Abstract
The Wistar Kyoto (WKY) rat strain is a putative genetic model of comorbid depression and anxiety. Previous research showing increased κ-opioid receptor (KOR) gene expression in the brains of WKY rats, combined with studies implicating the KOR in animal models of depression and anxiety, suggests that alterations in the KOR system could have a role in the WKY behavioral phenotype. Here, the effects of KOR antagonists in the forced swim test (FST) were compared with the WKY and the Sprague–Dawley (SD) rat strains. As previously reported, WKY rats showed more immobility behavior than SD rats. The KOR antagonists selectively produced antidepressant-like effects in the WKY rats. By contrast, the antidepressant desipramine reduced immobility in both strains. Brain regions potentially underlying the strain-specific effects of KOR antagonists in the FST were identified using c-fos expression as a marker of neuronal activity. The KOR antagonist nor-binaltorphimine produced differential effects on the number of c-fos-positive profiles in the piriform cortex and nucleus accumbens shell between SD and WKY rats. The piriform cortex and nucleus accumbens also contained higher levels of KOR protein and dynorphin A peptide, respectively, in the WKY strain. In addition, local administration of nor-binaltorphimine directly into the piriform cortex produced antidepressant-like effects in WKY rats further implicating this region in the antidepressant-like response to KOR antagonists. These results support the use of the WKY rat as a model of affective disorders potentially involving KOR overactivity and provide more evidence that KOR antagonists could potentially be used as novel antidepressants.
Similar content being viewed by others
INTRODUCTION
Depression is a debilitating illness that exerts a large cost, emotionally and economically, on society (Mauskopf et al, 2009). Despite the availability of a number of clinically effective treatments, a large segment of the population diagnosed with depression exhibits a treatment-resistant form of the disorder (Rush et al, 2006). Patients with comorbid depression and anxiety suffer from poorer overall outcomes in response to antidepressant treatment (Fava et al, 2008). These data suggest that potential antidepressant compounds should be tested in animal models of comorbid depression and anxiety to produce treatments that better address the unique aspects of this subtype of depression.
The Wistar Kyoto (WKY) rat strain was first developed as a normotensive control for the spontaneously hypertensive rat strain (Okamoto and Aoki, 1963). Evidence later emerged that the WKY strain exhibits a unique behavioral phenotype characterized by passive coping behavior and increased sensitivity to stress. For example, WKY rats exhibit increased development of stress-induced ulcers (Pare, 1989b), prolonged elevation of corticosterone after swim stress (Rittenhouse et al, 2002), exaggerated depression-like behavior in the forced swim test (FST) and learned helplessness test (Pare, 1994; Lopez-Rubalcava and Lucki, 2000), and increased anxiety-like behavior in the open-field test (Pare, 1989a), elevated plus maze (Pare, 1992), and defensive burying test (Pare, 1992, 1994). These results support the characterization of the WKY strain as a putative genetic model of comorbid depression and anxiety. In addition, this strain shows resistance to the antidepressant efficacy of selective serotonin reuptake inhibitors suggesting that it may provide insight into mechanisms that confer resistance to frontline antidepressant treatment (Lopez-Rubalcava and Lucki, 2000; Tejani-Butt et al, 2003; Will et al, 2003).
Given the potential utility of the WKY strain as a unique model of psychiatric dysfunction, the neurobiology of its phenotype could provide important understanding of the basis for depression or leads to novel targets for treatment. Quantitative trait loci and microarray analyses have been used to identify genes that are differentially expressed in WKY rats that could contribute to their behavioral phenotype (Ahmadiyeh et al, 2003, 2005; Solberg et al, 2004, 2006; Pearson et al, 2006). A key finding was the increased expression of the κ-opioid receptor (KOR) in the locus coeruleus of WKY rats compared to Sprague–Dawley (SD) rats, a result confirmed by real-time PCR (Pearson et al, 2006). These data are intriguing in the light of substantial literature linking the dynorphin–KOR system with stress and depression. Specifically, KOR activation mediates some endogenous neurobiological aspects of aversion and also comprises an important part of the response to environmental stressors (Iwamoto, 1985; Bals-Kubik et al, 1993; McLaughlin et al, 2003). Also, stressors that produce end points of depression in behavioral models (eg, learned helplessness and the FST) increase dynorphin expression in limbic regions (Shirayama et al, 2004) and, conversely, KOR agonists reproduce end points of depressive behavior (Newton et al, 2002; Mague et al, 2003; McLaughlin et al, 2006a). Consistent with a role for this system in stress-induced depression, disruption of the prodynorphin gene produces resistance to stress-induced immobility (McLaughlin et al, 2003). Finally, KOR antagonists produce antidepressant-like effects in a number of rodent models (Newton et al, 2002; Mague et al, 2003; McLaughlin et al, 2003, 2006b; Shirayama et al, 2004).
In this study, we examined the hypothesis that increased KOR function is a contributor to the depression-like features in WKY rats. The potential antidepressant-like effects of KOR antagonists in the FST were compared between WKY and SD rat strains. The SD strain was chosen because of its use as the comparison strain in the previously discussed microarray study (Pearson et al, 2006) and in the previous behavioral studies conducted by our laboratory (Lopez-Rubalcava and Lucki, 2000; Rittenhouse et al, 2002) and others (Tejani-Butt et al, 2003; Ma and Morilak, 2004). Using c-fos expression as a marker of neuronal activity after swim stress and KOR antagonist treatment, we identified the nucleus accumbens and piriform cortex as brain regions in which differences in KOR function may differentiate the strains. Consequently, KOR and dynorphin A protein levels in the nucleus accumbens and piriform cortex were compared in behaviorally naïve rats from both strains. Moreover, the local infusion of nor-binaltorphimine dihydrochloride (nor-BNI) in the piriform cortex of WKY rats was sufficient to produce antidepressant-like effects in this strain.
MATERIALS AND METHODS
Animals
Male SD (Charles River Laboratories, Wilmington, MA) and WKY (Taconic, Germantown, NY) rats, weighing 225–250 g on arrival, were used in these experiments. The subjects were housed two per cage in a temperature-controlled (22°C) colony room under a 12 : 12 h light–dark schedule (lights on at 0700 hours). Food and water were freely available. All rats were handled daily for a week before behavioral testing. The care and use of animals were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Drug Treatments
nor-BNI (Tocris Bioscience, Ellisville, MO), desipramine hydrochloride (Sigma-Aldrich, St Louis, MO), and sodium pentobarbital (Sigma-Aldrich) were dissolved in distilled water. 2-(3,4-Dichlorophenyl)-N-methyl-N-[(1S)-1(3-isothiocyanatophenyl)-2-(1-pyrrolidinyl)ethyl]acetamide hydrochloride (DIPPA; Tocris Bioscience, Ellisville, MO, USA) was dissolved in a mixture of 20% DMSO and 80% distilled water. All injection solutions were administered at a volume of 2 ml/kg. All systemic injections were given subcutaneously except for pentobarbital, which was given intraperitoneally. In the piriform cortex microinjection study, nor-BNI was dissolved in artificial cerebrospinal fluid (aCSF).
Forced Swim Test Procedures
The modified rat FST used in these experiments was similar to the protocol used previously in this laboratory (Detke et al, 1995). Rats were placed into a glass cylinder (21 cm diameter) filled with water (23–25°C; 30 cm depth) for 15 min. After the 15 min swim session, rats were removed, dried with paper towels, and placed into a polycarbonate cage located on a heating pad for 15 min. The rats were then returned to their home cage. A 5 min swim test was conducted 24 h after the 15 min session. This test was videotaped and scored for the frequency of climbing, swimming, and immobility behaviors by an observer masked to treatment using a time-sampling technique previously described (Detke et al, 1995). Climbing was defined as upward-directed movement of the forepaws aimed toward the sides of the cylinder. Swimming was defined as horizontal movement throughout the cylinder. Immobility consisted of minimum movement required to keep the rat's head above water in the absence of the other two behaviors.
In the first two experiments, owing to their long duration of action (Jones and Holtzman, 1992; Chang et al, 1994), nor-BNI (1, 5, and 10 mg/kg) and DIPPA (1, 2.5, 5, and 10 mg/kg) were administered only once, 0.5 h after the first swim session and 23.5 h before the 5 min swim test. The study with desipramine (20 mg/kg) used the traditional antidepressant treatment regimen with injections given at 23.5, 5, and 1 h before the 5 min swim test. Rats in the control groups received 0.9% saline injections.
nor-BNI Microinjection in the Piriform Cortex
Rats used in this study were anesthetized using sodium pentobarbital (60 mg/kg), mounted into a stereotaxic device (Kopf, San Diego, CA), and bilateral stainless steel guide cannulae (22 gauge; Plastics One, Roanoke, VA) were implanted within the piriform cortex (AP+1.5, ML±4.2, DV −7.0 mm, relative to bregma (Paxinos and Watson, 2005). The cannulae were kept in place using cranioplastic cement anchored to the skull by two screws. Stainless steel dummy cannulae were inserted after completion of the surgery to prevent blockage.
Rats were given 7 days to recover from the surgery before being tested in the modified FST. After being exposed to the first 15 min swim session, nor-BNI (2.5 and 10 μg per side) was infused 23.5 h before the 5 min test, the same time point as the systemic nor-BNI injections in the previous FST experiment. Rats in the control group received aCSF infusions. Nor-BNI or aCSF (1 μl) was infused bilaterally through injection cannulae (26 gauge) extending 1 mm beyond the previously implanted guide cannulae, using polyethylene tubing connected to a 10 μl syringe (Hamilton, Reno, NV). An infusion pump (Instech, Plymouth Meeting, PA) controlled the flow rate (0.1 μl/min) of the solution. The injection cannulae were left in place for an additional 5 min to allow for diffusion and then were replaced by dummy cannulae.
After completion of the FST, rats were anesthetized with an overdose of sodium pentobarbital. Fast Green dye (1 μl) was infused using the same parameters as the aCSF and nor-BNI injections given before testing. The rats were then decapitated and their brains were removed and stored at −80°C until processing. Sections through the piriform cortex (40 μm) were obtained using a cryostat and an observer masked to treatment and behavioral performance verified the location of the Fast Green dye.
Locomotor Activity
Locomotor activity was measured in a Plexiglas open-field arena (L 46 cm × W 38 cm × H 38 cm) located in a room lit by two 15 W lightbulbs pointed away from the testing arena (75 lux). Total distance traveled (cm) was measured over a 15 min time period using the SMART video tracking system (San Diego Instruments, San Diego, CA). A single systemic injection of saline, nor-BNI (10 mg/kg) or DIPPA (10 mg/kg) was given 23.5 h before testing.
c-Fos Immunohistochemistry
At 2 h after exposure to the 5 min FST session, rats from the saline and nor-BNI (10 mg/kg) treatment groups (both strains) were anesthetized with sodium pentobarbital (60 mg/kg) and transcardially perfused with a heparinized saline solution followed by a 4% paraformaldehyde solution. The brains were removed and stored in 4% paraformaldehyde at a temperature of 4°C for 24 h. Brains were then stored in a 20% sucrose solution until sectioning. A second set of subjects used to examine the effects of the FST procedure on c-fos expression did not receive any drug injections (naïve vs FST experienced) and were perfused in an identical manner. Both sets of tissues were processed using a protocol previously described (Roche et al, 2003).
Coronal sections (30 μm thick) were cut on a cryostat and collected into six–well plates. The sections were rinsed in phosphate buffer (PB; 0.1 M) and stored in cryoprotectant solution at −80°C. For the reaction, sections were rinsed three times in PB and incubated with 0.75% H2O2 for 30 min followed by three 10 min rinses in phosphate buffered saline (PBS). Sections were incubated in rabbit anti-c-fos (1 : 1000; Abcam, Cambridge, MA) for 3 days at 4°C. Sections were then rinsed three times and incubated in biotinylated donkey anti-rabbit antiserum (1 : 200; Jackson Labs, West Grove, PA) for 90 min followed by avidin–biotin complex (1 : 600, ABC Elite Kit; Vector Labs, Burlingame, CA) for 90 min at room temperature. Sections were immersed in a solution containing 0.02% 3,3′-diaminobenzidine-4HCl (Sigma-Aldrich), 0.01% H2O2, and 0.6% nickel in Tris buffer (0.05 M) for 10–15 min. The reaction was terminated by rinses in Tris buffer.
Sections were mounted and photomicrographs were taken using a Zeiss Axiovert 25 and Leica DFC 480 camera and imaging software by an individual masked to the experimental condition. At least two sections per animal per area were used to count c-fos profiles. For a particular brain region, the number of c-fos profiles per section was averaged for each subject and the group mean determined from these values.
Western Blot Analysis of KOR Levels
Rats were handled for 7 days before study but did not participate in any behavioral experiments. Brains were placed in a block from which 3 mm slices containing the nucleus accumbens and piriform cortex were microdissected using a trephine. The tissue was stored at −80°C until analysis. Samples were homogenized in a RIPA buffer (Sigma, St Louis, MO) with a protease inhibitor cocktail (Halt; Pierce, Rockford, IL) and centrifuged at 15 000 g for 15 min (4°C). Protein content was determined using the bicinchoninic acid method. Samples (50 μg per condition) were subjected to SDS–PAGE and proteins were transferred to polyvinylidene fluoride membranes as previously described (Curtis et al, 2006). After blocking the membranes with Odyssey buffer (1 h, diluted in PBS 1 : 1), membranes were incubated with rabbit anti-KT2 (KOR tail) antibody (3 mg/ml, provided by Dr Charles Chavkin, University of Washington) for 15 h at 4°C. Membranes were then incubated with the loading control antibody, mouse anti-β-actin (1.5 h, 1 : 5000; Sigma). After rinsing, membranes were incubated with two infrared fluorescent secondary antibodies with different wavelengths for 1 h (1 : 5000, donkey anti-rabbit IRDye 680CW and donkey anti-mouse IRDye 8000CW; LiCor, Lincoln, NE) for simultaneous detection of both primaries.
Membranes were scanned and proteins were detected using the Odyssey Infrared Imaging System (LiCor). Odyssey Infrared Imaging software was used to quantify the integrated intensity of each band and to determine the molecular weights of the proteins (based on Bio-Rad Precision Plus Protein Standards). A ratio of KOR to the loading control was calculated for each sample and the mean ratios were compared between groups.
ELISA Analysis of Dynorphin A Levels
Dynorphin A levels were measured in the same rats that were used for western blot analysis. Brains were placed in a block from which 3 mm slices containing the nucleus accumbens and piriform cortex were microdissected using a trephine. The tissue was stored at −80°C until analysis. Dynorphin A protein levels were quantified using a commercially available ELISA kit (Phoenix Pharmaceuticals, Burlingame, CA). The tissue was homogenized in lysis buffer (100 mM Tris, 1 M NaCl, 4 mM EDTA, 0.1% sodium azide, 2% bovine serum albumin, 2% Triton X-100, 5 μg/ml aprotinin, 0.1 μg/ml pepstatin A, 0.5 μg/ml antipain (pH 7.0)) at a concentration of 20 ml/g of wet tissue weight. The homogenate was then centrifuged for 30 min (15 000 g at 4°C). The supernatant was then processed according to the manufacturer's instructions. Each sample was run in duplicate.
Statistical Analyses
PASW Statistics 17.0 (SPSS, Chicago, IL) software was used for all statistical analysis. The nor-BNI and desipramine FST studies were analyzed using two-way ANOVAs (strain × treatment). The DIPPA FST studies were analyzed using one-way ANOVAs investigating the effects of treatment on behavior within each strain. The c-fos immunohistochemistry experiments were analyzed using two-way ANOVAs (strain × treatment). Pair-wise follow-up comparisons were conducted for regions with a significant or trend for a strain × treatment interaction (p<0.10) because of predetermined planned comparisons. The KOR western blot and dynorphin A ELISA studies were analyzed using Student's t-tests. The piriform cortex microinjection study was analyzed using a one-way ANOVA. Significance was established at p<0.05. Fisher's PLSD test was used for post hoc analyses.
RESULTS
KOR Antagonists Selectively Decrease Immobility in WKY Rats in the FST
WKY rats exhibited significantly higher counts of immobility (F(1,65)=26.41, p<0.001) behavior and lower counts of climbing behavior (F(1,65)=46.68, p<0.001; Figure 1). Saline-treated WKY rats showed about 50% more immobility than saline-treated SD rats (p<0.001). Nor-BNI treatment significantly reduced immobility (F(3,65)=4.11, p=0.010) and increased swimming behavior (F(3,65)=3.80, p=0.014). None of the doses of nor-BNI tested (1, 5, and 10 mg/kg) significantly altered the incidence of immobility in SD rats compared to the saline-treated strain control. In contrast, nor-BNI (5 and 10 mg/kg) produced significant decreases in immobility counts (p=0.014 and 0.013, respectively) and increases in swimming counts (p=0.023 and <0.001, respectively) in WKY rats. Nor-BNI had no effect on climbing in either strain. Nor-BNI (10 mg/kg) also did not produce any significant behavioral effects in SD rats when it was injected more frequently (23.5, 5, and 1 h before testing) according to a standard screening protocol used to measure the effects of tricyclic antidepressants (data not shown).
The effects of nor-binaltorphimine dihydrochloride (nor-BNI) in the forced swim test (FST) in Sprague–Dawley (SD) and Wistar Kyoto (WKY) rats. At baseline, WKY rats exhibit more immobility and less climbing behavior compared to SD rats. Systemic administration of nor-BNI (1, 5, or 10 mg/kg, s.c.) had no significant effects on any of the FST behaviors in SD rats. In contrast, nor-BNI (5 and 10 mg/kg) reduced immobility and increased swimming behavior in WKY rats. All data are depicted as the mean+SEM. n=8–10 per group. The number symbol represents significant differences between the saline-treated groups across strain, #p<0.001. Asterisks represent differences within strain from the saline-treated control group, *p<0.05 and **p<0.01.
The second KOR antagonist tested, DIPPA, also produced strain-specific effects in the FST. DIPPA had no significant effect on immobility or swimming behavior in SD rats (Figure 2a). However, DIPPA significantly decreased climbing behavior (F(2,27)=4.04, p=0.029) in this strain at both the 5 and 10 mg/kg doses (p=0.028 and 0.016, respectively). DIPPA decreased immobility behavior in WKY rats (F(4,64)=3.49, p=0.012; Figure 2b). Treatment groups of 5 and 10 mg/kg showed significantly lower counts of immobility behavior compared to the saline-treated control group (p=0.021 and 0.029, respectively). DIPPA treatment also increased swimming (F(4,64)=3.02, p=0.024). The 10 mg/kg treatment group exhibited significantly more swimming behavior than the control group (p=0.004). DIPPA did not significantly alter climbing behavior in WKY rats.
The effects of DIPPA in the forced swim test (FST) in Sprague–Dawley (SD) and Wistar Kyoto (WKY) rats. (a) Systemic administration of DIPPA (5 and 10 mg/kg) did not produce antidepressant-like effects in SD rats. Both doses significantly decreased climbing behavior. n=9–11 per group. (b) DIPPA (5 and 10 mg/kg) significantly decreased immobility in WKY rats. The 10 mg/kg dose also significantly increased swimming behavior. n=12–16 per group. All data are depicted as the mean+SEM. Asterisks represent significant differences from the saline-treated control group. *p<0.05 and **p<0.01.
Tricyclic Antidepressant Desipramine is Effective in both WKY and SD Rats
The tricyclic antidepressant desipramine was injected according to a standard screening protocol (23.5, 5, and 1 h before testing) and produced a different profile of effects compared to the KOR antagonists (Figure 3). There were significant effects of both strain (F(1,32)=16.96, p<0.001) and treatment (F(1,32)=26.36, p<0.001) on immobility behavior and no significant interaction. Post hoc analysis showed that the saline-treated WKY group exhibited significantly higher immobility counts than the saline-treated SD rats (p<0.001). Also, desipramine treatment decreased immobility in both SD and WKY rats when compared with their within-strain control groups (p<0.001 and p=0.011, respectively). There were also significant effects of strain (F(1,32)=16.88, p<0.001) and treatment (F(1,32)=23.45, p<0.001) on climbing behavior. As previously shown in the nor-BNI FST experiment, saline-treated WKY rats exhibited significantly lower counts of climbing behavior compared to saline-treated SD rats (p<0.001). Desipramine treatment significantly increased climbing behavior in SD and WKY rats compared to their within-strain control groups (p=0.001 and 0.012, respectively). There were no effects of strain or treatment on swimming behavior. Desipramine did not produce any significant behavioral effects when it was injected only once (23.5 h before testing), the same time that nor-BNI was administered (data not shown).
The effects of desipramine in the forced swim test (FST) in Sprague–Dawley (SD) and Wistar Kyoto (WKY) rats. WKY rats exhibited increased immobility and decreased climbing behavior at baseline compared to SD rats. Systemic administration of desipramine (20 mg/kg) significantly decreased immobility and also increased climbing behavior in both strains. All data are depicted as the mean+SEM. n=8–10 per group. The number symbol represents significant differences between the saline-treated groups across strain, #p<0.001. Asterisks represent significant differences from the saline-treated control group within strain. *p<0.05, **p<0.01, and ***p<0.001.
KOR Antagonists do not Produce a Stimulant-Like Increase in Locomotor Activity
Doses of nor-BNI and DIPPA (10 mg/kg) that reduced FST immobility in WKY rats were tested for their effects on locomotor activity in both strains. There were significant main effects of strain (F(1,42)=4.20, p=0.047) and treatment (F(2,42)=19.92, p<0.001) along with a significant strain × treatment interaction (F(2,42)=15.11, p<0.001) on locomotor activity (Table 1). Saline-treated WKY rats exhibited a lower level of baseline activity when compared to saline-treated SD rats (p=0.003). In SD rats, nor-BNI treatment did not alter locomotor activity whereas DIPPA treatment produced a significant decrease (p<0.001). Neither KOR antagonist altered locomotor activity in WKY rats compared to the saline-treated control group.
Differential Induction of c-fos by nor-BNI in WKY Rats Exposed to Swim Stress
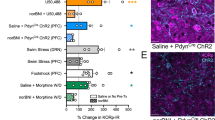
c-Fos expression was quantified in 14 brain regions (Figure 4; descriptive statistics in Table 2) in a subset of the rats (saline and 10 mg/kg groups) from the nor-BNI FST experiment. WKY rats exhibited overall higher numbers of c-fos-positive cells in the locus coeruleus (F(1,30)=6.77, p=0.014) and the piriform cortex (F(1,22)=4.58, p=0.044). Nor-BNI treatment did not alter c-fos expression differentially between strains in the locus coeruleus. Only two brain regions, the piriform cortex and nucleus accumbens shell, showed a significant or trend for interaction between strain and drug treatment sufficient to justify follow-up pair-wise comparisons (p<0.10). Nor-BNI treatment significantly increased c-fos-positive profiles in the piriform cortex in WKY rats (p=0.003) whereas it produced no significant change in SD rats (representative sections shown in Figure 5a). Nor-BNI treatment also increased c-fos-positive profiles in the nucleus accumbens shell in WKY rats (p=0.002) but produced no significant effect in SD rats (representative sections in Figure 5b). None of the other brain regions showed any significant differences in patterns of c-fos expression.
Localization of brain regions analyzed in c-fos studies. These schematics represent the approximate areas used for the quantification of c-fos profiles for each brain region. The images, from the top left image, correspond to Figures 20, 24, 35, 45, 47, 60, 94, and 115, respectively from Paxinos and Watson (2005). The numbers underneath each image represent the anterior/posterior distance from bregma. Abbreviations: AcbSh, nucleus accumbens shell; Cg1, cingulate cortex area 1; Cg2, cingulate cortex area 2; DG, dentate gyrus; DR, dorsal raphe; LC, locus coeruleus; LH, lateral habenula; Pir, piriform cortex; PaV, paraventricular nucleus of the hypothalamus; PVA, paraventricular thalamic nucleus; ST, bed nucleus of the stria terminalis.
Swim Stress Effects on c-fos Expression
To compare the effects of the FST procedure alone on c-fos expression between strains, we quantified c-fos profiles in a subset of the brain regions from the previous experiment in separate groups of rats that were either FST experienced or FST naïve. These animals did not receive pretreatment injections. There were no significant interaction effects for any of the brain regions between strain and swim stress (Table 3). The FST procedure produced significant increases in c-fos expression in the piriform cortex (F(1,20)=14.87, p=0.001), nucleus accumbens shell (F(1,20)=11.34, p=0.003), dentate gyrus (F(1,19)=22.95, p<0.001), and the CA3 area of the hippocampus (F(1,20)=13.54, p<0.001). There was a significant overall difference in c-fos expression between strains in the piriform cortex (F(1,20)=4.54, p<0.046) and the dentate gyrus (F(1,19)=11.54, p=0.003).
Strain Differences in KOR and Dynorphin A Expression
Baseline levels of the KOR protein in experimentally naïve subjects from both strains were measured in the nucleus accumbens and piriform cortex. KOR protein levels were elevated in the piriform cortex of WKY rats compared to SD rats (t(28)=2.87, p=0.008; Figure 6a and c). There was no difference in the amount of KOR protein in the nucleus accumbens between the two strains (t(18)=0.55, p=0.590; Figure 6b).
Western blot analysis of κ-opioid receptor (KOR) expression in the nucleus accumbens and piriform cortex. (a) Representative blots show the expression of KOR protein (MW=72 kDa), and β-actin (MW=42 kDa) in the piriform cortex. (b and c) Graphs show the mean ratio of the integrated intensity of each band of KOR protein to the corresponding band of β-actin from the same sample. KOR expression was greater in the piriform cortex, but not nucleus accumbens, of Wistar Kyoto (WKY) rats compared to Sprague–Dawley rats. All data are depicted as the mean+SEM. n=10–15 per group. Asterisks represent significant differences between groups, *p<0.05.
Baseline levels of dynorphin A, the main ligand of the KOR, were also measured in the nucleus accumbens and piriform cortex (Figure 7). Dynorphin A levels were greater in the nucleus accumbens of WKY rats compared to SD rats (t(18)=4.03, p<0.001; Figure 7a). There was no strain difference in dynorphin A content in the piriform cortex (t(18)=0.14, p=0.89; Figure 7b).
Dynorphin A expression levels in the nucleus accumbens and piriform cortex. (a) Wistar Kyoto (WKY) rats had higher baseline tissue content levels of dynorphin A in the nucleus accumbens. (b) There were no significant differences in dynorphin A content in the piriform cortex. All data are depicted as the mean+SEM. n=10 per group. Asterisks represent significant differences between groups, ***p<0.001.
Infusion of nor-BNI Within the Piriform Cortex Produces Antidepressant-Like Effects in WKY Rats
WKY rats were given local infusions of nor-BNI within the piriform cortex and subsequently tested in the FST. The infusion of nor-BNI into the piriform cortex reduced immobility in the FST (F(3,19)=3.76, p=0.028; Figure 8a). Both doses tested (2.5 and 10 μg per side) produced significant decreases in immobility counts (p=0.03 and 0.01, respectively) compared with the aCSF control group. There were no significant effects on swimming or climbing as the increase in active behavior was split between the two categories. To evaluate the site specificity of the antidepressant-like effect, the data from rats that received 10 μg per side outside the piriform cortex (‘misses’ group; Figure 8b) were compared with the aCSF controls. There was no difference in immobility between these two groups (p=0.586).
The effects of local infusion of nor-binaltorphimine dihydrochloride (nor-BNI) into the piriform cortex in the forced swim test (FST) in Wistar Kyoto (WKY) rats. (a) Both doses tested (2.5 μg per side (n=4) and 10 μg per side (n=7)) produced significant decreases in immobility. The ‘misses’ group (n=5), 10 μg per side dorsal with respect to the piriform cortex, did not exhibit any significant changes in behavior compared to the artificial cerebrospinal fluid (aCSF)-treated controls (n=7). (b) The location of the injections for the rats used in this study. Filled circles represent placements within the piriform cortex and open circles represent placements outside the region. All data depicted as the mean+SEM. Asterisks represent significant differences from the aCSF-treated control group, *p<0.05.
DISCUSSION
This study showed selective antidepressant efficacy of KOR antagonists in the FST in the WKY rat strain compared to the SD strain. Distinct c-fos activation patterns in WKY and SD rats treated with the KOR antagonist nor-BNI implicated the nucleus accumbens shell and piriform cortex as sites of action that may be involved in the strain-related pharmacodynamic differences. Consistent with those findings, we also measured baseline differences in dynorphin A and KOR protein levels in these regions between SD and WKY rats. In addition, local infusion of nor-BNI into the piriform cortex of WKY rats produced antidepressant-like effects in the FST further implicating this region in the behavioral response of the strain to KOR antagonists. Together, these findings suggest that the dynorphin–KOR system contributes to the stress-sensitive phenotype of WKY rats and that KOR antagonists could be a novel therapy for affective disorders.
The KOR system has been previously implicated in preclinical models of depression and KOR antagonists have been investigated as potential antidepressant treatments (Pliakas et al, 2001; Mague et al, 2003; McLaughlin et al, 2003; Shirayama et al, 2004). Antidepressant-like effects of KOR antagonists have been detected in SD rats under different conditions. Central administration of nor-BNI produced antidepressant-like effects in previous studies (Pliakas et al, 2001; Mague et al, 2003; Shirayama et al, 2004). The results of systemic administration of nor-BNI have been mixed. In agreement with our findings, Zhang et al (2007) reported that systemic administration of nor-BNI did not produce antidepressant-like effects in SD rats in a single trial version of the rat FST. In contrast, Beardsley et al (2005) reported that systemic administration of nor-BNI produces antidepressant-like effects in the modified rat FST. One possible explanation for the seemingly contradictory findings could be the high level of baseline immobility in their study. The high amount of immobility behavior present in their saline-treated group, compared to the control groups from this study, and the other studies previously cited, could be indicative of increased stress or other environmental conditions that contributed to the antidepressant-like response of the SD rats. These effects could be viewed as additional evidence that KOR antagonists may prove to be effective treatments in other rat models that are characterized by high baseline immobility (Becker et al, 2008). In support of this theory, a recent study shows that systemic nor-BNI can block the increase in immobility caused by early life methylphenidate exposure (Wiley et al, 2009).
Mague et al (2003) showed that the KOR antagonist GNTI did not produce antidepressant-like effects when administered systemically, but did produce effects when given centrally. In addition, systemic administration of the KOR antagonist 5′-acetamidinoethylnaltrindole (ANTI), with greater hypothesized central availability, produces antidepressant-like effects in the FST suggesting that insufficient availability in the brain may be a problem for some KOR antagonists. Although a dose of systemic nor-BNI higher than 10 mg/kg might still produce antidepressant-like effects in SD rats, administering this dose of nor-BNI more frequently to SD rats according to a standard screening protocol (23.5, 5, and 1 h before testing) failed to produce any behavioral effects in the FST. The effects of nor-BNI and DIPPA were longer lasting than most antidepressants, producing significant effects in WKY rats when tested 24 h after a single dose, whereas three injections within 24 h using the standard screening protocol are usually required to produce behavioral effects of established antidepressants. The lengthy time course of KOR antagonists in the FST is in agreement with the long-lasting (days) time course for their blockade of KOR agonist-induced analgesia (Jones and Holtzman, 1992; Chang et al, 1994).
Our c-fos analysis highlighted the nucleus accumbens shell and piriform cortex as two regions that are activated differentially by nor-BNI between the strains and may be involved in the antidepressant-like behavioral response to KOR antagonist treatment. Owing to the limitations associated with the use of c-fos induction as a measure of neuronal activity (Dragunow and Faull, 1989) and the complex nature of FST-associated behaviors, these two regions most likely do not represent the sole areas of functional divergence between the strains. However, previously published research does support a potential role for both regions in the antidepressant-like behavioral response in the FST.
The nucleus accumbens is thought to be involved in the integration of both rewarding and aversive stimuli (Carlezon and Thomas, 2009). This region has previously been implicated in the effects of both KOR agonists and antagonists in the FST (Pliakas et al, 2001; Shirayama et al, 2004). Therefore, it was not surprising for the area to be highlighted as a region of interest in response to KOR antagonist treatment. Although there was no effect of nor-BNI treatment in SD rats, a significant decrease in immobility (33%) in WKY rats coincided with the large increase in c-fos-positive cells in the region. In addition, WKY rats had much higher levels of dynorphin A protein in the region. The behavioral effects of agonists at the KOR are believed to be mediated through presynaptic inhibition of neurotransmitter release (Bals-Kubik et al, 1993; Svingos et al, 2001; Li and van den Pol, 2006; Kreibich et al, 2008). This could lead to a decreased activation of cells in the region that would be counteracted by KOR antagonist treatment. Interestingly, this inhibitory effect of KOR activation in the nucleus accumbens is thought to have a role in the expression of the aversive properties of KOR agonists (Bals-Kubik et al, 1993; Hjelmstad and Fields, 2003; Land et al, 2008).
The present findings also highlighted the piriform cortex as a potential site of action of KOR antagonists. WKY rats exhibited increased expression of KOR protein in the piriform cortex compared to SD rats and local infusion of nor-BNI in the region was sufficient to produce antidepressant-like effects in WKY rats. Although the piriform cortex is primarily considered an olfaction-associated brain region (Haberly, 2001), this is not the first study in which it has been implicated in the behavioral response to antidepressant-like treatments in rodents (Sibille et al, 1997; Bechtholt et al, 2008; Stone and Lin, 2008). These studies all measured an increase in c-fos activation in the region in response to treatment that produced antidepressant-like effects in their respective behavioral assays. The piriform cortex has also been identified as a region that shows plasticity in response to antidepressant treatment (Sun et al, 2005; Zhou et al, 2006; Hjaeresen et al, 2008). Interestingly, electroconvulsive shock causes the upregulation of brain-derived neurotrophic factor gene expression in the region (Nibuya et al, 1995), a growth factor shown to have antidepressant-like properties (Shirayama et al, 2002; Hoshaw et al, 2005). In addition, degeneration of the piriform is thought to contribute to the behavioral phenotype seen in the olfactory bulbectomy model of depression (Song and Leonard, 2005; Wang et al, 2007). The extensive connections of the piriform cortex to the amygdala, nucleus accumbens, thalamus, and prefrontal cortex in both rodents and primates suggest that the region is well placed to influence the behavioral response to stressful situations (Ray and Price, 1992; Carmichael et al, 1994; Haberly, 2001).
The locus coeruleus, a region in which WKY rats have been previously reported to show increased KOR gene expression in comparison to SD rats (Pearson et al, 2006), was also highlighted as a region of interest by the c-fos activation study. Given that the KOR–dynorphin system has been shown to presynaptically inhibit the activity of the locus coeruleus (Kreibich et al, 2008), our findings that WKY rats had higher levels of c-fos-positive profiles were initially surprising. However, these results are in agreement with previous research that suggests the regulation of norepinephrine release in WKY rats in response to stress depends on the duration of the stress. After acute stress, WKY rats exhibit a blunted norepinephrine response compared to SD rats (Sands et al, 2000; Ma and Morilak, 2004). In contrast, repeated stress leads to an increased norepinephrine response in WKY rats (Pardon et al, 2003). The fact that we measured c-fos expression after repeated swim stress may account for the increased number of c-fos-positive profiles in the locus coeruleus. More research into the electrophysiological effects of KOR-specific ligands in WKY rats will need to be conducted.
The WKY rat strain has been proposed as a model of comorbid depression and anxiety. Given the difficulties associated with therapy for comorbid depression and anxiety (Fava et al, 2008), it is important to identify novel treatments that may be effective against this subtype of depression. The current studies showed that WKY rats displayed increased sensitivity to the antidepressant-like effects of KOR antagonists. In addition, endogenous alterations in the dynorphin–KOR system in the nucleus accumbens and piriform cortex may have a role in the increased efficacy of KOR antagonists in the strain. Further studies are required to determine if the dynorphin–KOR system is involved in the anxiogenic component of the WKY phenotype. Given the increased difficulty of finding effective treatments for the comorbid depression and anxiety population, genetic animal models that recapitulate this unique behavioral profile can be used to further the development of effective clinical treatments.
References
Ahmadiyeh N, Churchill GA, Shimomura K, Solberg LC, Takahashi JS, Redei EE (2003). X-linked and lineage-dependent inheritance of coping responses to stress. Mamm Genome 14: 748–757.
Ahmadiyeh N, Churchill GA, Solberg LC, Baum AE, Shimomura K, Takahashi JS et al (2005). Lineage is an epigenetic modifier of QTL influencing behavioral coping with stress. Behav Genet 35: 189–198.
Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS (1993). Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther 264: 489–495.
Beardsley PM, Howard JL, Shelton KL, Carroll FI (2005). Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 183: 118–126.
Bechtholt AJ, Valentino RJ, Lucki I (2008). Overlapping and distinct brain regions associated with the anxiolytic effects of chlordiazepoxide and chronic fluoxetine. Neuropsychopharmacology 33: 2117–2130.
Becker C, Zeau B, Rivat C, Blugeot A, Hamon M, Benoliel JJ (2008). Repeated social defeat-induced depression-like behavioral and biological alterations in rats: involvement of cholecystokinin. Mol Psychiatry 13: 1079–1092.
Carlezon Jr WA, Thomas MJ (2009). Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology 56 (Suppl 1): 122–132.
Carmichael ST, Clugnet MC, Price JL (1994). Central olfactory connections in the macaque monkey. J Comp Neurol 346: 403–434.
Chang AC, Takemori AE, Portoghese PS (1994). 2-(3,4-Dichlorophenyl)-N-methyl-N-[(1S)-1-(3-isothiocyanatophenyl)-2-(1-pyrrolidinyl)ethyl]acetamide: an opioid receptor affinity label that produces selective and long-lasting kappa antagonism in mice. J Med Chem 37: 1547–1549.
Curtis AL, Bethea T, Valentino RJ (2006). Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology 31: 544–554.
Detke MJ, Rickels M, Lucki I (1995). Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 121: 66–72.
Dragunow M, Faull R (1989). The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods 29: 261–265.
Fava M, Rush AJ, Alpert JE, Balasubramani GK, Wisniewski SR, Carmin CN et al (2008). Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry 165: 342–351.
Haberly LB (2001). Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses 26: 551–576.
Hjaeresen ML, Hageman I, Wortwein G, Plenge P, Jorgensen MB (2008). Chronic electroconvulsive stimulation but not chronic restraint stress modulates mRNA expression of voltage-dependent potassium channels Kv7.2 and Kv11.1 in the rat piriform cortex. Brain Res 1217: 179–184.
Hjelmstad GO, Fields HL (2003). Kappa opioid receptor activation in the nucleus accumbens inhibits glutamate and GABA release through different mechanisms. J Neurophysiol 89: 2389–2395.
Hoshaw BA, Malberg JE, Lucki I (2005). Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res 1037: 204–208.
Iwamoto ET (1985). Place-conditioning properties of mu, kappa, and sigma opioid agonists. Alcohol Drug Res 6: 327–339.
Jones DN, Holtzman SG (1992). Long term kappa-opioid receptor blockade following nor-binaltorphimine. Eur J Pharmacol 215: 345–348.
Kreibich A, Reyes BA, Curtis AL, Ecke L, Chavkin C, Van Bockstaele EJ et al (2008). Presynaptic inhibition of diverse afferents to the locus ceruleus by kappa-opiate receptors: a novel mechanism for regulating the central norepinephrine system. J Neurosci 28: 6516–6525.
Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C (2008). The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci 28: 407–414.
Li Y, van den Pol AN (2006). Differential target-dependent actions of coexpressed inhibitory dynorphin and excitatory hypocretin/orexin neuropeptides. J Neurosci 26: 13037–13047.
Lopez-Rubalcava C, Lucki I (2000). Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology 22: 191–199.
Ma S, Morilak DA (2004). Induction of FOS expression by acute immobilization stress is reduced in locus coeruleus and medial amygdala of Wistar-Kyoto rats compared to Sprague-Dawley rats. Neuroscience 124: 963–972.
Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens Jr WC et al (2003). Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther 305: 323–330.
Mauskopf JA, Simon GE, Kalsekar A, Nimsch C, Dunayevich E, Cameron A (2009). Nonresponse, partial response, and failure to achieve remission: humanistic and cost burden in major depressive disorder. Depress Anxiety 26: 83–97.
McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C (2006a). Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology 31: 787–794.
McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C (2006b). Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology 31: 1241–1248.
McLaughlin JP, Marton-Popovici M, Chavkin C (2003). Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci 23: 5674–5683.
Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N et al (2002). Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci 22: 10883–10890.
Nibuya M, Morinobu S, Duman RS (1995). Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 15: 7539–7547.
Okamoto K, Aoki K (1963). Development of a strain of spontaneously hypertensive rats. Jpn Circ J 27: 282–293.
Pardon MC, Ma S, Morilak DA (2003). Chronic cold stress sensitizes brain noradrenergic reactivity and noradrenergic facilitation of the HPA stress response in Wistar Kyoto rats. Brain Res 971: 55–65.
Pare WP (1989a). Stress ulcer susceptibility and depression in Wistar Kyoto (WKY) rats. Physiol Behav 46: 993–998.
Pare WP (1989b). Strain, age, but not gender, influence ulcer severity induced by water-restraint stress. Physiol Behav 45: 627–632.
Pare WP (1992). The performance of WKY rats on three tests of emotional behavior. Physiol Behav 51: 1051–1056.
Pare WP (1994). Open field, learned helplessness, conditioned defensive burying, and forced-swim tests in WKY rats. Physiol Behav 55: 433–439.
Paxinos G, Watson C (2005). The Rat Brain in Stereotaxic Coordinates—The New Coronal Set, 5th edn. Academic Press: New York, NY.
Pearson KA, Stephen A, Beck SG, Valentino RJ (2006). Identifying genes in monoamine nuclei that may determine stress vulnerability and depressive behavior in Wistar-Kyoto rats. Neuropsychopharmacology 31: 2449–2461.
Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon Jr WA (2001). Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci 21: 7397–7403.
Ray JP, Price JL (1992). The organization of the thalamocortical connections of the mediodorsal thalamic nucleus in the rat, related to the ventral forebrain–prefrontal cortex topography. J Comp Neurol 323: 167–197.
Rittenhouse PA, Lopez-Rubalcava C, Stanwood GD, Lucki I (2002). Amplified behavioral and endocrine responses to forced swim stress in the Wistar-Kyoto rat. Psychoneuroendocrinology 27: 303–318.
Roche M, Commons KG, Peoples A, Valentino RJ (2003). Circuitry underlying regulation of the serotonergic system by swim stress. J Neurosci 23: 970–977.
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D et al (2006). Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163: 1905–1917.
Sands SA, Strong R, Corbitt J, Morilak DA (2000). Effects of acute restraint stress on tyrosine hydroxylase mRNA expression in locus coeruleus of Wistar and Wistar-Kyoto rats. Brain Res Mol Brain Res 75: 1–7.
Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS (2002). Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 22: 3251–3261.
Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS (2004). Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem 90: 1258–1268.
Sibille E, Sarnyai Z, Benjamin D, Gal J, Baker H, Toth M (1997). Antisense inhibition of 5-hydroxytryptamine2a receptor induces an antidepressant-like effect in mice. Mol Pharmacol 52: 1056–1063.
Solberg LC, Baum AE, Ahmadiyeh N, Shimomura K, Li R, Turek FW et al (2004). Sex- and lineage-specific inheritance of depression-like behavior in the rat. Mamm Genome 15: 648–662.
Solberg LC, Baum AE, Ahmadiyeh N, Shimomura K, Li R, Turek FW et al (2006). Genetic analysis of the stress-responsive adrenocortical axis. Physiol Genomics 27: 362–369.
Song C, Leonard BE (2005). The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev 29: 627–647.
Stone EA, Lin Y (2008). An anti-immobility effect of exogenous corticosterone in mice. Eur J Pharmacol 580: 135–142.
Sun W, Park KW, Choe J, Rhyu IJ, Kim IH, Park SK et al (2005). Identification of novel electroconvulsive shock-induced and activity-dependent genes in the rat brain. Biochem Biophys Res Commun 327: 848–856.
Svingos AL, Chavkin C, Colago EE, Pickel VM (2001). Major coexpression of kappa-opioid receptors and the dopamine transporter in nucleus accumbens axonal profiles. Synapse 42: 185–192.
Tejani-Butt S, Kluczynski J, Pare WP (2003). Strain-dependent modification of behavior following antidepressant treatment. Prog Neuropsychopharmacol Biol Psychiatry 27: 7–14.
Wang HH, Li LY, Wang LW, Liang CC (2007). Morphological and histological studies on the telencephalon of the salamander Onychodactylus fischeri. Neurosci Bull 23: 170–174.
Wiley MD, Poveromo LB, Antapasis J, Herrera CM, Bolanos Guzman CA (2009). Kappa-opioid system regulates the long-lasting behavioral adaptations induced by early-life exposure to methylphenidate. Neuropsychopharmacology 34: 1339–1350.
Will CC, Aird F, Redei EE (2003). Selectively bred Wistar-Kyoto rats: an animal model of depression and hyper-responsiveness to antidepressants. Mol Psychiatry 8: 925–932.
Zhang H, Shi YG, Woods JH, Watson SJ, Ko MC (2007). Central kappa-opioid receptor-mediated antidepressant-like effects of nor-binaltorphimine: behavioral and BDNF mRNA expression studies. Eur J Pharmacol 570: 89–96.
Zhou L, Huang KX, Kecojevic A, Welsh AM, Koliatsos VE (2006). Evidence that serotonin reuptake modulators increase the density of serotonin innervation in the forebrain. J Neurochem 96: 396–406.
Acknowledgements
This work was supported by a research grant provided by AstraZeneca (IL, RJV). Additional support was provided by National Institutes of Health Grants DA09082 (RJV), MH084423 (DAB), and MH14652 (GVC and DAB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure
Irwin Lucki is on the scientific advisory board for Wyeth and has received research support from AstraZeneca, Wyeth, Forest, and Epix pharmaceutical companies during the past 3 years. Rita Valentino has received support from AstraZeneca. There are no disclosures from other authors.
Rights and permissions
About this article
Cite this article
Carr, G., Bangasser, D., Bethea, T. et al. Antidepressant-Like Effects of κ-Opioid Receptor Antagonists in Wistar Kyoto Rats. Neuropsychopharmacol 35, 752–763 (2010). https://doi.org/10.1038/npp.2009.183
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2009.183
Keywords
This article is cited by
-
Suicide Risk and Addiction: The Impact of Alcohol and Opioid Use Disorders
Current Addiction Reports (2021)
-
Ablation of olfactory bulb glutamatergic neurons induces depressive-like behaviors and sleep disturbances in mice
Psychopharmacology (2020)
-
Endogenous opioid system dysregulation in depression: implications for new therapeutic approaches
Molecular Psychiatry (2019)
-
The importance of buprenorphine research in the opioid crisis
Molecular Psychiatry (2019)
-
The Behavioral Effects of the Antidepressant Tianeptine Require the Mu-Opioid Receptor
Neuropsychopharmacology (2017)