Abstract
Central oxytocin (OXT) has anxiolytic and pro-social properties both in humans and rodents, and has been proposed as a therapeutic option for anxiety and social dysfunctions. Here, we utilized a mouse model of social fear conditioning (SFC) to study the effects of OXT on social fear, and to determine whether SFC causes alterations in central OXT receptor (OXTR) binding and local OXT release. Central infusion of OXT, but not arginine vasopressin, prior to social fear extinction training completely abolished social fear expression in an OXTR-mediated fashion without affecting general anxiety or locomotion. SFC caused increased OXTR binding in the dorso-lateral septum (DLS), central amygdala, dentate gyrus, and cornu ammunis 1, which normalized after social fear extinction, suggesting that these areas form part of a brain network involved in the development and neural support of social fear. Microdialysis revealed that the increase in OXT release observed in unconditioned mice within the DLS during social fear extinction training was attenuated in conditioned mice. Consequently, increasing the availability of local OXT by infusion of OXT into the DLS reversed social fear. Thus, alterations in the brain OXT system, including altered OXTR binding and OXT release within the DLS, play an important role in SFC and social fear extinction. Thus, we suggest that the OXT system is adversely affected in disorders associated with social fear, such as social anxiety disorder and reinstalling an appropriate balance of the OXT system may alleviate some of the symptoms.
Similar content being viewed by others
INTRODUCTION
The appropriate display of social behaviors is essential for the well-being and survival of social species, and disorders associated with social deficits, such as social anxiety disorder (SAD) are highly debilitating (Blanco et al, 2002; Labuschagne et al, 2010; Morrison and Heimberg, 2013). Therefore, substantial research efforts have been undertaken in order to identify the neuronal circuitries involved in the control of social behaviors under both normal and pathological conditions. Fear and avoidance of social situations are the main behavioral symptoms of SAD. Cognitive-behavioral therapy, especially exposure therapy, leads to gradual fear extinction as a result of repeated exposure to the feared situation and represents the best current therapeutic option (Blanco et al, 2013). However, this is not effective in all patients, and pharmacotherapies originally designed for depression or generalized anxiety, such as antidepressants and benzodiazepines, can also be used—either alone or in addition. A major problem with both treatment options is that many SAD patients achieve only partial remission of symptoms or show a high rate of relapse (Blanco et al, 2002, 2013). Therefore, development of approaches that combine psychotherapy with novel pharmacotherapies is required.
One potential therapeutic target is oxytocin (OXT), a neuropeptide synthesized within the hypothalamic paraventricular (PVN) and supraoptic nuclei, which acts on OXT receptors (OXTR) throughout the brain (Gimpl and Fahrenholz, 2001). Numerous animal studies have revealed anxiolytic (Bale et al, 2001; Waldherr and Neumann 2007; Blume et al, 2008; Knobloch et al, 2012; for review see Neumann and Landgraf, 2012) and pro-social (Donaldson and Young, 2008; Neumann, 2009) properties of OXT. For example, we have recently shown that brain OXT is essential for naturally-occurring social investigation in rodents, which is relevant in the context of SAD (Lukas et al, 2011). In humans, intranasal OXT has been shown to facilitate social encounters (Bartz and Hollander, 2006) and to attenuate anxiety and fear responses in social contexts both in healthy individuals (Petrovic et al, 2008; Kirsch et al, 2005) and in SAD patients (Guastella et al, 2009; Labuschagne et al, 2010). These OXT effects appear to involve attenuation of amygdala hyperactivity and its coupling with brainstem regions implicated in autonomic manifestations of fear (Kirsch et al, 2005; Viviani et al, 2011).
Here, we studied the effects of OXT in an animal model of social fear, namely social fear conditioning (SFC), that was established to mimic the major behavioral symptoms of SAD, ie, reduced social investigation and avoidance of conspecifics as indicative of social fear (Toth et al, 2013). In this murine model, social fear is induced by administration of foot shocks during the investigation of a conspecific (Toth et al, 2012b). Importantly, treatment of socially fear-conditioned (SFC+) mice with medication used for SAD patients, such as diazepam and paroxetine, reversed social fear, providing predictive validity to the SFC model (Toth et al, 2012b).
In the present study, we used the SFC model to investigate the involvement of the brain OXT system in social fear. We first assessed the effects of synthetic intracerebroventricular (i.c.v.) OXT infusion on social fear extinction, and the effects of SFC on OXTR binding in relevant brain regions. As OXTR binding was increased within the dorso-lateral septum (DLS), central amygdala (CeA) and hippocampus in SFC+ mice and the septum receives input from both these regions, we monitored local OXT release within the DLS during the SFC paradigm, and aimed to localize the effects of synthetic OXT on extinction within the DLS.
MATERIALS AND METHODS
Animals
Male CD1 mice (Charles River, Sulzfeld, Germany, 8 weeks old) were individually housed for 1 week and transferred to observation cages (30 × 23 × 36 cm) 3 days before the experiments started. Age- and weight-matched male CD1 mice were used as social stimuli. Mice were kept under standard laboratory conditions (12 : 12 light/dark cycle, lights on at 0600 hours, 22 °C, 60% humidity, food and water ad libitum). Experiments were performed between 0800 and 1200 hours in accordance with the Guide for the Care and Use of Laboratory Animals of the Government of Oberpfalz and the guidelines of the NIH.
SFC Paradigm
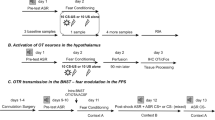
SFC (day 1)
Mice were carried from their home cage into a neighboring room and placed in the conditioning chamber (45 × 22 × 40 cm; transparent perspex box with a stainless steel grid floor) and, after a 30-s adaptation period, an empty wire mesh cage (7 × 7 × 6 cm) was placed as a non-social stimulus near one of the short walls of the chamber for 3 min. Immediately thereafter, the non-social stimulus was replaced by an identical cage containing an unfamiliar conspecific. Unconditioned control mice (SFC−) were allowed to freely investigate the social stimulus for 3 min without receiving any foot shocks, whereas SFC+ mice were given a 1-s electric foot shock (0.7 mA) each time they investigated, ie, made direct contact with the social stimulus. Thus, SFC+ mice could control the number of foot shocks received (between 2 and 5) and the inter-shock interval by approaching the social stimulus. Mice were returned to their home cage when no further social contact was made for 2 min. SFC− and SFC+ mice were videotaped, and the time mice spent investigating the stimuli was analysed by an observer blind to treatment using the JWatcher program (V 1.0, Macquarie University and UCLA). The time investigating the non-social stimulus was considered as a pre-conditioning measure of non-social anxiety, whereas investigation of the social stimulus indicated social interest and social fear, respectively (Toth et al, 2012b).
Social fear extinction training (day 2)
One day after SFC, mice were exposed in their home cage to three non-social stimuli, ie, three different, but technically identical, empty cages used during day 1, to assess non-social investigation as a parameter of non-social fear and general anxiety-related behavior. Mice were then exposed to six different unfamiliar social stimuli, ie, male mice, each in a different small cage (see day 1) to assess social investigation as a parameter of social fear. Each stimulus was placed near a short wall of the home cage and presented for 3 min, with a 3-min (for i.c.v. and DLS infusions) or 5-min (for microdialysis) inter-exposure interval.
Social fear extinction recall (day 3)
One day after extinction training, mice were exposed in their home cage to six different unfamiliar social stimuli, each in a different small cage (see days 1 and 2), for 3 min, with a 3-min inter-exposure interval.
Elevated Plus-Maze (EPM)
Possible drug effects on general, non-social anxiety were tested on the EPM 10 min after drug infusion as described before (Pellow et al, 1985; Toth et al, 2012b) in separate cohorts of naive mice. Increased time spent on the open arms (110 lux), calculated as a percentage from the total time spent on the EPM indicated reduced anxiety. The number of entries into the closed arms (25 lux) during the 5-min testing period indicated locomotor activity.
Home Cage Locomotion
Possible drug effects on locomotion (total distance travelled) were monitored in separate groups of naive mice in their home cage for 1 h using the Noldus system (Noldus Information Technology, Wageningen, NL) starting immediately after drug infusion (Toth et al, 2012a, 2012b).
Stereotaxic Implantations
Implantation of guide cannulas (21 G, 8 mm length; Injecta GmbH, Germany) for i.c.v. (from Bregma +0.2 mm, lateral +1.0 mm, depth −1.4 mm) or local bilateral (DLS; −0.3 mm, ±0.5 mm, −1.6 mm) infusions, and of microdialysis probes for monitoring OXT release within the DLS (U-shaped, molecular cutoff 10 kDa; −0.3 mm, +0.4 mm, −3.5 mm) was performed under isoflurane anesthesia (Forene, Abbott GmbH, Wiesbaden, Germany) according to Paxinos and Franklin, 1997, as previously described (Toth et al, 2012a; Neumann et al, 2013). To avoid post-surgical infections, mice received subcutaneous antibiotics (3 mg/30 μl Baytril, Bayer GmbH, Leverkusen, Germany). After surgery, mice were repeatedly handled before experiments started.
Intracerebral Infusions
Mice received i.c.v. or DLS infusions of either vehicle (Veh; sterile Ringer solution), synthetic OXT or arginine vasopressin (AVP; Sigma-Aldrich, Germany; to verify peptide specificity; i.c.v. OXT and AVP: 0.1 or 0.5 μg/2 μl; DLS OXT: 5 ng/0.2 μl/side), or of a selective OXTR antagonist (OXTR-A; desGly-NH2,d(CH2)5[Tyr(Me)2,Thr4]OVT; i.c.v.: 2 μg/2 μl) via an infusion cannula inserted into the guide cannula and connected to a Hamilton syringe. I.c.v. and local infusions were performed either 10 min before social fear extinction training and EPM testing or immediately before home cage locomotion testing.
The correct infusion site was histologically verified; accordingly, one DLS-infused mouse was removed from statistical analysis. OXT, AVP, and OXTR-A doses and timing were selected based on previous studies (Lukas et al, 2011; Kessler et al, 2011; Toth et al, 2012a).
Monitoring OXT Release by Microdialysis
One day after surgery (Nyuyki et al, 2012 for validation of experimental schedules), the microdialysis probe was perfused for 2 h with sterile Ringer solution (pH 7.4; 3.3 μl/min) to establish equilibrium between the inside and outside of the microdialysis membrane. Then, four consecutive 30-min dialysates were collected before (dialysates 1 and 2; basal conditions), during (dialysate 3), and after (dialysate 4; baseline conditions) SFC. On day 2, six dialysates were collected before (dialysates 1 and 2; basal conditions), during (dialysate 3 during exposure to the first three social stimuli, dialysate 4 during exposure to the next three social stimuli), and after (dialysate 5; basal conditions) extinction training. Dialysate 6 was collected during exposure to three non-social stimuli. Dialysates were immediately frozen and stored at −20 °C until quantification of OXT by a radioimmunoassay (sensitivity 0.3 pg/sample; RIAgnosis, Munich; Germany; for details: Neumann et al, 2013).
OXTR Autoradiography
The influence of SFC and its extinction on OXTR binding was studied in five groups of (1) naive mice, (2) SFC− mice without extinction training (SFC−/ext−), (3) SFC− mice with prolonged extinction training (SFC−/ext+), ie, exposure to 12 social stimuli instead of six, (4) SFC+ mice without extinction training (SFC+/ext−), (5) SFC+ mice with prolonged extinction training (SFC+/ext+). A prolonged social fear extinction training was applied to ensure complete extinction of social fear in all mice. We have previously shown that social fear is completely extinguished 24 h after the extinction procedure (Toth et al, 2012b).
Forty-eight hours after SFC (Litvin et al, 2011), and 24 h after social fear extinction training, all brains were removed, snap frozen, and stored at −20 °C. Brains were cut in 16-μm coronal sections targeting the infralimbic (IL) and prelimbic (PL) prefrontal cortex (Bregma 1.9–1.3 mm), cingulate (CC) and insular (INS) cortex, nucleus accumbens (NAc), medial septum (MS), ventro- (VLS) and dorso- (DLS) lateral septum (Bregma 1.3–0.6 mm), medial preoptic area (MPOA), bed nucleus of the stria terminalis (BNST) (Bregma 0.0–−0.6 mm), CeA, basolateral (BLA) and medial (MeA) amygdala, dentate gyrus (DG), cornu ammunis 1 (CA1), and CA3 (Bregma −1.2 to −1.8 mm). These brain regions were selected based on their role in conditioned fear and social behavior (Ledoux and Muller, 1997; Neumann, 2008; Knobloch et al, 2012). OXTR autoradiography was performed according to Lukas et al (2010) using a linear OXTR antagonist [125I]-d(CH2)5[Tyr(Me)2-Tyr-Nh2]9-OVT (Perkin Elmer). The exposure time varied between 10 days and 4 weeks depending on the OXTR density in the region of interest. OXTR binding was analysed using the ImageJ program (V1.37i, National Institute of Health) and was calculated for each mouse by taking the mean of 4–6 sections per region of interest. Background signal was subtracted to control for non-specific binding. Left and right regions were scored separately and pooled if no significant hemispheric difference was found.
Statistical Analysis
PASW/SPSS (Version 17) was used for data analysis employing either Student’s t-tests (Figure 2; Supplementary Figure S2D, E, J and K), Mann–Whitney U-tests (Figure 2 DLS), or one- (Supplementary Figure S2A, B, G and H), two- (Figure 1c and d; Figure 2; Figure 4; Figure 5; Supplementary Figure S2C,F and I), or three- (Figure 1a and b; Supplementary Figure S1) way ANOVA for repeated measures followed by a Bonferroni post hoc analysis, whenever appropriate. Statistical significance was set at p<0.05.
Social fear conditioning (SFC) and social fear extinction training alter oxytocin receptor (OXTR) binding. Male mice were exposed to SFC, with naive mice as control. Unconditioned (SFC−) and conditioned (SFC+) mice were exposed (ext+) or not (ext−) to social fear extinction training 24 h after SFC. Forty-eight hours after SFC, brains were collected and processed for OXTR autoradiography. DLS, dorso-lateral septum; DG, dentate gyrus; CA1, cornu ammunis 1; MPOA, medial preoptic area; CeA, central amygdala. Data represent means±SEM. *p<0.05 after analysis of variance and post hoc test; (*)p<0.05, Mann–Whitney U-test.
Intracerebroventricular oxytocin (OXT) abolishes social fear expression after social fear conditioning (SFC) in male mice via OXT receptors (OXTR), and brain OXT is required for social investigation. Percentage investigation of the non-social (empty cage) and social (cage with different conspecific) stimuli during social fear extinction training (a and c; day 2) and social fear extinction recall (b and d; day 3) after exposure to SFC on day 1. (a and b) Unconditioned (SFC−) and conditioned (SFC+) mice were infused i.c.v. with either vehicle (Veh) or OXT (0.1 or 0.5 μg/2 μl) 10 min before extinction training. (c and d) SFC− and SFC+ mice were infused i.c.v. with either Veh or OXTR antagonist (OXTR-A; 2 μg/2 μl) 40 min before extinction training. After 30 min, Veh-treated mice were infused with Veh, whereas OXTR-A-treated mice were infused with either Veh or OXT (0.1 μg/2 μl). Data represent means±SEM. (a and b) *p<0.05 vs SFC−/Veh and #p<0.01 vs SFC+/OXT 0.1 and SFC+/OXT 0.5. (c and d) *p<0.05 vs all groups; #p<0.05 vs all groups except SFC− OXTR-A/Veh;+p<0.05 vs SFC+/Veh/Veh.
Social fear conditioning (SFC) impairs oxytocin (OXT) release in the dorso-lateral septum. Mice were exposed to SFC on day 1 and to social fear extinction training on day 2. On day 1, four consecutive 30-min dialysates were collected before (dialysates 1 and 2; basal conditions), during (dialysate 3), and after (dialysate 4) exposure to SFC (SFC+) or to social stimuli without foot shocks (SFC−). On day 2, six dialysates were collected before (dialysates 1 and 2), during (dialysate 3 during exposure to social stimuli #1–3, dialysate 4 during exposure to social stimuli #4–6), and after (dialysate 5; basal conditions) extinction training. Dialysate 6 was collected during exposure to three non-social stimuli. Right panel: percentage investigation of the social (cage with conspecific) and non-social (empty cage) stimuli during extinction training on day 2. Data represent means±SEM. *p<0.05 vs SFC+ (day 2); #p<0.05 vs all other dialysates in SFC− mice.
Oxytocin (OXT) abolishes social fear expression within the dorso-lateral septum (DLS). Percentage investigation of the non-social (empty cage) and social (cage with conspecific) stimuli during social fear extinction training (a) and social fear extinction recall (b). Unconditioned (SFC−) and conditioned (SFC+) mice were infused bilaterally into the DLS with either vehicle (Veh) or OXT (5 ng/0.2 μl/side) 10 min before extinction training. Data represent means±SEM. *p<0.05 vs both other groups.
RESULTS
All mice showed similar investigation of the non-social stimuli during SFC in all experiments. All SFC+ mice received a similar number of foot shocks during SFC.
I.c.v. OXT Abolished Social Fear Expression
To verify whether OXT affects extinction, SFC+ and SFC− mice (n=6–10/group; 6 groups) were infused i.c.v. with either Veh or OXT (0.1 or 0.5 μg) 10 min before extinction training.
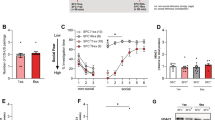
During extinction training (stimulus × SFC × treatment F(16,368)=3.70, p<0.01), Veh-treated SFC+ mice showed reduced social investigation reflecting social fear compared with SFC− controls (p<0.05 vs SFC−/Veh; Figure 1a). Both doses of OXT increased social investigation in SFC+ mice to levels found in SFC− mice already during the first exposure (p<0.01 vs SFC−/Veh; Figure 1a) reflecting abolishment of fear expression. During recall (SFC × treatment F(2,46)=2.98, p<0.05; stimulus × treatment F(10,230)=2.28, p<0.01), Veh-, but not OXT-treated SFC+ mice, still showed reduced social investigation compared with their respective SFC− controls (p<0.05; Figure 1b).
OXT Abolishes Social Fear Expression via OXTR
To verify whether OXT effects are mediated by OXTR, SFC+ and SFC− mice (n=7/group; five groups) were infused i.c.v. with either Veh or OXTR-A 40 min before extinction training. After 30 min, Veh-treated mice were infused with Veh, whereas OXTR-A-treated mice were infused with either Veh or OXT (0.1 μg).
During extinction training (stimulus × group F(32,240)=2.61, p<0.01), reduced social investigation was found in both OXTR-A/Veh-treated SFC− and OXTR-A/OXT-treated SFC+ mice (p<0.05 vs SFC−/Veh/Veh mice; Figure 1c). During recall (group F(4,30)=3.87, p<0.01), Veh/Veh-treated SFC+ mice still showed reduced social investigation (p<0.05 vs SFC−/Veh/Veh mice; Figure 1d).
I.c.v. AVP does not Affect Social Fear Expression
To confirm peptide specificity, separate SFC+ and SFC− mice (n=5–6/group; four groups) were infused i.c.v. with either Veh or AVP 10 min before extinction training.
During both extinction training (SFC F(1,19)=20.95, p<0.01) and recall (SFC F(1,19)=9.95, p<0.01), SFC+ mice showed reduced social investigation compared with SFC− mice, independent of treatment (p<0.05 vs SFC− mice; Supplementary Figure S1).
SFC and Extinction Training Alter OXTR Binding
To determine whether SFC and extinction training alter OXTR binding, receptor autoradiography was used. There was no difference between naive and SFC−/ext− mice in any of the brain regions investigated (Figure 2). A two-way ANOVA performed on SFC−/ext−, SFC−/ext+, SFC+/ext−, and SFC+/ext+ mice revealed a significant SFC × extinction interaction bilaterally in the DLS (F(1,19)=5.77, p<0.05), and in the right DG (F(1,19)=10.30, p<0.01), CA1 (F(1,19)=5.18, p<0.05), and CeA (F(1,18)=5.56, p<0.05). In these regions SFC+/ext− mice showed an increased OXTR binding compared with SFC−/ext− mice (p<0.05; Figure 2; Figure 3). After extinction of social fear, however, OXTR binding no longer differed in these regions between SFC+ and SFC− mice. Separate statistics revealed a decreased OXTR binding in the DLS of SFC+/ext+ compared with SFC+/ext− mice (p<0.05; Mann–Whitney U-test). Moreover, a significant extinction effect was observed within the MPOA (F(1,13)=54.71, p<0.01; Figure 2), where OXTR binding was increased in both SFC+ and SFC− mice (p<0.05 vs respective ext− group). No significant effects were found in the IL, PL, CC, INS, NAc, VLS, MS, BNST, BLA, MeA, and CA3 (Supplementary Table S1).
Representative images of oxytocin receptor (OXTR) binding at different brain levels. Mice were exposed to social fear conditioning (SFC), with naive mice as control. Unconditioned (SFC−) and conditioned (SFC+) mice were exposed (ext+) or not (ext−) to social fear extinction training 24 h after SFC. Forty-eight hours after SFC, brains were collected and processed for OXTR autoradiography. DLS, dorso-lateral septum; MPOA, medial preoptic area; CA1, cornu ammunis 1; DG, dentate gyrus; CeA, central amygdala.
SFC Blocks the Increase in OXT Release within the DLS
To verify whether SFC alters OXT release within the DLS, microdialysates were collected from SFC− and SFC+ mice (n=8–9/group) during SFC and extinction training.
During SFC, SFC− and SFC+ mice showed similar OXT release within the DLS, which was not significantly altered by social exploration (SFC−) or SFC exposure (SFC+; Figure 4, day 1). During extinction training on day 2 (SFC × time F(5,75)=5.49, p<0.01), however, OXT release was increased in SFC−, but not in SFC+ mice (p<0.05 vs basal; Figure 4, day 2 left panel). The SFC paradigm was succesful (stimulus × SFC F(8,120)=6.47, p<0.01), as indicated by the reduced social investigation in SFC+ mice during extinction training (p<0.05 vs SFC− mice; Figure 4, day 2 right panel).
OXT Abolishes Social Fear Expression within the DLS
To determine whether alterations in OXTR binding and OXT release in the DLS are involved in social fear, SFC− and SFC+ mice (n=7–8/group; three groups) were infused bilaterally into the DLS with either Veh or OXT 10 min before extinction training.
During extinction training (stimulus × group F(16,160)=5.58, p<0.01) Veh-treated SFC+ mice showed reduced social investigation (p<0.01 vs SFC−/Veh and SFC+/OXT groups; Figure 5a) reflecting social fear, whereas OXT increased social investigation to levels found in SFC− mice, starting from the first social stimulus, reflecting a complete abolishment of social fear expression. During recall (stimulus × group F(10,100)=2.17, p<0.01) Veh-treated SFC+ mice still showed reduced social investigation (p<0.05 vs both SFC−/Veh and SFC+/OXT groups; Figure 5b).
Effects of OXT, OXTR-A, and AVP on Non-Social Anxiety and Locomotion
To verify the specificity of neuropeptide effects on SFC, separate groups of mice (n=6–9/group) were infused i.c.v. or within the DLS with either Veh, OXT, OXTR-A, or AVP before testing on the EPM or in the home cage.
Neither i.c.v.- nor DLS-infused OXT affected anxiety or locomotion on the EPM (Supplementary Figure S2). Home cage locomotion (F(2,17)=6.88, p<0.01) was increased by 0.5 μg, but not by 0.1 μg i.c.v. OXT (p<0.05 vs Veh-treated mice) as previously reported (Toth et al, 2012a). Also, i.c.v. OXTR-A did not affect non-social anxiety, or EPM and home cage locomotion. Compared with Veh-treated mice, both doses of i.c.v. AVP increased anxiety on the EPM (F(2,19)=19.76, p<0.01; p<0.05), whereas 0.5 μg, but not 0.1 μg, AVP decreased EPM (F(2,19)=4.39, p<0.01; p<0.5) and home cage (treatment × time F(10,75)=3.17, p<0.01; p<0.05) locomotion (Supplementary Figure S2).
DISCUSSION
The present study demonstrates that OXT, particularly within the DLS, abolishes SFC-induced fear of unknown conspecifics. The reversal of social fear in male SFC+ mice by OXT is an OXTR-mediated effect, which is not accompanied by any confounding behavioral alterations. The SFC-induced social fear was accompanied by increased OXTR binding in the DLS, CeA, DG, and CA1; effects that were normalized by social fear extinction. Further, a lack of increase in OXT release within the DLS of SFC+ mice was found during repeated exposure to unknown conspecifics during social fear extinction training, indicating that a lack of local OXT availability might cause social fear. Indeed, bilateral infusion of OXT into the DLS before extinction training reversed social fear. These results suggest that the DLS OXT system is part of a brain network importantly involved in the expression and extinction of social fear.
OXT increased social investigation only in SFC+, but not in SFC− mice. This is in agreement with our findings in male rats, where OXT reinstated social preference in socially defeated rats (Lukas et al, 2011), and findings in male goldfish, in which isotocin (the teleost correspondent of OXT) increased social approach only in individuals with low sociability (Thompson and Walton, 2004). Although synthetic OXT was shown to decrease general anxiety, when infused directly into the PVN (Blume et al, 2008; Jurek et al, 2012) or CeA (Bale et al, 2001), i.c.v. OXT did not alter non-social anxiety on the EPM, as previously reported under unstressed conditions (Slattery and Neumann, 2010). Thus, the reversal of social fear by i.c.v. OXT is unlikely due to its general anxiolytic properties. In agreement with the specific reversal of social fear by i.c.v. OXT in the present study, we have recently shown that, in contrast, OXT significantly delays cued fear extinction when applied i.c.v. at a comparable time point (Toth et al, 2012a). In support, pharmacogenetic overexpression of the OXTR within the lateral septum resulted in increased contextual fear expression (Guzman et al, 2013). Therefore, the effect of OXT on social fear may relate to the social aspect of the SFC paradigm and the pro-social properties of OXT.
Furthermore, the increased social investigation in OXT-treated SFC+ mice was not due to an increased locomotion, as neither EPM nor home cage locomotion was altered after OXT (0.1 μg) treatment. However, the higher OXT dose (0.5 μg) increased home cage locomotion, but slightly decreased social investigation in both SFC+ and SFC− mice, indicating that, if anything, treatment-induced increases in locomotion have a rather detrimental effect on social investigation. However, given that OXT effects on non-social anxiety and locomotion were only assessed in naive mice, it is possible that SFC+ mice are more sensitive to the stimulatory and anxiolytic properties of OXT, which will be investigated in future studies.
We also showed that the OXT-induced abolishment of social fear was mediated via the OXTR, as pre-administration of an OXTR-A blocked this effect, and infusion of the closely-related neuropeptide AVP did not affect social fear. However, the anxiogenic effect of AVP on the EPM could be confirmed (Bhattacharya et al, 1998; Kessler et al, 2011), again revealing a separation between general- and social-anxiety. Interestingly, OXTR-A administration itself decreased social investigation in SFC− mice, thus confirming the essential contribution of endogenous OXT to naturally occurring social investigation (Lukas et al, 2011).
Considering these findings and previous studies showing social interaction (Murakami et al, 2011) and social defeat-induced (Litvin et al, 2011) alterations in OXTR expression, we next determined the effect of SFC on OXTR binding in brain regions involved in social behavior and conditioned fear (Ledoux and Muller, 1997; Neumann, 2008). Whereas a 3-min exposure to a social stimulus on day 1 was not sufficient to alter OXTR binding in SFC− mice, OXTR binding was altered as a result of repeated social exposure during extinction training on day 2, ie, it increased in the MPOA of both SFC+ and SFC− mice. As previous studies showed increased c-Fos expression in the MPOA in response to same-sex social stimuli (Ferguson et al, 2001), and OXT infusion into the MPOA facilitated social recognition (Popik and van Ree, 1991), the increased OXTR binding might be relevant for recognition of social stimuli, particularly as it occurs in both SFC+ and SFC− mice.
Importantly, OXTR binding was increased in the CeA, DG, CA1, and DLS of SFC+/ext− mice, which normalized after extinction of social fear. Thus, alterations in OXTR binding might be involved in the development, extinction, and/or maintenance of social fear. In detail, the CeA coordinates the behavioral and physiological correlates of fear expression (LeDoux et al, 1988; Kalin et al, 1998). Both local infusion (Roozendaal et al, 1992; Viviani et al, 2011) and evoked axonal release (Knobloch et al, 2012) of OXT attenuated the fear response in rodents. Interestingly, SAD patients show hyperactivity of the amygdala in response to threatening faces, which can be attenuated by intranasal OXT (Kirsch et al, 2005; Labuschagne et al, 2010). Therefore, the increase in OXTR binding may be relevant for processing threatening stimuli and for the display of social fear. The DG and CA1 are important regions for memory processing both in humans (Nadel et al, 2007) and rodents (Corcoran and Maren, 2001) and facilitate, together with the amygdala, contextual fear conditioning (Kim et al, 1993). Therefore, the increase in OXTR binding may be relevant for processing context-dependent information related to social fear. Although the mechanisms underlying the unilateral changes observed in the CeA, DG, and CA1 are unclear, hemispheric differences in stress responses have repeatedly been demonstrated (for review see Sullivan, 2004).
Our finding of increased DLS OXTR binding is in accordance with the observation of increased OXTR mRNA in the lateral septum of chronically defeated mice (Litvin et al, 2011). The DLS is also involved in social and associative memory, where OXT facilitates (Popik et al, 1992) and OXTR-A impairs (Lukas et al, 2013) male social recognition. Taken together, these findings suggest that the DLS is relevant for recognition of stimuli associated with the social conditioning procedure (Calandreau et al, 2007). Considering that the same pattern of OXTR-binding alterations was found in the CeA, hippocampus, and DLS and the interconnectivity between these regions (Sheehan et al, 2004), these limbic areas seem to be part of a brain network involved in the development, extinction, and/or maintenance of social fear.
The increased OXTR binding in the DLS of SFC+/ext− mice implies a compensatory mechanism for the lack of local OXT release and, thus, extracellular OXT availability. Septal OXT release, from projections originating in the hypothalamus (De Vries and Buijs, 1983), has been described during stressful social interactions, such as social defeat in male rats (Ebner et al, 2000). Here, we could not detect a significant increase in DLS OXT release in response to either brief social investigation in SFC− mice or during exposure to foot shocks in SFC+ mice. In contrast, during repeated social investigation during extinction training, a significant increase in OXT release was observed in SFC−, but not in SFC+ mice. The lack of increase in OXT release within the DLS in response to repeated social stimulation appears to be important for social fear expression, as substitution of OXT by bilateral infusion of synthetic OXT abolished social fear expression. However, future studies are needed to determine local OXT release and local OXT effects also within the CeA, DG, or CA1—other regions of altered OXTR binding in SFC+/ext− mice and known components of the fear circuitry.
Although most human studies focus on the amygdala in relation to social fear (Kirsch et al, 2005; Labuschagne et al, 2010), our findings suggest the involvement of a more complex brain network and of the OXT system in the development and extinction of social fear. While our data implicate OXT as a treatment option for disorders associated with social fear, such as SAD, more detailed studies regarding acute and long-term treatment effects on molecular, neuronal, and behavioral levels are required. This is more evident as chronic central as well as peripheral OXT treatment in mice affected brain OXTR binding (Huang et al, 2013; Peters et al, 2014).
In summary, we have shown that the brain OXT system including OXTR binding and OXT release is altered in socially fearful mice, and that these alterations normalized after extinction of social fear. Further, we have shown that OXT can reverse social fear, an effect that we localized within the DLS. Thus, our findings suggest that the brain OXT system, particularly within the DLS, is involved in the acquisition and extinction of social fear.
FUNDING AND DISCLOSURE
IZ received financial support from the Bayerische Forschungsstiftung. IDN received financial support from BMBF, EU (7th FP), and Deutsche Forschungsgemeinschaft. The authors declare no conflict of interest.
References
Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM (2001). CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci 21: 2546–2552.
Bartz JA, Hollander E (2006). The neuroscience of affiliation: forging links between basic and clinical research on neuropeptides and social behaviour. Horm Behav 50: 518–528.
Bhattacharya SK, Bhttacharya A, Chakrabarti A (1998). Anxiogenic activity in intraventricularly administered arginine vasopressin in the rat. Biogenic Amines 14: 367–385.
Blanco C, Antia SX, Liebowitz MR (2002). Pharmacotherapy of social anxiety disorder. Biol Psychiatry 51: 109–120.
Blanco C, Bragdon LB, Schneier FR, Liebowitz MR (2013). The evidence-based pharmacotherapy of social anxiety disorder. Int J Neuropsychopharmacol 16: 235–249.
Blume A, Bosch OJ, Miklos S, Torner L, Wales L, Waldherr M et al (2008). Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricularnucleus. Eur J Neurosci 27: 1947–1956.
Calandreau L, Jaffard R, Desmedt A (2007). Dissociated roles for the lateral and medial septum in elemental and contextual fear conditioning. Learn Mem 14: 422–429.
Corcoran KA, Maren S (2001). Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci 21: 1720–1726.
De Vries GJ, Buijs RM (1983). The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res 273: 307–317.
Donaldson ZR, Young LJ (2008). Oxytocin, vasopressin, and the neurogenetics of sociality. Science 322: 900–904.
Ebner K, Wotjak CT, Landgraf R, Engelmann M (2000). A single social defeat experience selectively stimulatesthe release of oxytocin, but not vasopressin, within the septal brain area of male rats. Brain Res 872: 87–92.
Ferguson JN, Aldag JM, Insel TR, Young LJ (2001). Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci 21: 8278–8285.
Gimpl G, Fahrenholz F (2001). The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81: 629–683.
Guastella AJ, Howard AL, Dadds MR, Mitchell P, Carson DS (2009). A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinol 34: 917–923.
Guzman YF, Tronson NC, Jovasevic V, Sato K, Guedea AL, Mizukami H et al (2013). Fear-enhancing effects of septal oxytocin receptors. Nature Neurosci 16: 1185–1187.
Jurek B, Slattery DA, Maloumby R, Hillerer KA, Koszinowski S, Neumann ID et al (2012). Differential contribution of hypothalamic MAPK activity to anxiety-like behaviour in virgin and lactating rats. PLoS One 7: e37060.
Huang H, Michetti C, Busnelli M, Managò F, Sannino S, Scheggia D et al (2013). Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacol 39: 1102–1114.
Kalin NH, Larson C, Shelton SE, Davidson RJ (1998). Asymmetric frontal brain activity, cortisol, and behavior associated with fearful temperaments in rhesus monkeys. Behav Neurosci 112: 286–292.
Kessler MS, Bosch OJ, Bunck M, Landgraf R, Neumann ID (2011). Maternal care differs in mice bred for high vs low trait anxiety: impact of brain vasopressin and cross-fostering. Soc Neurosci 6: 156–168.
Kim JJ, Rison RA, Fanselow MS (1993). Effects of amygdala, hippocampus, and periaqueductal grey lesions on short- and long-term contextual fear. Behav Neurosci 107: 1093–1098.
Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S et al (2005). Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci 25: 11489–11493.
Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH et al (2012). Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73: 553–566.
Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M et al (2010). Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacol 35: 2403–2413.
LeDoux JE, Iwata J, Cicchetti P, Reis DJ (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 8: 2517–2529.
Ledoux JE, Muller J (1997). Emotional memory and psychopathology. Philos Trans R Soc Lond B Biol Sci 352: 1719–1726.
Litvin Y, Murakami G, Pfaff DW (2011). Effects of chronic social defeat on behavioral and neural correlates of sociality: vasopressin, oxytocin and the vasopressinergic V1b receptor. Physiol Behav 103: 393–403.
Lukas M, Bredewold R, Neumann ID, Veenema AH (2010). Maternal separation interferes with developmental changes in brain vasopressin and oxytocin receptor binding in male rats. Neuropharmacol 58: 78–87.
Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID (2011). The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacol 36: 2159–2168.
Lukas M, Toth I, Veenema AH, Neumann ID (2013). Oxytocin mediates rodent social memory within the lateral septum and the medial amygdala depending on the relevance of the social stimulus: male juvenile versus female adult conspecifics. Psychoneuroendocrinol 38: 916–926.
Morrison AS, Heimberg RG (2013). Social anxiety and social anxiety disorder. Annu Rev Clin Psychol 9: 249–274.
Murakami G, Hunter RG, Fontaine C, Ribeiro A, Pfaff D (2011). Relationships among estrogen receptor, oxytocin and vasopressin gene expression and social interaction in male mice. Eur J Neurosci 34: 469–477.
Nadel L, Campbell J, Ryan L (2007). Autobiographical memory retrieval and hippocampal activation as a function of repetition and the passage of time. Neural Plast 2007: 90472.
Neumann ID (2008). Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol 20: 858–865.
Neumann ID (2009). The advantage of social living: brain neuropeptides mediate the beneficial consequences of sex and motherhood. Front Neuroendocrinol 30: 483–496.
Neumann ID, Landgraf R (2012). Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends Neurosci 35: 649–659.
Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R (2013). Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinol 38: 1985–1993.
Nyuyki KD, Maloumby R, Reber SO, Neumann ID (2012). Comparison of corticosterone responses to acute stressors: chronic jugular vein versus trunk blood samples in mice. Stress 15: 618–626.
Paxinos G, Franklin KBJ (1997) The mouse brain in stereotaxic coordinates. Academic Press: San Diego.
Pellow S, Chopin P, File SE, Briley M (1985). Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14: 149–167.
Peters S, Slattery DA, Uschold-Schmidt N, Reber SO, Neumann ID (2014). Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology 42: 225–236.
Petrovic P, Kalisch R, Singer T, Dolan RJ (2008). Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci 28: 6607–6615.
Popik P, van Ree JM (1991). Oxytocin but not vasopressin facilitates social recognition following injection into the medial preoptic area of the rat brain. Eur Neuropsychopharmacol 1: 555–560.
Popik P, Vos PE, Van Ree JM (1992). Neurohypophyseal hormone receptors in the septum are implicated in social recognition in the rat. Behav Pharmacol 3: 351–358.
Roozendaal B, Schoorlemmer GH, Wiersma A, Sluyter S, Driscoll P, Koolhaas JM et al (1992). Opposite effects of central amygdaloid vasopressin and oxytocin on the regulation of conditioned stress responses in male rats. Ann NY Acad Sci 652: 460–461.
Sheehan TP, Chambers RA, Russell DS (2004). Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res Rev 46: 71–117.
Slattery DA, Neumann ID (2010). Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacol 58: 56–61.
Sullivan RM (2004). Hemispheric asymmetry in stress processing in rat prefrontal cortex and the role of mesocortical dopamine. Review. Stress 7: 131–143.
Thompson RR, Walton JC (2004). Peptide effects on social behavior: effects of vasotocin and isotocin on social approach behavior in male goldfish (Carassiusauratus). Behav Neurosci 118: 620–626.
Toth I, Neumann ID, Slattery DA (2012a). Central administration of oxytocin receptor ligands affects cued fear extinction in rats and mice in a timepoint-dependent manner. Psychopharmacol (Berl) 223: 149–158.
Toth I, Neumann ID, Slattery DA (2012b). Social fear conditioning:a novel and specific animal model to study social anxiety disorder. Neuropsychopharmacology 37: 1433–1443.
Toth I, Neumann ID, Slattery DA (2013). Social fear conditioning as an animal model of social anxiety disorder. Curr Protoc Neurosci Chapter 9: Unit 9:42.
Viviani D, Charlet A, van den Burg EH, Robinet C, Hurni N, Abatis M et al (2011). Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science 333: 104–107.
Waldherr M, Neumann ID (2007). Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc Natl Acad Sci USA 104: 16681–16684.
Acknowledgements
We thank Dr Maurice Manning for generously providing the OXTR-A, and Gabriele Schindler, Martina Fuchs, Andrea Havasi, Lena Bockreiss, and Giulia Lia for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Zoicas, I., Slattery, D. & Neumann, I. Brain Oxytocin in Social Fear Conditioning and Its Extinction: Involvement of the Lateral Septum. Neuropsychopharmacol 39, 3027–3035 (2014). https://doi.org/10.1038/npp.2014.156
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2014.156
This article is cited by
-
A dedicated hypothalamic oxytocin circuit controls aversive social learning
Nature (2024)
-
Functional involvement of septal miR-132 in extinction and oxytocin-mediated reversal of social fear
Molecular Psychiatry (2023)
-
Detection, processing and reinforcement of social cues: regulation by the oxytocin system
Nature Reviews Neuroscience (2023)
-
HDAC1-mediated regulation of GABA signaling within the lateral septum facilitates long-lasting social fear extinction in male mice
Translational Psychiatry (2023)
-
Advances in human oxytocin measurement: challenges and proposed solutions
Molecular Psychiatry (2023)