Abstract
Highly palatable foods and dieting are major contributing factors for the development of compulsive eating in obesity and eating disorders. We previously demonstrated that intermittent access to palatable food results in corticotropin-releasing factor-1 (CRF1) receptor antagonist-reversible behaviors, which include excessive palatable food intake, hypophagia of regular chow, and anxiety-like behavior. However, the brain areas mediating these effects are still unknown. Male Wistar rats were either fed chow continuously for 7 days/week (Chow/Chow group), or fed chow intermittently 5 days/week, followed by a sucrose, palatable diet 2 days/week (Chow/Palatable group). Following chronic diet alternation, the effects of microinfusing the CRF1 receptor antagonist R121919 (0, 0.5, 1.5 μg/side) in the central nucleus of the amygdala (CeA), the basolateral nucleus of the amygdala (BlA), or the bed nucleus of the stria terminalis (BNST) were evaluated on excessive intake of the palatable diet, chow hypophagia, and anxiety-like behavior. Furthermore, CRF immunostaining was evaluated in the brain of diet cycled rats. Intra-CeA R121919 blocked both excessive palatable food intake and anxiety-like behavior in Chow/Palatable rats, without affecting chow hypophagia. Conversely, intra-BlA R121919 reduced the chow hypophagia in Chow/Palatable rats, without affecting excessive palatable food intake or anxiety-like behavior. Intra-BNST treatment had no effect. The treatments did not modify the behavior of Chow/Chow rats. Immunohistochemistry revealed an increased number of CRF-positive cells in CeA—but not in BlA or BNST—of Chow/Palatable rats, during both withdrawal and renewed access to the palatable diet, compared with controls. These results provide functional evidence that the CRF–CRF1 receptor system in CeA and BlA has a differential role in mediating maladaptive behaviors resulting from palatable diet cycling.
Similar content being viewed by others
INTRODUCTION
Highly palatable foods (eg, foods rich in sugars and/or fats) are believed to be a major contributing factors in the emergence of compulsive eating in certain forms of obesity and eating disorders (Corwin, 2006; Yach et al, 2006). Many analogies exist between drug addiction and excessive intake of highly palatable foods, including loss of control over drug/food, inability to terminate use/overeating despite negative consequences, distress, and dysphoria when attempting to abstain from drug/food (Parylak et al, 2011; Volkow and O'Brien, 2007). These common symptoms have been proposed to arise from dysfunctions of brain circuits, which overlap in drug addiction and compulsive eating.
Corticotropin-releasing factor type 1 (CRF1) receptor antagonists have been proposed as novel therapeutic targets for addictive disorders because of their ability to reduce the motivational effects of withdrawal (Logrip et al, 2011). CRF is a critical mediator of endocrine, sympathetic, and behavioral responses to stress (Bakshi and Kalin, 2000; Vale et al, 1981). CRF in the paraventricular nucleus of the hypothalamus controls the hypothalamic–pituitary–adrenal (HPA) response to stress, whereas the behavioral effects of CRF are HPA independent and mediated by extrahypothalamic brain regions (Koob and Heinrichs, 1999). The extrahypothalamic CRF–CRF1 receptor system is recruited in dependence to all known drugs of abuse via cycles of intoxication/withdrawal, and this hyperactivation is considered a common element, promoting excessive drug intake through a negatively reinforced mechanism (ie, compulsive drug intake produced by the removal of withdrawal-induced negative emotional state; Heilig and Koob, 2007; Koob, 2010; Shalev et al, 2010).
Although similarities between drugs of abuse and food have been widely studied with respect to their positive reinforcing properties (ie, excessive food intake produced by obtaining a pleasant effect; Avena et al, 2012; Blasio et al, 2013b; Corwin and Grigson, 2009; Cottone et al, 2012; Hagan et al, 2003), the hypothesis that excessive food intake may result as a form of ‘self-medication’ to relieve the negative emotional state associated with withdrawal from highly palatable foods is relatively understudied (Bale, 2005; Cottone et al, 2009a; Shalev et al, 2010).
We have previously shown that withdrawal from chronic, intermittent access to highly palatable foods causes the recruitment of the extrahypothalamic CRF system and the emergence of CRF1 receptor-dependent maladaptive behaviors, which include excessive food intake upon renewed access to the highly palatable diet, hypophagia of the otherwise acceptable chow diet, and anxiety-like behavior during abstinence (Cottone et al, 2009a).
However, direct functional evidence regarding which brain area is responsible for the CRF1 receptor-dependent behavioral adaptations induced by palatable diet cycling is missing. This study, therefore, aimed at determining whether site-specific antagonism of CRF1 receptors within the central nucleus of the amygdala (CeA), the basolateral nucleus of the amygdala (BlA) or the bed nucleus of the stria terminalis (BNST) was able to block excessive intake of highly palatable food, withdrawal-induced hypophagia of the regular chow, and anxiety-like behavior. In addition, this study was aimed at determining whether the expression of CRF in CeA, BlA, and BNST was increased in diet cycled rats as compared with controls, using immunohistochemistry. Although we have previously shown that withdrawal from palatable food is associated with an increased CRF expression in CeA, how BlA and BNST are affected by diet cycling is currently unknown.
MATERIALS AND METHODS
Subjects
Male Wistar rats (n=140, of which 33 rats for CeA experiments, 46 rats for BlA experiments, 39 rats for BNST experiments, and 22 rats for the immunohistochemistry experiment; Supplementary Table 1), weighing 180–230 g and 41–47 days old at arrival (Charles River, Wilmington, MA, USA), were single housed in wire topped, plastic cages (27 × 48 × 20 cm) on a 12-h reverse light cycle (lights off at 1100 hours), in an AAALAC-approved humidity- (60%) and temperature-controlled (22 °C) vivarium. Rats had ad libitum access to corn-based chow (Harlan Teklad LM-485 Diet 7012; 65% kcal carbohydrate, 13% fat, 21% protein, metabolizable energy 310 cal/100 g; Harlan, Indianapolis, IN, USA) and water, unless otherwise specified. The procedures used in this study adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication number 85-23, revised 1996) and the Principles of Laboratory Animal Care, and were approved by Boston University Medical Campus Institutional Animal Care and Use Committee.
Drugs
R121919 (3-[6-(dimethylamino)-4-methyl-pyrid-3-yl]-2,5-dimethyl-N,N-dipropyl-pyrazolo[2,3-a]pyrimidin-7-amine, NBI 30775) was synthesized as described in Chen et al (2004). R121919 is a potent, non-peptide, high-affinity CRF1 receptor antagonist (Ki=2–5 nM), which shows over 1000-fold weaker activity at the CRF2 receptor, CRF-binding protein, or 70 other receptor types (Grigoriadis et al, 2000). R121919 was solubilized using a 18 : 1 : 1 mixture of saline:ethanol:cremophor.
Behavioral Tests
Ad libitum palatable diet alternation access
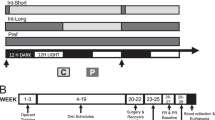
The access to the ad libitum palatable diet alternation was performed as previously described (Cottone et al, 2008, 2009a, 2009b; Iemolo et al, 2012). Briefly, after acclimation, rats were divided into two groups matched for food intake, body weight, and feed efficiency of the previous 3–4 days. One group was then provided with ad libitum access to a chow diet (Chow) for 7 days a week (Chow/Chow, the control group of this study) while a second group was provided with free access to chow for 5 days a week, followed by 2 days of ad libitum to a highly palatable, chocolate-flavored, high-sucrose diet (Palatable; Chow/Palatable group). All the behavioral tests were performed in rats that had been diet cycled for at least 7 weeks. The ‘chow’ diet was the above-described corn-based chow from Harlan, whereas the palatable diet was a nutritionally complete, chocolate-flavored, high-sucrose (50% kcal), AIN-76A-based diet that is comparable in macronutrient proportions and energy density to the chow diet (chocolate-flavored formula 5TUL: 66.7% kcal carbohydrate, 12.7% fat, 20.6% protein, metabolizable energy 344 kcal/100 g (Test Diet, Richmond, IN, USA) formulated as 45 mg precision food pellets to increase its preferredness). For brevity, the first 5 days (chow only) and the last 2 days (chow or palatable according to the experimental group) of each week are referred to in all experiments as C and P phases. Palatable diet was provided in GPF20 ‘J’-feeders (Ancare, Bellmore, NY, USA). Diets were never concurrently available.
Food intake experiments
Rats were provided with pre-weighed food in their home cages at the dark cycle onset. Treatments were given in rats that were diet cycled for at least 7 weeks upon renewing access to the palatable diet (C→P phase), or to the chow diet (P→C phase). R121919 was microinfused bilaterally within the CeA, the BlA, or the BNST (0, 0.5, and 1.5 μg/side, 0.5 μl/side, 30-min pre-treatment time) using randomized within-subject Latin square designs.
Light–dark box test
Rats were tested for 10 min in a light–dark rectangular box (50 × 100 × 35 cm) in which the aversive light compartment (50 × 70 × 35 cm) was illuminated by a 60 lux light. The dark side (50 × 30 × 35 cm) had an opaque cover and ∼0 lux of light. The two compartments were connected by an open doorway, which allowed the subjects to move freely between the two. Testing took place following at least 7 weeks of diet alternation, 5–9 h after the switch from the palatable diet to the chow diet (P→C phase); this time point ensures the occurrence of anxiety-like behavior induced by withdrawal from palatable food in Chow/Palatable rats (Cottone et al, 2009a, 2009b). Rats were kept in the quiet, dark anteroom for at least 2 h before testing. White noise was present throughout habituation and testing. On the day of testing, rats were microinfused bilaterally with R121919 within the CeA, the BlA, or the BNST (0, 0.5, and 1.5 μg/side, 0.5 μl/side) 30 min before being placed into the dark compartment facing the doorway and behavior was video recorded for later scoring. Treatments were given using a between-subject design. The time spent in the open compartment was measured as an index of anxiety-like behavior. The apparatus was wiped clean with water and dried after each subject.
Intracranial Surgeries, Microinfusion Procedure, and Cannula Placement
Intracranial surgeries
Rats were stereotaxically implanted with bilateral, intracranial cannulas as described previously (Cottone et al, 2007; Iemolo et al, 2012; Sabino et al, 2007). Briefly, stainless steel, guide cannulas (24 gauge, Plastics One, Roanoke, VA, USA) were lowered bilaterally 2.0 mm above the CeA, the BlA, or the BNST. Four stainless steel jeweler’s screws were fastened to the rat’s skull around the cannula. Dental restorative filled resin (Henry Schein, Melville, NY, USA) and acrylic cement were applied, forming a pedestal firmly anchoring the cannula. The cannula coordinates from bregma used for the CeA were: AP +0.2, ML ±4.2, DV −7 (from skull) with the incisor bar set 5.0 mm above the interaural line, according to the atlas of Pellegrino (1979). The cannula coordinates used for the BlA were: AP −2.64, ML ±4.8, DV −6.5 (from skull) with flat skull, according to Paxinos and Watson (2007). The cannula coordinates used for the BNST were: AP −0.6, ML ±3.5, DV −4.8 (from skull) with flat skull and tilt angle of 14°. A stainless steel dummy stylet (Plastics One) maintained patency of the cannula. After surgery, the rats were allowed a 7-day recovery period, during which they were handled daily.
Microinfusion procedure
Drug was microinfused in the brain of rats as previously described (Blasio et al, 2013b; Dore et al, 2013). For intracranial microinfusion, the dummy stylet was removed from the guide cannula, and was replaced with a 31-gauge stainless steel injector projecting 2 mm beyond the tip of the guide cannula; the injector was connected via PE 20 tubing to a Hamilton microsyringe (Hamilton, Reno, Nevada) driven by a multi-syringe microinfusion pump (KD Scientific/Biological Instruments, Holliston, MA, USA). Microinfusions were performed in 0.5 μl volume delivered over 2 min; injectors were left in place for 1 additional minute to minimize backflow.
Cannula placement
Cannula placement was verified at the conclusion of all testing (see Figure 1). Subjects were anesthetized (isoflurane, 2–3% in oxygen) and transcardially perfused with ice-cold 4% paraformaldehyde (PFA) in water (pH 7.4) and microinfused with Cresyl violet (0.5 μl/side). Brains were then fixed overnight in 4% PFA and equilibrated in 30% sucrose in PFA. Coronal sections of 40 μm were collected using a cryostat (Thermo Scientific HM-525) and placements were verified under a microscope. Forty subjects (14 for CeA, 16 for BlA, and 10 for BNST) were excluded from analysis because of incorrect cannula placement. Data from incorrect placements were analyzed to help interpret the site specificity of effects.
Drawing of coronal rats’ brain slices. Dots represent the injection sites in the central nucleus of the amygdala (CeA) (a), basolateral nucleus of the amygdala (BlA) (b) and bed nucleus of the stria terminalis (BNST) (c) included in the data analysis. Photomicrographs show coronal sections of the brain of a rat with representative injection sites in the CeA (d), BlA (e), and BNST (f).
CRF Immunohistochemistry
Behavioral procedure, perfusions, and immunohistochemistry
Rats (n=22) were diet cycled for 7 weeks, anesthetized, and perfused 2–4 h after being switched either from the palatable diet to the chow diet (P→C phase) or from the chow diet to the palatable diet (C→P phase). Rats were anesthetized and then transcardially perfused with saline+2% (w/v) sodium nitrite (pH=7.4) first, and with 4% paraformaldehyde buffered in Borax (pH=9.5) next. Rats were then decapitated and the brains immediately collected, placed in ∼20 ml of 4% PFA, and stored in a 30% sucrose in 4% PFA solution at 4 °C until saturation.
For CRF visualization, brains were cut into 40 μm coronal sections using a cryostat and subsequently stored in a cryoprotectant at −20 °C. Every sixth section (240 μm apart) of the entire CeA, BlA, and BNST was chosen in a systematic random manner and processed for immunocytochemistry. Free-floating sections were washed in potassium phosphate buffer saline (KPBS). After the initial wash, sections received incubation in 0.3% hydrogen peroxide KPBS solution for 30 min to block endogenous peroxidases. Sections were then washed again and placed in blocking solution (3% normal goat serum, 0.25% Triton X100, and 0.1% bovine serum albumin) for 2 h. Sections were then transferred into primary antibody (1 : 100 dilution, anti-CRF (sc-10718), Santa Cruz Biotechnology) in blocking solution and incubated for 72 h at 4 °C. Following an additional wash, sections were incubated into secondary antibody (1 : 1000 dilution, biotinylated anti-rabbit (BA-1000) Vector Laboratories, Burlingame, California) in blocking solution for 2 h at room temperature. Sections were washed and then incubated in an avidin–biotin horseradish peroxidase ABC solution (Vector Laboratories) in blocking solution for 1 h. Sections were then incubated using a diaminobenzidine substrate kit (Vector Laboratories) according to the manufacturer’s instructions and once the reaction was complete sections were rinsed in KPBS, mounted onto slides and allowed to dry overnight. The following day, slides were dehydrated using graded alcohol concentrations and coverslipped using DPX mountant (Electron Microscopy Sciences, Hatfield, PA, USA).
Quantification of CRF+ cell bodies
Quantification of CRF+ cell bodies was performed in accordance with the unbiased stereology approach. The series of sections was analyzed for each staining batch. Sections were analyzed using an Olympus (Center Valley, PA, USA) BX-51 microscope equipped with a Rotiga 2000R live video camera (QImaging, Surrey, BC, Canada), a three-axis MAC6000 XYZ motorized stage (Ludl Electronics, Hawthorne, NY, USA), and a personal computer workstation. All cell counts were made on coded slides by an investigator blind to the treatment conditions. Each region was outlined virtually on the digitized image of each randomly chosen section using the optical fractionator workflow module of Stereo Investigator software (MicroBrightField, Williston, VT, USA). All contours were drawn at low magnification using an Olympus PlanApo N 2X objective with numerical aperture 0.08 and counted using an Olympus UPlanFL N 40X objective with numerical aperture 0.75. The grid frame and the counting frame were set to 275 × 160 μm. A guard zone of 2 μm and a dissector height of 20 μm were used. The frozen sections were originally cut at a nominal thickness of 40 μm. Immunostaining and mounting result in altered section thickness, which was measured at each counting site. An average section thickness was computed by the software and used to estimate the total volume of the sample region and total number of CRF+ cells.
Statistical Analysis
Student’s t-tests were used to analyze factors with two levels. ANOVAs were performed to analyze factors with more than two levels. Following significant omnibus effect of ANOVAs (p<0.05), Fisher’s LSD post hoc comparison tests were used. Dunnett’s test was used to determine whether R121919 normalized intake of Chow/Palatable rats to vehicle-treated Chow/Chow-fed levels. The software/graphic packages used were Systat 11.0, SigmaPlot 11.0 (Systat Software, Chicago, IL, USA), InStat 3.0 (GraphPad, San Diego, CA, USA), Statistica 7.0 (Statsoft, Tulsa, OK, USA), and PASW Statistics 18.0 (SPSS, Chicago, IL, USA).
RESULTS
Effects of Microinfusion of R121919 into the CeA
Excessive intake of palatable food
To determine whether CRF1 receptors in the CeA mediate excessive intake of palatable food in diet cycled rats, we microinfused site specifically the selective CRF1 receptor antagonist R121919 into this brain area and measured food intake at the beginning of the P phase. As shown in Figure 2a, the intake of vehicle-treated palatable diet-fed Chow/Palatable rats was twofold higher than that of chow-fed control Chow/Chow rats. Antagonism of CeA CRF1 receptors fully blocked this excessive eating of palatable food in Chow/Palatable rats, without affecting food intake in control rats (Chow/Chow, F(2, 20)=0.72, NS; Chow/Palatable, F(2, 14)=5.02, p<0.05). Post hoc comparison revealed that the highest dose of R121919 (1.5 μg/side) significantly reduced palatable food intake compared with vehicle in Chow/Palatable rats. Intake of Chow/Palatable rats following the microinfusion of the 1.5 μg/side dose did not significantly differ from the intake of vehicle-treated Chow/Chow rats. Confirming the specificity of the effects for CRF1 receptors in the CeA, no effect was observed in food intake of subjects with misplaced cannulae (Chow/Palatable, F(2, 2)=4.32, NS).
Effects of microinfusion of the selective corticotropin-releasing factor-1 (CRF1) receptor antagonist R121919 (0, 0.5, 1.5 μg/side) in the central nucleus of the amygdala (CeA) on excessive eating of palatable food, hypophagia of regular chow diet, and anxiety-like behavior in male Wistar rats (n=33). Rats were tested after 7 weeks of diet cycling. (a) Intra-CeA R121919 fully blocked the excessive eating of palatable food in Chow/Palatable rats (right panel), without affecting regular chow intake in control Chow/Chow rats (left panel). (b) Intra-CeA R121919 did not affect the intake of the regular chow diet in either Chow/Chow the (left panel) or Chow/Palatable (right panel) group. (c) Intra-CeA R121919 fully blocked the reduction of the time spent in the light compartment of a light–dark box induced by withdrawing the palatable food in Chow/Palatable rats. Drug treatment did not affect the performance of control Chow/Chow rats. Panel represents M±SEM. Please note the different scales for panels a and b. Symbols denote: *significant difference from vehicle condition p<0.05, **p<0.01; ##significant difference from Chow/Chow vehicle p<0.01.
Hypophagia of the regular chow diet
To determine whether CRF1 receptors in the CeA mediate the hypophagia of chow diet in diet cycled rats, we microinfused R121919 into this brain area and measured food intake at the beginning of the C phase. As shown in Figure 2b, the intake of vehicle-treated Chow/Palatable rats was ∼1/3 of the intake of vehicle-treated Chow/Chow rats (hypophagia). R121919 treatment did not affect the hypophagia of the regular chow in Chow/Palatable rats (Chow/Palatable, F(2, 12)=0.14, NS). Confirming the results obtained in P phase, R121919 microinfusion in the CeA did not affect chow intake in control Chow/Chow rats (Chow/Chow, F(2, 20)=0.01, NS).
Acute withdrawal-induced anxiety-like behavior
To determine whether CeA CRF1 receptors mediate the negative emotional state induced by withdrawing the palatable food in cycled rats, we microinfused site specifically R121919 into this brain area and measured anxiety-like behavior using the light–dark box test 5 h into the C phase. As shown in Figure 2c, rats acutely withdrawn from chronic, intermittent access to the highly palatable diet showed a significant decrease in the time spent in the light compartment of the light–dark box. Microinfusion of 1.5 μg/side of R121919 in the CeA, the dose that effectively reduced excessive eating of palatable food, fully blocked anxiety-like behavior by increasing the time spent in the light area of the box in Chow/Palatable rats, without affecting the behavior in Chow/Chow rats (DOSE: F(1, 24)=4.40, p<0.05). Confirming the specificity of the effects for CRF1 receptors in the CeA, no effect was observed in food intake of subjects with misplaced cannulae (DOSE: F(2, 2)=4.32, NS).
Effects of Microinfusion of R121919 into the BlA
Excessive intake of palatable food
To determine whether BlA CRF1 receptors mediate excessive eating of palatable food in diet cycled rats, we microinfused site specifically R121919 into this brain area and measured food intake at the beginning of the P phase. Unlike what was observed following administration of R1219191 into the CeA, as shown in Figure 3a bilateral microinfusion of the selective CRF1 receptor antagonist into the BlA did not significantly affect palatable food intake in Chow/Palatable rats (Chow/Palatable, F(2, 26)=1.56, NS). Similarly, regular chow consumption in Chow/Chow rats was not affected by R121919 microinfusion (Chow/Chow, F(2, 18)=0.52, NS).
Effects of microinfusion of the selective corticotropin-releasing factor-1 (CRF1) receptor antagonist R121919 (0, 0.5, 1.5 μg/side) in the basolateral nucleus of the amygdala (BlA) on excessive eating of palatable food, hypophagia of regular chow diet and anxiety-like behavior in male Wistar rats (n=46). Rats were tested after 7 weeks of diet cycling. (a) Intra-BlA R121919 did not affect the intake of the regular chow diet in Chow/Chow (left panel) or the excessive eating of palatable food in Chow/Palatable (right panel). (b) Intra-BlA R121919 reduced the hypophagia of regular chow diet in Chow/Palatable rats (right panel), without affecting it in Chow/Chow rats (left panel). (c) Intra-BlA R121919 did not affect the time spent in the light compartment of a light–dark box in Chow/Chow and Chow/Palatable rats. Panel represents M±SEM. Please note the different scales for panels a and b. Symbols denote: **significant difference from vehicle condition p<0.01, ##significant difference from Chow/Chow vehicle p<0.01; $significant main effect of Diet p<0.05.
Hypophagia of the regular chow diet
To determine whether CRF1 receptors in the BlA mediate the hypophagia of chow in cycled rats, we microinfused R121919 into this brain area and measured food intake at the beginning of the C phase. As shown in Figure 3b, a significant increase in regular chow intake was observed following microinfusion of the CRF1 receptor antagonist in the BlA of Chow/Palatable rats (Chow/Palatable, F(2, 26)=4.46, p<0.05). Indeed, the highest dose (1.5 μg) of R121919 microinfused in the BlA during C phase significantly increased the consumption of the regular chow diet by 221.1±33.1 (M±SEM) percent when compared with vehicle-treated Chow/Chow rats. R121919 attenuated, but did not completely block, the withdrawal-induced hypophagia at the highest dose injected. Confirming the data obtained in P phase, R121919 microinfusion did not affect regular chow intake in Chow/Chow rats (Chow/Chow, F(2, 20)=0.25, NS). Confirming the specificity of the effects for CRF1 receptors in the BlA, no effect was observed in food intake of subjects with misplaced cannulae (Chow/Palatable, F(2, 8)=0.50, NS).
Acute withdrawal-induced anxiety-like behavior
To determine whether BlA CRF1 receptors mediate the negative emotional state induced by acutely withdrawing the palatable food in cycled rats, we microinfused site specifically R121919 into this brain area and measured anxiety-like behavior 5 h into the C phase. As shown in Figure 3c, palatable food-withdrawn Chow/Palatable rats spent less time in the light compartment compared with Chow/Chow rats (DIET: F(1, 23)=84.03, p<0.001). R121919, microinfused into the BlA, did not significantly affect the time spent in the light area (DOSE: F(1, 39)=0.01, NS).
Effects of Microinfusion of R121919 into the BNST
Excessive intake of palatable food
To determine whether BNST CRF1 receptors mediate excessive eating of palatable food in diet cycled rats, R121919 was site specifically microinfused into this brain area and food intake was measured at the beginning of the P phase. As shown in Figure 4b, bilateral microinfusion of the selective CRF1 receptor antagonist into the BNST did not significantly affect palatable food intake in Chow/Palatable rats (Chow/Palatable, F(2, 18)=0.33, NS). Similarly, regular chow consumption in Chow/Chow rats was not affected by R121919 microinfusion (Chow/Chow, F(2, 20)=1.03, NS).
Effects of microinfusion of the selective corticotropin-releasing factor-1 (CRF1) receptor antagonist R121919 (0, 0.5, 1.5 μg/side) in the bed nucleus of the stria terminalis (BNST) on excessive eating of palatable food, hypophagia of regular chow diet, and anxiety-like behavior in male Wistar rats (n=39). Rats were tested after 7 weeks of diet cycling. (a) Intra-BNST R121919 did not affect the intake of the regular chow diet in Chow/Chow (left panel) or excessive eating of palatable food in Chow/Palatable (right panel). (b) Intra-BNST R121919 did not affect the intake of the regular chow diet in either the Chow/Chow (left panel) or Chow/Palatable (right panel) group. (c) Intra-BNST R121919 did not affect the time spent in the light compartment in Chow/Chow and Chow/Palatable rats. Panel represents M±SEM. Please note different scales for panels a and b. Symbols denote: #significant difference from Chow/Chow vehicle p<0.05, ##p<0.01; $significant main effect of Diet p<0.05.
Hypophagia of the regular chow diet
To determine whether BNST CRF1 receptors mediate the hypophagia of chow diet in cycled rats, we microinfused R121919 into this brain area and measured food intake at the beginning of the C phase. As shown in Figure 4a, R121919 microinfusion did not affect regular chow intake in Chow/Chow rats (Chow/Chow, F(2, 14)=0.03, NS). Similarly, R121919 treatment did not affect the hypophagia of the regular chow in Chow/Palatable rats (Chow/Palatable, F(2, 20)=0.27, NS).
Acute withdrawal-induced anxiety-like behavior
To determine whether BNST CRF1 receptors mediate the negative emotional state induced by acutely withdrawing the palatable food in cycled rats, we microinfused site specifically R121919 into this brain area and measured anxiety-like behavior 5 h after the switch from P→C phase. As shown in Figure 4c, palatable food-withdrawn Chow/Palatable rats spent less time in the light compartment compared with control Chow/Chow rats (DIET: F(1, 17)=17.11, p<0.01). R121919, bilaterally microinfused at the dose of 1.5 μg/side into the BNST did not significantly affect the time spent in the light area (DOSE: F(1, 33)=0.47, NS).
CRF Immunohistochemistry
Figure 5 illustrates representative micrographs of CRF+ cells in the CeA, BlA, and BNST in Chow/Chow and Chow/Palatable rats, following the ad libitum palatable diet alternation procedure. Analysis of CRF immunoreactivity of the CeA revealed that a significant difference between Chow/Palatable and Chow/Chow rats during both the C and the P phase (F(2, 19)=4.19, p<0.05). No statistically significant differences among groups were observed in either BlA (F(2, 17)=1.13, NS) or BNST (F(2, 19)=1.16, NS).
Representative micrographs of corticotropin-releasing factor (CRF) immunoreactivity in the central nucleus of the amygdala (CeA) (a–d), basolateral nucleus of the amygdala (BlA) (e–h), and bed nucleus of the stria terminalis (BNST) (i–l) of male Wistar rats (n=22) following 7 weeks of diet cycling. A 20 × representative images were taken from Chow/Chow rats (a, e, i) and Chow/Palatable rats, in C phase (b, f, j) and P phase (c, g, k). Bar graphs show the number of CRF+ cells. A significant increase in total CRF+ cells was observed in both C phase and P phase of Chow/Palatable rats when compared with Chow/Chow rats in the CeA. Statistical differences were not detected between groups in either the BlA, or the BNST. Panel represents M±SEM. Symbols denote: *significant difference from Chow/Chow condition p<0.05.
DISCUSSION
This study was designed to functionally identify the brain site responsible for the CRF-mediated excessive intake of highly palatable food in rats under diet alternation regimen. Our findings prove a major role for the CeA in mediating excessive eating of highly palatable food. In addition, we demonstrate that CRF system in the BlA, differently from the CeA, has a role in the devaluation process that occurs when downshifting in food reward magnitude.
We have previously shown that repeated cycles of access to and acute withdrawal from a sugary, highly palatable diet lead to excessive eating of palatable food as well as to acute withdrawal-dependent hypophagia of the regular chow diet and anxiety-like behavior (Blasio et al, 2013a; Cottone et al, 2009a, 2009b). Excessive eating observed here is hypothesized to be driven by the negative emotional state induced by repeated episodes of acute withdrawal from highly palatable foods via an extrahypothalamic CRF–CRF1 receptor system-mediated mechanism, which resembles the ‘kindling’-like process underlying addictive disorders (Breese et al, 2005; Cottone et al, 2009a; Koob, 2008; Parylak et al, 2011).
The results of this study demonstrate that CRF1 receptors of the CeA and BlA differentially mediate feeding adaptations and anxiety-like behavior of chronically diet cycled rats. Administration of the selective CRF1 receptor antagonist within the CeA blocked both excessive eating and anxiety-like behavior in Chow/Palatable rats, without affecting the hypophagia of the underaccepted, regular diet. Interestingly, administration of R121919 into the BlA, attenuated the hypophagia of the less appetitive chow diet (ie, increased regular chow intake) in Chow/Palatable rats, without affecting excessive eating or anxiety-like behavior. When microinfused in the BNST, R121919 did not affect any of the variables measured in Chow/Palatable rats (excessive eating of the highly palatable diet, intake of the regular chow diet and the acute withdrawal-induced anxiety-like behavior). The observed pharmacological effects were selective for Chow/Palatable rats because R121919, microinfused within the CeA, BlA, or BNST of Chow/Chow control rats, exerted no effect. Therefore, the CRF–CRF1 receptor system of the CeA and BlA appear to differentially mediate the behavioral outcomes resulting from chronic palatable diet cycling. On the other hand, the CRF–CRF1 receptor system of the BNST does not seem to be involved in the behavioral adaptations induced by palatable diet alternation.
Our behavioral and pharmacological findings were supported by the observation that CRF immunoreactivity within the CeA of Chow/Palatable rats was significantly increased compared with Chow/Chow control rats, during withdrawal from and following renewed access to the highly palatable diet (Cottone et al, 2009a). Interestingly, no significant difference in CRF immunoreactivity between groups was observed within the BlA or the BNST. The increased CRF immunoreactivity observed in the CeA of Chow/Palatable rats is consistent with our previous finding that acute withdrawal from the palatable diet is associated with an increased release of CRF in the CeA (Cottone et al, 2009a). However, contrarily to what was shown previously, renewing access to the palatable diet did not cause a return of CRF expression in the CeA to control levels. The discrepancy between the results obtained here and the previous observation may be related to the different time point of the brain collection, and the different anatomical resolution of the techniques adopted to measure CRF expression. Nonetheless, the observed increase in CRF expression in the CeA during withdrawal and following renewed access to the palatable diet is consistent with the selective effects of blockade of anxiety-like behavior (during withdrawal) and excessive eating (renewed access) in Chow/Palatable rats. The apparent inconsistency between the two studies can, therefore, be collectively interpreted as follows: during acute palatable food withdrawal, CRF expression increases in the CeA of diet cycled rats compared with controls and it is responsible for the emergence of a negative affect. The CeA CRF expression remains altered up to the first hours of highly palatable access, inducing excessive eating. Following excessive palatable food consumption, however, CRF returns back to control levels (Cottone et al, 2009a).
The behavioral, pharmacological and molecular results shown support the hypothesis that the CRF–CRF1 receptor system in the CeA has an important role in mediating the negative affective state and the excessive intake of palatable food in diet cycled rats, similarly to what has been extensively demonstrated for alcohol and drug dependence (Koob, 2010). Indeed, ethanol-dependent rats exhibit increased extracellular release of CRF in the CeA during withdrawal and the administration of a CRF receptor antagonist into the CeA is able to block escalated ethanol self-administration during withdrawal (Funk et al, 2006; Merlo Pich et al, 1995). Analogously, opiate-dependent animals show increased CRF expression in the CeA during withdrawal (Maj et al, 2003) and blockade of CRF receptors in the CeA, but not the BNST, reduces the behavioral signs of withdrawal (Heinrichs et al, 1995; McNally and Akil, 2002). A key role for CRF–CRF1 system in the CeA has also been demonstrated in nicotine dependence. Indeed, mecamylamine-precipitated nicotine withdrawal is associated with a hyperactivation of the CRF–CRF1 receptor system in CeA (George et al, 2007), and intra-CeA, but not intra-BlA, microinfusion of a CRF1 receptor antagonist reduces the nicotine withdrawal-dependent elevations in brain reward threshold (Bruijnzeel et al, 2012). In cannabinoid-dependent rats, precipitated withdrawal is associated with a marked elevation in extracellular CRF concentration in the CeA (Rodriguez de Fonseca et al, 1997). Altogether, this evidence strongly supports the hypothesis that the CRF–CRF1 receptor system in the CeA is a key mediator of acute withdrawal-induced negative affect, along with excessive drug and alcohol intake during dependence. Our results expand this knowledge to excessive eating of highly palatable food, suggesting that analogous neuroadaptations occur.
The results of this study show that the palatable food withdrawal-dependent hypophagia of the less appetitive chow diet is attenuated by microinfusion within the BlA of the selective CRF1 receptor antagonist, whereas excessive eating and anxiety-like behavior were not affected by the intra-BlA drug treatment. The differential involvement of the BlA CRF–CRF1 receptor system in the outcomes of diet-cycling suggests that hypophagia of chow may represent a behavioral process independent from anxiety-like behavior. Rather, these findings are consistent with the hypothesis that the BlA mediates sensory and incentive aspects of motivationally salient events. Indeed, considerable evidence exists that the BlA is critically important in mediating devaluation processes and aversive responses to reward reduction (ie, Crespi effect, successive negative contrast, reward devaluation, and so on; Hatfield et al, 1996; Salinas et al, 1996; Wellman et al, 2005), and, therefore, the hypophagia resulting from the switch from the highly palatable diet to the less appetitive chow diet may represent a hedonic devaluation process, rather than an energy homeostasis-dependent mechanism (ie, independent from previous energy intake or body weight gain; Cottone et al, 2008, 2009b). Blockade of CRF1 receptors within the BlA is, therefore, hypothesized to reduce chow hypophagia (ie, to increase chow intake) by attenuating the devaluation process that occurs when switching from the highly palatable food to the less appetitive chow. Relevant to this context is also the apparent inconsistency between the molecular and the behavioral/pharmacological results obtained in the BlA. Although the CRF1 receptor antagonist was able to reduce the magnitude of the chow hypophagia when injected within the BlA, no significant differences in CRF immunoreactivity were observed in this area when comparing control and diet cycled rats. This apparent discrepancy can be explained considering that BlA-dependent devaluation processes of alternative rewards occur physiologically and have an important evolutionary significance in the selection of foods that yields the highest reward/energy value (Murray et al, 2011). As such, it is arguable that the mediation of these processes in the BlA does not require neuroadaptations in the CRF system (similar to the ones observed in the CeA). In support of this hypothesis, while excessive eating requires chronic diet cycling to develop, hypophagia of the less preferred alternative chow occurs after the very first switch from the highly palatable diet back to the regular chow (Cottone et al, 2008). In addition, it is important to emphasize that, based on the results obtained by injecting the CRF1 receptor antagonist into the BlA and CeA, the CRF1 receptor-dependent hypophagia observed here appears to be a different behavioral process than the anhedonia seen in drug withdrawal. Nevertheless, acute withdrawal from intermittent access to palatable food has been demonstrated to induce other hypohedonic-like responses such as increased immobility in the forced-swim test and decreased responding in a progressive ratio schedule of reinforcement (Cottone et al, 2008; Iemolo et al, 2012).
It is noteworthy to mention that, although Chow/Palatable rats have been chronically diet cycled, the behavioral and neurochemical changes shown here occur during an acute, rather than a chronic, withdrawal from palatable diet. Emphasizing this aspect is particularly relevant as in addiction research profound differences in the behavioral, pharmacological, and neurochemical consequences of acute vs protracted abstinence have been observed (Heilig et al, 2010; Koob and Le Moal, 2008). Future studies will be valuable to determine how protracted withdrawal may influence the outcomes of diet cycling.
A relevant point of discussion is whether the excessive palatable food intake behavior we observe in the context of this animal model may be considered ‘compulsive’. In preclinical addiction research, the term ‘compulsive’ has been extensively used to describe excessive drug intake during withdrawal, which is driven by a negative affective state and is relieved upon renewing access to the drug (Ahmed and Koob, 2005; Koob and Le Moal, 2005). This acceptation of the term ‘compulsive’ is based on the conceptual framework that compulsive disorders are characterized by anxiety and stress before committing a compulsive behavior, and relief from the stress by performing the compulsive behavior (Koob, 2009; Koob and Volkow, 2010). In the context of the animal model used here, the excessive eating behavior could be interpreted as a form of ‘compulsive’ behavior given the previously published evidence that rats with intermittent access to the palatable diet show a negative emotional state during palatable food withdrawal, characterized by anxiety-like and depressive-like behaviors, which are relieved upon renewing access (Cottone et al, 2009a, 2009b; Iemolo et al, 2012).
In summary, the results of this study provide critical functional evidence that the CRF–CRF1 receptor system of the CeA and the BlA has a differential role in mediating maladaptive behaviors resulting from intermittent access to palatable food. In the CeA, the CRF–CRF1 receptor system is a key mediator of the excessive eating of palatable food and the withdrawal-dependent negative affect, whereas in the BlA it mediates the subjects’ aversive responses elicited by reward reduction.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
References
Ahmed SH, Koob GF (2005). Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology (Berl) 180: 473–490.
Avena NM, Bocarsly ME, Hoebel BG (2012). Animal models of sugar and fat bingeing: relationship to food addiction and increased body weight. Methods Mol Biol 829: 351–365.
Bakshi VP, Kalin NH (2000). Corticotropin-releasing hormone and animal models of anxiety: gene-environment interactions. Biol Psychiatry 48: 1175–1198.
Bale TL (2005). Sensitivity to stress: dysregulation of CRF pathways and disease development. Horm Behav 48: 1–10.
Blasio A, Iemolo A, Sabino V, Petrosino S, Steardo L, Rice KC (2013a). Rimonabant precipitates anxiety in rats withdrawn from palatable food: role of the central amygdala. Neuropsychopharmacology (doi:10.1038/npp.2013.153).
Blasio A, Steardo L, Sabino V, Cottone P (2013b). Opioid system in the medial prefrontal cortex mediates binge-like eating. Addict Biol (doi:10.1111/adb.12033).
Breese GR, Overstreet DH, Knapp DJ (2005). Conceptual framework for the etiology of alcoholism: a ‘kindling’/stress hypothesis. Psychopharmacology (Berl) 178: 367–380.
Bruijnzeel AW, Ford J, Rogers JA, Scheick S, Ji Y, Bishnoi M et al (2012). Blockade of CRF1 receptors in the central nucleus of the amygdala attenuates the dysphoria associated with nicotine withdrawal in rats. Pharmacol Biochem Behav 101: 62–68.
Chen C, Wilcoxen KM, Huang CQ, Xie YF, McCarthy JR, Webb TR et al (2004). Design of 2,5-dimethyl-3-(6-dimethyl-4-methylpyridin-3-yl)-7-dipropylaminopyrazolo[1,5-a]py rimidine (NBI 30775/R121919) and structure—activity relationships of a series of potent and orally active corticotropin-releasing factor receptor antagonists. J Med Chem 47: 4787–4798.
Corwin RL (2006). Bingeing rats: a model of intermittent excessive behavior? Appetite 46: 11–15.
Corwin RL, Grigson PS (2009). Symposium overview—food addiction: fact or fiction? J Nutr 139: 617–619.
Cottone P, Sabino V, Nagy TR, Coscina DV, Zorrilla EP (2007). Feeding microstructure in diet-induced obesity susceptible versus resistant rats: central effects of urocortin 2. J Physiol 583 (Pt 2): 487–504.
Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB et al (2009a). CRF system recruitment mediates dark side of compulsive eating. Proc Natl Acad Sci USA 106: 20016–20020.
Cottone P, Sabino V, Steardo L, Zorrilla EP (2008). Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats. Am J Physiol Regul Integr Comp Physiol 295: R1066–R1076.
Cottone P, Sabino V, Steardo L, Zorrilla EP (2009b). Consummatory, anxiety-related and metabolic adaptations in female rats with alternating access to preferred food. Psychoneuroendocrinology 34: 38–49.
Cottone P, Wang X, Park JW, Valenza M, Blasio A, Kwak J et al (2012). Antagonism of sigma-1 receptors blocks compulsive-like eating. Neuropsychopharmacology 37: 2593–2604.
Dore R, Iemolo A, Smith KL, Wang X, Cottone P, Sabino V (2013). CRF mediates the anxiogenic and anti-rewarding, but not the anorectic effects of PACAP. Neuropsychopharmacology (doi:10.1038/npp.2013.113).
Funk CK, O'Dell LE, Crawford EF, Koob GF (2006). Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci 26: 11324–11332.
George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH et al (2007). CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci USA 104: 17198–17203.
Grigoriadis DE, Chen C, Wilcoxen K, Chen T, Lorang MT, Bozigion H et al (2000). In vitro characterization of R121919: a novel non-peptide corticotropin-releasing factor1 (CRF1) receptor antagonist for the potential treatment of depression and anxiety-related disorders. Society for Neuroscience Abstract 807: 4–9.
Hagan MM, Chandler PC, Wauford PK, Rybak RJ, Oswald KD (2003). The role of palatable food and hunger as trigger factors in an animal model of stress induced binge eating. Int J Eat Disord 34: 183–197.
Hatfield T, Han JS, Conley M, Gallagher M, Holland P (1996). Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. J Neurosci 16: 5256–5265.
Heilig M, Egli M, Crabbe JC, Becker HC (2010). Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol 15: 169–184.
Heilig M, Koob GF (2007). A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci 30: 399–406.
Heinrichs SC, Menzaghi F, Schulteis G, Koob GF, Stinus L (1995). Suppression of corticotropin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behav Pharmacol 6: 74–80.
Iemolo A, Valenza M, Tozier L, Knapp CM, Kornetsky C, Steardo L et al (2012). Withdrawal from chronic, intermittent access to a highly palatable food induces depressive-like behavior in compulsive eating rats. Behav Pharmacol 23: 593–602.
Koob GF (2008). A role for brain stress systems in addiction. Neuron 59: 11–34.
Koob GF (2009). Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology 56 (Suppl 1): 18–31.
Koob GF (2010). The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res 1314: 3–14.
Koob GF, Heinrichs SC (1999). A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res 848: 141–152.
Koob GF, Le Moal M (2005). Plasticity of reward neurocircuitry and the 'dark side' of drug addiction. Nat Neurosci 8: 1442–1444.
Koob GF, Le Moal M (2008). Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci 363: 3113–3123.
Koob GF, Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238.
Logrip ML, Koob GF, Zorrilla EP (2011). Role of corticotropin-releasing factor in drug addiction: potential for pharmacological intervention. CNS Drugs 25: 271–287.
Maj M, Turchan J, Smialowska M, Przewlocka B (2003). Morphine and cocaine influence on CRF biosynthesis in the rat central nucleus of amygdala. Neuropeptides 37: 105–110.
McNally GP, Akil H (2002). Role of corticotropin-releasing hormone in the amygdala and bed nucleus of the stria terminalis in the behavioral, pain modulatory, and endocrine consequences of opiate withdrawal. Neuroscience 112: 605–617.
Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF et al (1995). Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci 15: 5439–5447.
Murray E, Wise S, Rhodes S (2011). What can different brains do with reward? In Gottfried JA (eds). Neurobiology of Sensation and Reward, Chapter 4. CRC Press: Boca Raton, FL, USA.
Parylak SL, Koob GF, Zorrilla EP (2011). The dark side of food addiction. Physiol Behav 104: 149–156.
Paxinos G, Watson C (2007) The Rat Brain in Stereotaxic Coordinates 6th edn. Academic Press.
Pellegrino A (1979) A Stereotaxic Atlas of the Rat Brain. Plenum: New York.
Rodriguez de Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F (1997). Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science 276: 2050–2054.
Sabino V, Cottone P, Steardo L, Schmidhammer H, Zorrilla EP (2007). 14-Methoxymetopon, a highly potent mu opioid agonist, biphasically affects ethanol intake in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 192: 537–546.
Salinas JA, Parent MB, McGaugh JL (1996). Ibotenic acid lesions of the amygdala basolateral complex or central nucleus differentially effect the response to reductions in reward. Brain Res 742: 283–293.
Shalev U, Erb S, Shaham Y (2010). Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res 1314: 15–28.
Vale W, Spiess J, Rivier C, Rivier J (1981). Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 213: 1394–1397.
Volkow ND, O'Brien CP (2007). Issues for DSM-V: should obesity be included as a brain disorder? Am J Psychiatry 164: 708–710.
Wellman LL, Gale K, Malkova L (2005). GABAA-mediated inhibition of basolateral amygdala blocks reward devaluation in macaques. J Neurosci 25: 4577–4586.
Yach D, Stuckler D, Brownell KD (2006). Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med 12: 62–66.
Acknowledgements
We thank Duncan Momaney, Aditi R Narayan, Jina Kwak for technical assistance, and Tamara Zeric for technical and editorial assistance. We also thank Elena F Crawford for helpful suggestions related to CRF immunohistochemistry. This publication was made possible by grant numbers DA023680, DA030425, MH091945, MH093650, and AA016731, from the National Institute on Drug Abuse (NIDA), the National Institute of Mental Health (NIMH), and the National Institute on Alcohol Abuse and Alcoholism (NIAAA), by the Peter Paul Career Development Professorship (PC) and by Boston University's Undergraduate Research Opportunities Program (UROP). This research was also supported by the NIH Intramural Research Programs of the National Institute on Drug Abuse, and the National Institute of Alcohol Abuse and Alcoholism, NIH, DHHS. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Iemolo, A., Blasio, A., St Cyr, S. et al. CRF–CRF1 Receptor System in the Central and Basolateral Nuclei of the Amygdala Differentially Mediates Excessive Eating of Palatable Food. Neuropsychopharmacol 38, 2456–2466 (2013). https://doi.org/10.1038/npp.2013.147
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2013.147
Keywords
This article is cited by
-
Current perspectives on brain circuits involved in food addiction-like behaviors
Journal of Neural Transmission (2024)
-
Pituitary adenylate cyclase-activating polypeptide (PACAP) modulates dependence-induced alcohol drinking and anxiety-like behavior in male rats
Neuropsychopharmacology (2021)
-
AMPK in the gut-liver-brain axis and its influence on OP rats in an HSHF intake and WTD rat model
Pflügers Archiv - European Journal of Physiology (2021)
-
Reward sensitivity deficits in a rat model of compulsive eating behavior
Neuropsychopharmacology (2020)
-
Oleoylethanolamide decreases frustration stress-induced binge-like eating in female rats: a novel potential treatment for binge eating disorder
Neuropsychopharmacology (2020)