Abstract
Animal models of relapse to drug seeking have focused primarily on relapse induced by exposure to drugs, drug-associated cues or contexts, and foot-shock stress. However, relapse in human drug abusers is often precipitated by loss of alternative non-drug reinforcement. The present experiment used a novel ‘resurgence’ paradigm to examine relapse to cocaine seeking of rats as a result of loss of an alternative source of non-drug reinforcement. Rats were first trained to press a lever for intravenous infusions of cocaine. Next, cocaine deliveries were omitted and food pellets were provided for an alternative nose-poke response. Once cocaine seeking was reduced to low levels, food pellets for the alternative response were also omitted. Cocaine seeking increased with the loss of the alternative non-drug reinforcer (ie, resurgence occurred) despite continued extinction conditions. The increase in cocaine seeking did not occur in another group of rats injected with SCH 23390 before the loss of the alternative reinforcer. These results suggest that removal of an alternative source of reinforcement may induce relapse of cocaine seeking and that the dopamine D1 receptor may have a role in this effect.
Similar content being viewed by others
INTRODUCTION
The propensity to relapse after a period of abstinence is one of the defining features of drug addiction (American Psychiatric Association, 1994). The goal of drug abuse treatment is to encourage abstinence from drug use and decrease the propensity to relapse. Relapse may be triggered by a variety of events including exposure to drug-related cues (Carter and Tiffany, 1999) or stress (Sinha, 2001). Behavioral theories of addiction suggest that drug use is critically dependent on the value of drug reinforcers relative to other non-drug sources of reinforcement (Vuchinich and Tucker, 1988; Bickel et al, 1998). In the laboratory, drug self-administration of both humans and non-humans decreases when an alternative non-drug reinforcer is available (eg, Shahan et al, 2001; see Carroll, 1996 for review). Furthermore, the availability of more frequent or larger non-drug reinforcers produces greater shifts in choice away from drug taking in animal models of drug self-administration (eg, Anderson et al, 2002; Nader and Woolverton, 1991, 1992). Consistent with such findings, treatments for substance abuse designed explicitly to decrease the value of drug use by arranging alternative sources of reinforcement have been quite successful (ie, contingency management; see Higgins et al, 2004; Petry, 2000). However, when such treatment ends, discontinuation of the alternative reinforcers may result in an increase in the relative value of drug reinforcement, and consequently relapse to drug use (see Silverman et al, 1998).
In animal models, examination of environmental and neural mechanisms of relapse have focused almost exclusively on exposure to drug, drug cues, or physical stressors using the reinstatement and renewal paradigms. In the reinstatement paradigm, animals learn to make a response for access to a drug and this response is subsequently extinguished by withholding access to the drug. Responding is then reinstated by exposure to drug, drug-cues, or foot-shock stress (see Shaham et al 2003, for review). In the renewal model, animals are trained to respond for a drug in one context, and subsequently that response is extinguished in another context by withholding the drug. A return to the original self-administration context produces relapse to drug seeking, even though drug remains unavailable, (see Crombag et al, 2008, for review). Although the reinstatement and renewal models allow assessment of several events that trigger relapse in humans, they do not allow assessment of the impact of losing an alternative source of non-drug reinforcement on relapse.
Both the reinstatement and renewal paradigms have their origins in the basic conditioning and learning literature. Another such phenomenon from the conditioning literature called ‘resurgence’ might provide an animal model of relapse following loss of an alternative non-drug reinforcer. In a resurgence paradigm, animals are trained to make a target response for a reinforcer, and that response is subsequently extinguished by withholding the reinforcer. During extinction of the target response, an alternative reinforcer is made available for a second, different response. When the reinforcer for the second response is subsequently withheld, the target response reappears (eg, Leitenberg et al, 1970; Winterbauer and Bouton, 2010; see Shahan and Sweeney, 2011, for review).
Although both reinstatement and renewal of drug seeking have been widely investigated, just one previous study has used the resurgence paradigm to study relapse of drug seeking (Podlesnik et al, 2006). Podlesnik et al (2006) examined resurgence of extinguished oral alcohol self-administration when an alternative food reinforcer produced by pulling a chain was also removed. However, because both the alcohol and the alternative food reinforcer provided calories, it is possible that resurgence occurred because the food reinforcer was a substitute for the calories provided by the alcohol solution. Thus, at present it is not known if the resurgence model can provide a more general model of relapse induced by loss of alternative non-drug reinforcement.
The purpose of the present experiment was to determine if the loss of an alternative non-drug reinforcer induces relapse to intravenous cocaine seeking in a resurgence paradigm. In addition, previous research has found that activity at dopamine (DA) D1 receptors has a role in relapse to drug seeking produced by drug priming or exposure to drug cues (Shalev et al, 2002). The DA D1 antagonist SCH 23390 attenuates both reinstatement and renewal of extinguished cocaine seeking (Norman et al, 1999; Crombag et al, 2002; Alleweireldt et al, 2002). Thus, in order to begin an examination of whether resurgence might share neurobiological mechanisms with reinstatement and renewal of cocaine seeking, we also examined whether SCH 23390 would attenuate resurgence of extinguished cocaine seeking.
MATERIALS AND METHODS
Animals and Housing
Subjects
Twelve experimentally naïve male Long-Evans rats obtained from Charles River (Portage, Michigan, USA) were used. Rats were randomly assigned to the control group or the SCH 23390 group. Rats were individually housed in a climate controlled room with a 12 : 12 light:dark cycle and water was continuously available. Food was continuously available before surgery. Following recovery from surgery, the amount of food the animals received after daily sessions was adjusted to maintain their 85% weights, despite variations in the availability of food in the different phases of the experiment. Care and use of these rats was approved by the Utah State University Institutional Animal Care and Use Committee.
Apparatus
Experimental sessions were conducted in four Med Associates operant self-administration chambers. Each chamber measured 30 cm long, 24 cm wide and 21 cm high, and was housed in a sound-attenuating cubicle. A 4 cm diameter hole in the roof of the chamber allowed a lead made of Tygon tubing encased in a metal spring to be attached to a connector attached to a rat's head and extend to a swivel (Med Associates) hanging ∼15 cm above the chamber. Another 60 cm length of Tygon tubing extended from the swivel to a 60 ml syringe in a Razel infusion pump located outside of the sound-attenuating chamber.
Each chamber contained a response panel with two levers positioned equidistant on either side of a food delivery aperture with an interior light and a tray for collection of 45 mg dustless precision food pellets (Bio-Serv, Frenchtown, New Jersey, USA). A houselight and a Sonalert (2900±500 Hz, 75–85 dB) were located above the food aperture. Three light emitting diodes (LEDs) were located above each lever on the response panel. The opposite side of the chamber contained five apertures evenly spaced horizontally across the bottom of the panel. Each of these apertures contained an interior light and a photobeam to record head entries. Only the left-most aperture and light were used in the present experiment. Med Associates interfacing and software was used for control of experimental events and recording of responses.
Surgery
Before training, each rat was implanted with an intravenous jugular catheter. Surgery was preceded by an injection of antibiotic solution (gentamicin, 2.0 mg/kg, IM) and an anticholinergic drug (atropine sulfate, 0.6 mg/ml, SC, 0.2 ml per rat). Rats were then anesthetized with sodium pentobarbital (65 mg/kg, IP) and prepared for surgery. During surgery, a silastic catheter was inserted into the right jugular vein through an incision made in the right ventral surface of the neck. The catheter was fed subcutaneous dorsally through the neck to the top of the head where it was attached to a 22-gauge metal cannula (PlasticsOne) secured to the skull using skull screws and dental cement. Following surgery, rats were given a non-steroidal anti-inflammatory drug (flunixin meglumine, 1.1 mg/kg, IM) approximately every 12 h for the first 2 days following surgery to minimize pain from the surgical procedures. Rats were allowed to recover at least 5 days after surgery before beginning behavioral training. Food restriction was not implemented until a rat exceeded its pre-surgical weight. Catheter patency was maintained by twice daily infusions of gentamicin heparinized saline solution.
Drug
Cocaine hydrochloride (NIDA/USA) was dissolved in sterile 0.9% saline solution to a concentration of 2.56 mg/ml. Individual infusion doses of cocaine were adjusted by changing the duration of a syringe pump administering 0.0527 ml/s of solution (ie, ∼1 s per 0.32 mg/kg infusion depending upon the rat's weight). SCH 23390 HCl was dissolved to a concentration of 10 μg/ml in 0.9% saline solution.
Methods
The experiment was divided into six phases: training, baseline, extinction+food, resurgence, extinction+food 2, and resurgence 2. Sessions were conducted 7 days per week at approximately the same time each day. Sessions lasted 60 min excluding reinforcer delivery time. Rats in the SCH 23390 group received SCH 23390 (10 μg/kg, SC) 15 min before each session in resurgence and resurgence 2 phases. This dose of SCH 23390 appears to suppress the renewal of extinguished sucrose and cocaine seeking but has little impact on operant responding maintained by food reinforcers (Hamlin et al, 2006; Crombag et al, 2002; Weissenborn et al, 1996).
Training
Rats were first exposed to four daily sessions of magazine training in which a food pellet was delivered approximately every 60 s on a variable time (VT) 60-s schedule. Pellet deliveries were accompanied by the lighting of the food aperture and darkening of other stimuli for 3 s. Following magazine training, rats were trained to press the right lever for infusions of cocaine solution (1.0 mg/kg per infusion) on a fixed ratio (FR) 1 schedule. The number of presses required for each cocaine infusions was increased by increasing the FR value across sessions to an FR 20. The dose of each infusion was then decreased from 1.0 to 0.5, and finally 0.32 across sessions. Rats were limited to obtaining 40 cocaine infusions per session at the 0.32 mg/kg per infusion dose. During training sessions, the light above the right lever was illuminated except during cocaine infusions.
Baseline
Baseline conditions were identical to training with the exception of the cocaine delivery schedule. The baseline schedule arranged a cocaine infusion for an average of 20 responses (ie, a variable ratio (VR) 20 schedule) on the right lever. Responses to the left lever were also recorded but had no other programmed effect. Rats remained in the baseline phase for at least 20 sessions and up to 25 sessions to obtain stability in response rates (ie, coefficient of variation <25% and absence of consistent increasing or decreasing response rates across 5 days).
Extinction+food phase
Following a stable baseline of responding on the cocaine lever, conditions were changed such that lever presses no longer produced cocaine deliveries (ie, extinction was in effect). With the start of extinction of lever pressing for cocaine, the light in the left aperture on the back wall of the chamber was illuminated and nose pokes produced food pellets. Initially, nose pokes were reinforced on a FR 1 schedule, but the schedule was gradually increased across four sessions to an FR 9. On the fifth session, the schedule of food delivery for nose poking was changed to a VR 10. This gradual escalation of the response requirement for food was used to ensure that nose poking for food was initially well trained but not resulting in excessive numbers of food pellets during the session. This phase lasted at least 10 sessions, or until response rates on the cocaine lever for an individual rat decreased to below 10% of baseline response rates. This phase never exceeded 25 sessions.
Resurgence phase
Resurgence of responding was tested by withholding food deliveries for nose pokes while extinction conditions associated with the cocaine lever remained in effect. The light within the nose-poke aperture remained lighted during this condition. This phase lasted at least 10 sessions and until response rates on the cocaine lever for individual rats returned to below 10% of baseline. This criterion was used in order to ensure that responding returned to low levels before the replication conditions to follow.
Extinction+food 2 and resurgence 2 phases
These replication phases were conducted in order to determine whether resurgence could be examined more than once in a sequence of conditions while cocaine-lever pressing remained on extinction. Immediately following the initial resurgence phase, food pellets were again made available contingent on nose pokes in the lighted aperture for 10 sessions. Food was again subsequently removed in the resurgence 2 phase while the aperture remained lighted. Extinction of responding on the cocaine lever remained in effect throughout these conditions. One rat from each group was euthanized because of catheter failure before these replication conditions. An additional rat from the SCH 23390 group was euthanized due to catheter following three sessions of exposure to the resurgence 2 phase. Thus, analyses of the resurgence 2 phase are limited to three sessions.
Statistical Methods
Total mg/kg per session cocaine delivered and responses per min in the last 5 days of baseline were compared for the control and SCH 23390 using 2 (group) × (session) mixed factor ANOVAs. Resurgence of extinguished cocaine-lever pressing with the loss of the alternative reinforcer was analyzed using a 2 (extinction+food versus resurgence) × 5 (session) × 2 (group) mixed factor ANOVA. This analysis focused on the first five sessions of the resurgence condition in order to permit comparison with low-rate responding during the previous extinction phase for the same number of sessions. A similar analysis was conducted on the food nose-poking response. Given the loss of one rat per group, separate mixed ANOVAs with the same factors were used to compare the last three sessions of the extinction+food 2 phase and the resurgence 2 phase. The magnitude of the resurgence effect obtained for the control group in the resurgence and resurgence 2 tests was compared using a 2 (Phase) × 3 (session) repeated measures ANOVA based on the first three sessions of each phase. Statistical significance was determined using α=0.05.
RESULTS
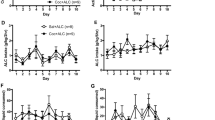
Figure 1 shows rates of lever pressing (cocaine) and nose poking (food) for the control and SCH 23390 groups across experimental phases. Data for the last five sessions are presented for the baseline and the two extinction+food phases. Data are presented for the first five sessions of the resurgence phase and the first three sessions of the resurgence 2 phase. There were no significant differences between the two groups in terms of response rates or total mg/kg per session cocaine earned across the last five sessions of the baseline phase.
Resurgence of cocaine seeking. Lever pressing (cocaine) and nose poking (food) for the control and SCH 23390 groups across experimental phases. When an alternative source of food reinforcement was removed during the resurgence and resurgence 2 conditions, rates of extinguished lever pressing for cocaine increased for the control group, but not for the SCH 23390 group. Data are from the last five sessions of the baseline and the two extinction+food phases and from the first five and three sessions of the resurgence and resurgence 2 phases, respectively.
Examination of cocaine-lever pressing rates during the extinction+food and resurgence phases in Figure 1 reveals that lever pressing increased with the removal of food reinforcement in the resurgence phase for the control group, but not for the SCH 23390 group. In addition, the initially increased rates of lever pressing for the control group in the resurgence phase decreased across sessions. These conclusions are supported by a significant phase × session × group interaction (F(4,40)=5.99, p=0.007), a significant phase × group interaction (F(1,10)=14.78, p<0.003), and main effects of phase (F(1,10)=5.82, p=0.036), session (F(4,40)=5.07, p<0.002), and group (F(1,10)=8.92, p=0.014). Irrelevant-lever pressing remained low across all phases (ie, <1 response per min) and showed no significant increase in the resurgence phase for either group. Nose-poking rates decreased for both groups when the pokes no longer produced food in the resurgence phase. This conclusion is supported by a significant phase × session interaction (F(1,10)=5.95, p<0.001) and a significant main effect of phase (F(1,10)=5.95, p<0.001). There were no significant differences between the control and SCH 23390 groups in terms of nose poking across the extinction+food and resurgence phases (ie, no main effect of group and no significant interactions involving group). Thus, although SCH 23390 blocked the increase in extinguished lever pressing for cocaine when the food reinforcer was removed, it did not affect rates of nose poking.
Figure 1 also shows that cocaine-lever pressing again increased for the control group but not the SCH 23390 group with the transition from extinction+food 2 to resurgence 2 in the replication phases, as evidenced by a significant phase × group interaction (F(1,8)=75.38, p<0.0001), and significant main effects of phase (F(1,8)=59.53, p<0.0001) and group (F(1,8)=31.30, p<0.001). Again, irrelevant-lever pressing remained low (ie, <1 response per min) and showed no significant increase in the resurgence 2 phase for either group. A direct comparison of the first three sessions of the two replications of the resurgence phase reveals that the obtained increase in cocaine-lever responding was larger for the control group in the resurgence than in the resurgence 2 phase (F(1,8)=5.51, p=0.047). Thus, although cocaine-lever responding again increased when the food reinforcer was removed in the replication phase, the magnitude of the resurgence effect decreased. Finally, nose poking also decreased when pokes no longer produced food, as evidenced by a main effect of phase (F(1,8)=48.61, p<0.0001). As in the initial resurgence phase, overall nose-poking rates did not differ for the control and SCH 23390 groups, and there was no interaction of phase and group. However, there was a significant session × group interaction (F(2,16)=8.24, p=0.003) resulting from the fact that nose-poking rates for the SCH23990 group tended to increase in the last three sessions of extinction+food 2, but decreased during the first three resurgence 2 conditions.
DISCUSSION
Extinguished lever pressing previously maintained by cocaine increased (ie, showed resurgence) when non-drug reinforcement for an alternative response was removed from the situation. This effect was replicable across a second set of phases in which food was again made available and then removed, but the magnitude of the effect was smaller. In addition, rats that received the DA D1 antagonist SCH 23390 before resurgence tests did not exhibit an increase in cocaine seeking in either phase in which food was removed.
The present finding that extinguished lever pressing for cocaine increased with the loss of an alternative source of reinforcement is consistent with a larger literature on such resurgence effects in the basic learning literature (Leitenberg et al, 1970; Winterbauer and Bouton, 2010; see Shahan and Sweeney, 2011, for review). Like other similar relapse-like effects (ie, reinstatement, renewal, spontaneous recovery), the resurgence effect is consistent with contemporary theories suggesting that extinction does not involve unlearning (see Bouton, 2004, for review). One notable finding of the present experiment was that the magnitude of the resurgence effect was smaller when the alternative reinforcer was reintroduced and then again removed under continued extinction of cocaine-lever pressing. This effect is consistent with a recently proposed quantitative model of resurgence based on behavioral momentum theory (Shahan and Sweeney, 2011). In short, the model suggests that extinction involves the disruption (rather than unlearning) of previously learned behavior and that resurgence occurs because of the removal of the additional disruptive impact of having an alternative reinforcer present during extinction. The effects of repeated resurgence tests are predicted to be smaller because the disruptive impact of extinction continues to grow with time, and thus, the relative release from disruption associated with removing the alternative reinforcer is lessened (see Shahan and Sweeney, in press, for simulations). Regardless of the details of the model, the present findings suggest that the basic conditioning literature on extinction might continue to provide a rich source of ideas about behavioral processes involved in relapse to drug taking.
One potential complication with interpreting the neurobiological significance of the finding that SCH 23390 prevented resurgence of extinguished cocaine seeking is that the drug might have reduced responding via non-specific motor effects. Although more generally dissociating potential motor effects from motivational effects of SCH 23390 in relapse is difficult (see Crombag et al, 2002), several lines of evidence suggest that the prevention of resurgence in the present experiment likely was not due to motor impairment alone. First, rates of nose poking did not differ significantly for the SCH 23390 and control groups during the resurgence tests. Obviously, the failure to detect any such difference might have been due to the relatively small sample size. Nonetheless, in some cases, nose poking of the SCH 23390 group in the resurgence tests occurred at rates near those of cocaine-lever pressing in the control group. Second, Alleweireldt et al 2002 suggested that evidence against non-specific motor effects of SCH 23390 can be provided by showing that the drug affects persistence across the session, rather than the tendency to begin responding during the session. To examine this possibility, Figure 2 shows the pattern of responding across 2-min bins of the first session of the resurgence phase in the present experiment. Responding across the last day of the extinction+food condition did not differ significantly for the two groups and was thus combined for the analysis. A mixed ANOVA revealed a significant phase × group × bin interaction (F(29, 290)=7.85, p<0.0001), with all other two way interactions and main effects being significant (all Fs>7, p<0.002). Importantly, cumulative responses increased across time in extinction+food and resurgence phases for the SCH 23390 group (F(29, 145)=7.59, p<0.0001), and the pattern of the increase across the session did not differ for the two phases (ie, no main effect of phase or interaction). Finally, a significant difference in responding between the SCH 23390 and control groups did not emerge until the 4th 2-min bin into the session (t(10)=2.67, p=0.023). Thus, SCH 23390 appears not to have impacted the tendency to initiate responding in the session during resurgence. Combined with the nose-poke data above, these finding are consistent with the suggestion that SCH 23390 likely did not prevent resurgence due to motor impairment alone.
Within-session patterns of responding. Cumulative lever presses across successive 2-min bins of the first resurgence session for the control and SCH 23390 groups. Similar data for the last session of exposure to the extinction+food phase (combined across groups) are provided for comparison. Lever pressing for the control and SCH 23390 groups did not differ until the 4th 2-min bin. In addition, lever pressing for the SCH 23390 continued to occur at a low rate throughout the session, as was true for the last extinction+food session.
Previous research has also implicated the DA D1 receptor in drug- and cue-induced reinstatement (Norman et al, 1999; Alleweireldt et al, 2002), and in context-induced renewal of drug seeking (Crombag et al, 2002). On the contrary, DA D1 receptors are not believed to mediate stress-induced relapse (Shaham et al, 2000). This trigger-specific role of DA D1 receptors is potentially important because the loss of alternative reinforcement that induced relapse in the present experiment could be likened to a stressful event. Indeed, many potentially stress-related sources of relapse like job loss or divorce (eg, Temple et al, 1991; Gallo et al, 2001; Falba et al, 2005) could be characterized as involving reinforcement loss. However, considerable uncertainty remains about potentially shared mechanisms in stress-induced relapse and resurgence induced by reinforcement loss. On one hand, it is true that both extinction of operant behavior (De Boer et al, 1990) and exposure to footshock stress (Friedman et al, 1967) are associated with increases in the stress hormone corticosterone. On the other hand, although minimal basal levels of corticosterone are necessary for footshock or food-deprivation stress-induced reinstatement of cocaine seeking, increases in corticosterone do not appear to be crucial (Erb et al, 1998; Shalev et al, 2003). Rather, extrahypothalamic corticotropin-releasing factor appears to have the critical role (see Shalev et al, 2010, for review). At present, the respective roles of corticosterone and corticotropin-releasing factor in extinction of even simple food-maintained operant behavior appear to be unknown. In addition, despite renewed interest in resurgence as a relapse phenomenon (eg, Winterbauer and Bouton, 2010; Shahan and Sweeney, 2011) the relevant neurobiological mechanisms have never been examined—even with food maintained behavior.
Regardless of the specific behavioral or neurobiological mechanisms involved in resurgence, the present experiment suggests that the resurgence paradigm may hold promise as an animal model of relapse induced by loss of an alternative non-drug source of reward. The availability of such an animal model is important because, as noted above, treatments that provide alternative sources of reinforcement have been quite effective. Nonetheless, the long-term efficacy of such treatments in reducing relapse is less clear (Prendergast et al, 2006). Despite reports of greater abstinence following contingency management treatment than control therapies (see Higgins et al, 2000), cessation of treatment is often associated with increased levels of drug use relative to treatment (Silverman et al, 1998). The resurgence model appears to provide a novel way to examine the behavioral and neurobiological mechanisms of such relapse, and to perhaps understand it within the context of existing accounts of relapse.
References
Alleweireldt AT, Weber SM, Kirschner KF, Bullok BL, Neiswander JL (2002). Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 159: 284–293.
American Psychiatric Association (1994). Diagnostic and statistical manual of mental disorders: Fourth edition. American Psychiatric Association: Washington, DC, pp 175–183.
Anderson KG, Velkey AJ, Woolverton WL (2002). The generalized matching law as a predictor of choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 163: 319–326.
Bickel WK, Madden GJ, Petry NM (1998). The price of change: the behavioral economics of drug dependence. Behav Ther 29: 545–565.
Bouton ME (2004). Context and behavioral processes in extinction. Learn Mem 11: 485–494.
Carroll ME (1996). Reducing drug abuse by enriching the environment with alternative nondrug reinforcers. In: Green L, Kagel JH (eds). Advances in Behavioral Economics Volume 3: Substance Use and Abuse. Ablex Publishing Corporation: Norwood, NJ, pp 37–68.
Carter BL, Tiffany ST (1999). Meta-analysis of cue-reactivity in addiction research. Addiction 94: 327–340.
Crombag HS, Bossert JM, Koya E, Shaham Y (2008). Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci 363: 3233–3243.
Crombag HS, Grimm JW, Shaham YS (2002). Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology 27: 1006–1015.
De Boer SF, De Beun R, Slangen JL, Van Der Gugten J (1990). Dynamics of plasma catecholamine and corticosterone concentrations during reinforced and extinguished operant behavior in rats. Physiol Behav 47: 691–698.
Erb S, Shaham Y, Stewart J (1998). The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci 18: 5529–5536.
Falba T, Teng H, Sindelar JL, Gallo WT (2005). The effect of involuntary job loss on smoking intensity and relapse. Addiction 100: 1330–1339.
Friedman SB, Ader R, Grota LJ, Larson T (1967). Plasma corticosterone response to parameters of electric shock stimulation in the rat. Psychosom Med 29: 323–328.
Gallo WT, Bradley EH, Siegel M, Kasl SV (2001). The impact of involuntary job loss on subsequent alcohol consumption by older workers: findings from the health and retirement survey. J Gerontol B Psychol Sci 56: S3–S9.
Hamlin AS, Blatchford KE, McNally GP (2006). Renewal of an extinguished instrumental response: neural correlates and the role of D1 dopamine receptors. Neuroscience 143: 25–38.
Higgins ST, Heil SH, Lussier JP (2004). Clinical implications of reinforcement as a determinant of substance use disorders. Annu Rev Psychol 55: 431–461.
Higgins ST, Wong CJ, Badger GJ, Ogden DEH, Dantona RL (2000). Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. J Consult Clin Psychol 68: 64–72.
Leitenberg H, Rawson RA, Bath K (1970). Reinforcement of competing behavior during extinction. Science 169: 301–303.
Nader MA, Woolverton WL (1991). Effects of increasing the magnitude of an alternative reinforcer on drug choice in a discrete-trials choice procedure. Psychopharmacology (Berl) 105: 167–174.
Nader MA, Woolverton WL (1992). Effects of increasing response requirement on choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 108: 295–300.
Norman AB, Norman MK, Hall JF, Tsibulsky VL (1999). Priming threshold: a novel quantitative measure of the reinstatement of cocaine self-administration. Brain Res 831: 165–174.
Petry NM (2000). A comprehensive guide to the application of contingency management procedures in clinical settings. Drug Alcohol Depend 58: 9–25.
Podlesnik CA, Jimenez-Gomez C, Shahan TA (2006). Resurgence of alcohol seeking produced by discontinuing non-drug reinforcement as an animal model of drug relapse. Behav Pharmacol 17: 369–374.
Prendergast M, Podus D, Finney J, Greenwell L, Roll J (2006). Contingency management for treatment of substance use disorders: a meta-analysis. Addiction 101: 1546–1560.
Shaham Y, Erb S, Stewart J (2000). Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Rev 33: 13–33.
Shaham Y, Shalev U, Lu L, de Witt H, Stewart J (2003). The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 168: 1432–2072.
Shahan TA, Bickel WK, Badger GJ, Giordano LA (2001). Sensitivity of nicotine-containing and de-nicotinized cigarette consumption to alternative non-drug reinforcement: a behavioral economic analysis. Behav Pharmacol 12: 277–284.
Shahan TA, Sweeney MM (2011). A model of resurgence based on behavioral momentum. J Exp Anal Behav 95: 91–108.
Shalev U, Erb S, Shaham Y (2010). Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res 1314: 15–28.
Shalev U, Grimm JW, Shaham Y (2002). Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54: 1–42.
Shalev U, Marinelli M, Baumann MH, Piazza PV, Shaham Y (2003). The role of corticosterone in food deprivation-induced reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 168: 170–176.
Silverman K, Wong CJ, Umbricht-Schneiter A, Montoya ID, Schuster CR, Preston KL (1998). Broad beneficial effects of cocaine abstinence reinforcement among methadone patients. J Consult Clin Psychol 66: 811–824.
Sinha R (2001). How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 158: 343–359.
Temple MT, Fillmore KM, Hartka E, Johnstone B, Leino EV, Motoyoshi M (1991). A meta-analysis of change in marital and employment status as predictors of alcohol consumption on a typical occasion. Br J Addict 86: 1269–1281.
Vuchinich RE, Tucker JA (1988). Contributions from behavioral theories of choice to an analysis of alcohol abuse. J Abnorm Psychol 97: 181–195.
Weissenborn R, Deroche V, Koob GF, Weiss F (1996). Effects of dopamine agonists and antagonists on cocaine-induced operant responding for a cocaine-associated stimulus. Psychopharmacology (Berl) 126: 311–322.
Winterbauer NE, Bouton ME (2010). Mechanisms of resurgence of an extinguished instrumental behavior. J Exp Psychol Anim Behav Process 36: 343–353.
Acknowledgements
Cocaine was kindly supplied by NIDA. The authors thank Eric A Thrailkill for his help in conducting this experiment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
This research was funded by National Institute of Drug Abuse grant R21DA026497 (TAS). The author(s) declares that, except for income received from the primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Quick, S., Pyszczynski, A., Colston, K. et al. Loss of Alternative Non-Drug Reinforcement Induces Relapse of Cocaine-Seeking in Rats: Role of Dopamine D1 Receptors. Neuropsychopharmacol 36, 1015–1020 (2011). https://doi.org/10.1038/npp.2010.239
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2010.239
Keywords
This article is cited by
-
A Comprehensive Systematic Review of Procedures and Analyses Used in Basic and Preclinical Studies of Resurgence, 1970–2020
Perspectives on Behavior Science (2023)
-
Lever-press duration as a measure of frustration in sucrose and drug reinforcement
Psychopharmacology (2021)
-
Resurgence of Challenging Behavior Following Functional Communication Training for Children with Disabilities: a Literature Review
Journal of Developmental and Physical Disabilities (2020)
-
Drug abstinence: exploring animal models and behavioral treatment strategies
Psychopharmacology (2014)