1983). Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36: 165–186.
Swanson LW, Simmons DM (1989). Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: a hybridization histochemical study in the rat. J Comp Neurol 285: 413–435.
Tropea D, Capsoni S, Tongiorgi E, Giannotta S, Cattaneo A, Domenici L (2001). Mismatch between BDNF mRNA and protein expression in the developing visual cortex: the role of visual experience. Eur J Neurosci 13: 709–721.
Turesson HK, Rodriguez-Sierra OE, Pare D (2013). Intrinsic connections in the anterior part of the bed nucleus of the stria terminalis. J Neurophysiol 109: 2438–2450.
Valjent E, Pages C, Herve D, Girault J-A, Caboche J (2004). Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci 19: 1826–1836.
Valjent E, Pascoli V, Pascoli V, Svenningsson P, Svenningsson P, Paul S et al (2005). Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA 102: 491–496.
Van Bockstaele EJ, Peoples J, Valentino RJ (1999). A.E. Bennett Research Award. Anatomic basis for differential regulation of the rostrolateral peri-locus coeruleus region by limbic afferents. Biol Psychiatry 46: 1352–1363.
Vranjkovic O, Hang S, Baker DA, Mantsch JR (2012). β-adrenergic receptor mediation of stress-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: roles for β1 and β2 adrenergic receptors. J Pharmacol Exp Ther 342: 541–551.
Vyas A, Bernal S, Chattarji S (2003). Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res 965: 290–294. Chronic stress increases dendritic arborization of neurons in the BNST but not central amygdala.
Walker DL, Davis M (2008). Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct 213: 29–42. A review on the role of the BNST in sustained fear responses, implicating the BNST in anxiety responses.
Walker DL, Miles LA, Davis M (2009). Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry 33: 1291–1308.
Walsh JJ, Han MH (2014). The heterogeneity of ventral tegmental area neurons: projection functions in a mood-related context. Neuroscience 282C: 101–108.
Walter A, Mai JK, Lanta L, Görcs T (1991). Differential distribution of immunohistochemical markers in the bed nucleus of the stria terminalis in the human brain. J Chem Neuroanat 4: 281–298.
Watts AG, Kelly AB, Sanchez-Watts G (1995). Neuropeptides and thirst: the temporal response of corticotropin-releasing hormone and neurotensin/neuromedin N gene expression in rat limbic forebrain neurons to drinking hypertonic saline. Behav Neurosci 109: 1146–1157.
Weller KL, Smith DA (1982). Afferent connections to the bed nucleus of the stria terminalis. Brain Res 232: 255–270.
Wilde MI, Benfield P (1995). Tianeptine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in depression and coexisting anxiety and depression. Drugs 49: 411–439.
Yang C-H, Huang C-C, Hsu K-S (2012). A critical role for protein tyrosine phosphatase nonreceptor type 5 in determining individual susceptibility to develop stress-related cognitive and morphological changes. J Neurosci 32: 7550–7562.
Yassa MA, Hazlett RL, Stark CEL, Hoehn-Saric R (2012). Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. J Psychiatr Res 46: 1045–1052.
Zimmerman JM, Maren S (2011). The bed nucleus of the stria terminalis is required for the expression of contextual but not auditory freezing in rats with basolateral amygdala lesions. Neurobiol Learn Mem 95: 199–205.
Acknowledgements
We thank laboratory members for helpful discussions and critical reading of the manuscript. Especially, we thank Jidong Guo and Joanna Dabrowska for insightful comments and suggestions for this review article.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Daniel, S., Rainnie, D. Stress Modulation of Opposing Circuits in the Bed Nucleus of the Stria Terminalis. Neuropsychopharmacol 41, 103–125 (2016). https://doi.org/10.1038/npp.2015.178
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2015.178
This article is cited by
-
SIRT1 in the BNST modulates chronic stress-induced anxiety of male mice via FKBP5 and corticotropin-releasing factor signaling
Molecular Psychiatry (2023)
-
Constitutive 5-HT2C receptor knock-out facilitates fear extinction through altered activity of a dorsal raphe-bed nucleus of the stria terminalis pathway
Translational Psychiatry (2022)
-
Neuroendocrine control of appetite and metabolism
Experimental & Molecular Medicine (2021)
-
Functional deletion of neuropeptide Y receptors type 2 in local synaptic networks of anteroventral BNST facilitates recall and increases return of fear
Molecular Psychiatry (2021)
-
Effects of neural estrogen receptor beta deletion on social and mood-related behaviors and underlying mechanisms in male mice
Scientific Reports (2020)