Abstract
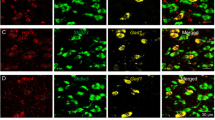
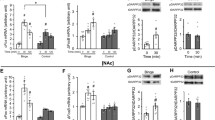
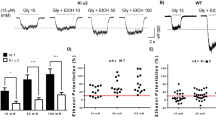
Agonists of GABAB receptors exert a bi-directional effect on the activity of dopamine (DA) neurons of the ventral tegmental area, which can be explained by the fact that coupling between GABAB receptors and G protein-gated inwardly rectifying potassium (GIRK) channels is significantly weaker in DA neurons than in GABA neurons. Thus, low concentrations of agonists preferentially inhibit GABA neurons and thereby disinhibit DA neurons. This disinhibition might confer reinforcing properties on addictive GABAB receptor agonists such as γ-hydroxybutyrate (GHB) and its derivatives. Here we show that, in DA neurons of mice, the low coupling efficiency reflects the selective expression of heteromeric GIRK2/3 channels and is dynamically modulated by a member of the regulator of G protein signaling (RGS) protein family. Moreover, repetitive exposure to GHB increases the GABAB receptor-GIRK channel coupling efficiency through downregulation of RGS2. Finally, oral self-administration of GHB at a concentration that is normally rewarding becomes aversive after chronic exposure. On the basis of these results, we propose a mechanism that might underlie tolerance to GHB.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
Change history
14 November 2007
In the version of this article initially published online, the corresponding author's email address was incorrect. The correct email address is Christian.Luscher@medecine.unige.ch. The error has been corrected for all versions of the article.
References
Lüscher, C., Jan, L.Y., Stoffel, M., Malenka, R.C. & Nicoll, R.A. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron 19, 687–695 (1997).
Dascal, N. et al. Atrial G protein-activated K+ channel: expression cloning and molecular properties. Proc. Natl. Acad. Sci. USA 90, 10235–10239 (1993).
Krapivinsky, G. et al. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K(+)-channel proteins. Nature 374, 135–141 (1995).
Kubo, Y., Reuveny, E., Slesinger, P.A., Jan, Y.N. & Jan, L.Y. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature 364, 802–806 (1993).
Lesage, F. et al. Cloning provides evidence for a family of inward rectifier and G-protein-coupled K+ channels in the brain. FEBS Lett. 353, 37–42 (1994).
Liao, Y.J., Jan, Y.N. & Jan, L.Y. Heteromultimerization of G-protein-gated inwardly rectifying K+ channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain. J. Neurosci. 16, 7137–7150 (1996).
Jelacic, T.M., Kennedy, M.E., Wickman, K. & Clapham, D.E. Functional and biochemical evidence for G-protein-gated inwardly rectifying K+ (GIRK) channels composed of GIRK2 and GIRK3. J. Biol. Chem. 275, 36211–36216 (2000).
Inanobe, A. et al. Characterization of G-protein-gated K+ channels composed of Kir3.2 subunits in dopaminergic neurons of the substantia nigra. J. Neurosci. 19, 1006–1017 (1999).
Schoots, O. et al. Co-expression of human Kir3 subunits can yield channels with different functional properties. Cell. Signal. 11, 871–883 (1999).
Slesinger, P.A. et al. Functional effects of the mouse weaver mutation on G protein-gated inwardly rectifying K+ channels. Neuron 16, 321–331 (1996).
Karschin, C., Dissmann, E., Stuhmer, W. & Karschin, A. IRK(1–3) and GIRK(1–4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J. Neurosci. 16, 3559–3570 (1996).
Wickman, K., Karschin, C., Karschin, A., Picciotto, M.R. & Clapham, D.E. Brain localization and behavioral impact of the G-protein-gated K+ channel subunit GIRK4. J. Neurosci. 20, 5608–5615 (2000).
Snead, O.C., III & Gibson, K.M. Gamma-hydroxybutyric acid. N. Engl. J. Med. 352, 2721–2732 (2005).
Crunelli, V., Emri, Z. & Leresche, N. Unravelling the brain targets of gamma-hydroxybutyric acid. Curr. Opin. Pharmacol. 6, 44–52 (2006).
Nestler, E.J. Is there a common molecular pathway for addiction? Nat. Neurosci. 8, 1445–1449 (2005).
Luscher, C. & Ungless, M.A. The mechanistic classification of addictive drugs. PLoS Med. 3, e437 (2006).
Colombo, G. et al. Oral self-administration of gamma-hydroxybutyric acid in the rat. Eur. J. Pharmacol. 285, 103–107 (1995).
Martellotta, M.C., Fattore, L., Cossu, G. & Fratta, W. Rewarding properties of gamma-hydroxybutyric acid: an evaluation through place preference paradigm. Psychopharmacology (Berl.) 132, 1–5 (1997).
Brebner, K., Childress, A.R. & Roberts, D.C. A potential role for GABA(B) agonists in the treatment of psychostimulant addiction. Alcohol Alcohol. 37, 478–484 (2002).
Cousins, M.S., Roberts, D.C. & de Wit, H. GABA(B) receptor agonists for the treatment of drug addiction: a review of recent findings. Drug Alcohol Depend. 65, 209–220 (2002).
Andriamampandry, C. et al. Cloning and characterization of a rat brain receptor that binds the endogenous neuromodulator gamma-hydroxybutyrate (GHB). FASEB J. 17, 1691–1693 (2003).
Kaupmann, K. et al. Specific γ-hydroxybutyrate (GHB) binding sites but loss of pharmacological effects of GHB in GABAB(1)-deficient mice. Eur. J. Neurosci. 18, 2722–2730 (2003).
Cruz, H.G. et al. Bi-directional effects of GABA(B) receptor agonists on the mesolimbic dopamine system. Nat. Neurosci. 7, 153–159 (2004).
Shea, L. & : Linderman, J.J. Mechanistic model of G-protein signal transduction. Determinants of efficacy and effect of precoupled receptors. Biochem. Pharmacol. 53, 519–530 (1997).
Doupnik, C.A., Jaen, C. & Zhang, Q. Measuring the modulatory effects of RGS proteins on GIRK channels. Methods Enzymol. 389, 131–154 (2004).
Ford, C.P., Mark, G.P. & Williams, J.T. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J. Neurosci. 26, 2788–2797 (2006).
Margolis, E.B. et al. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc. Natl. Acad. Sci. USA 103, 2938–2942 (2006).
Wallen, A. & Perlmann, T. Transcriptional control of dopamine neuron development. Ann. NY Acad. Sci. 991, 48–60 (2003).
Zhao, S. et al. Generation of embryonic stem cells and transgenic mice expressing green fluorescence protein in midbrain dopaminergic neurons. Eur. J. Neurosci. 19, 1133–1140 (2004).
Tamamaki, N. et al. Green fluorescent protein expression and colocalization with calretinin, parvalbumin and somatostatin in the GAD67-GFP knock-in mouse. J. Comp. Neurol. 467, 60–79 (2003).
Torrecilla, M. : et al. G-protein-gated potassium channels containing Kir3.2 and Kir3.3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons. J. Neurosci. 22, 4328–4334 (2002).
Popov, S.G., Krishna, U.M., Falck, J.R. & Wilkie, T.M. Ca2+/Calmodulin reverses phosphatidylinositol 3,4,5-trisphosphate-dependent inhibition of regulators of G protein-signaling GTPase-activating protein activity. J. Biol. Chem. 275, 18962–18968 (2000).
Traynor, J.R. & Neubig, R.R. Regulator of G protein signaling and drugs of abuse. Mol. Interv. 5, 30–41 (2005).
Rahman, Z. et al. RGS9 modulates dopamine signaling in the basal ganglia. Neuron 38, 941–952 (2003).
Gold, S.J., Ni, Y.G., Dohlman, H.G. & Nestler, E.J. Regulators of G-protein signaling (RGS) proteins: region-specific expression of nine subtypes in rat brain. J. Neurosci. 17, 8024–8037 (1997).
Axelrod, D., Thompson, N.L. & Burghardt, T.P. Total internal inflection fluorescent microscopy. J. Microsc. 129, 19–28 (1983).
Takanishi, C.L., Bykova, E.A., Cheng, W. & Zheng, J. GFP-based FRET analysis in live cells. Brain Res. 1091, 132–139 (2006).
Fowler, C.E., Aryal, P., Suen, K.F. & Slesinger, P.A. Evidence for association of GABA(B) receptors with Kir3 channels and regulators of G protein signalling (RGS4) proteins. J. Physiol. 580, 51–65 (2007).
Kaupmann, K. et al. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature 396, 683–687 (1998).
Pfaff, T. et al. Alternative splicing generates a novel isoform of the rat metabotropic GABA(B)R1 receptor. Eur. J. Neurosci. 11, 2874–2882 (1999).
Chuang, H.H., Yu, M., Jan, Y.N. & Jan, L.Y. Evidence that the nucleotide exchange and hydrolysis cycle of G proteins causes acute desensitization of G-protein-gated inward rectifier K+ channels. Proc. Natl. Acad. Sci. USA 95, 11727–11732 (1998).
Jaen, C. & Doupnik, C.A. RGS3 and RGS4 differentially associate with G protein-coupled receptor-Kir3 channel signaling complexes revealing two modes of RGS modulation. Precoupling and collision coupling. J. Biol. Chem. 281, 34549–34560 (2006).
Riven, I., Iwanir, S. & Reuveny, E. GIRK channel activation involves a local rearrangement of a preformed G protein channel complex. Neuron 51, 561–573 (2006).
Gold, S.J. et al. Regulation of RGS proteins by chronic morphine in rat locus coeruleus. Eur. J. Neurosci. 17, 971–980 (2003).
Zachariou, V. et al. Essential role for RGS9 in opiate action. Proc. Natl. Acad. Sci. USA 100, 13656–13661 (2003).
Taymans, J.M., Leysen, J.E. & Langlois, X. Striatal gene expression of RGS2 and RGS4 is specifically mediated by dopamine D1 and D2 receptors: clues for RGS2 and RGS4 functions. J. Neurochem. 84, 1118–1127 (2003).
Bettahi, I., Marker, C.L., Roman, M.I. & Wickman, K. Contribution of the Kir3.1 subunit to the muscarinic-gated atrial potassium channel IKACh. J. Biol. Chem. 277, 48282–48288 (2002).
Signorini, S., Liao, Y.J., Duncan, S.A., Jan, L.Y. & Stoffel, M. Normal cerebellar development but susceptibility to seizures in mice lacking G protein–coupled, inwardly rectifying K+ channel GIRK2. Proc. Natl. Acad. Sci. USA 94, 923–927 (1997).
Koyrakh, L. et al. Molecular and cellular diversity of neuronal G protein–gated potassium channels. J. Neurosci. 25, 11468–11478 (2005).
Acknowledgements
We thank M. Serafin and the members of the Lüscher lab for discussions, F. Loctin and M. J. Cabañero for technical assistance and V. Ossipow for help with the real-time PCR. We thank M. Stoffel for the GIRK2−/− mice and J. Penninger for the RGS2−/− mice. CFP-Kir3.4 and Kir3.1-YFP cDNAs were provided by E. Reuveny. This work was supported by the Swiss National Science Foundation (C.L.), NIDA (P.A.S. and C.L.), and a grant from the Spanish Ministry of Education and Science (R.L.). Additional support comes from DA011806 and MH61933 (K.W.), and by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare, Japan (Y.Y.).
Author information
Authors and Affiliations
Contributions
G.L., M. Lomazzi and H.G.C. carried out all the electrophysiology experiments and participated in the preparation of the manuscript. G.L. performed the stereotactic viral injections. M. Lomazzi did all the PCR experiments. C.C. carried out the behavioral experiments. R.L. did the light microscope immunohistochemistry and the double-labeling electron microscope immunoreactions using an antibody raised by M.W., M. Li, Y.Y., K.O. and K.W. provided transgenic mouse lines. S.B.B. did the FRET experiments. C.L. designed the study in collaboration with P.A.S. and wrote the core of the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1, Table 1 and Methods (PDF 735 kb)
Rights and permissions
About this article
Cite this article
Labouèbe, G., Lomazzi, M., Cruz, H. et al. RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat Neurosci 10, 1559–1568 (2007). https://doi.org/10.1038/nn2006
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn2006
This article is cited by
-
Characterization of orexin input to dopamine neurons of the ventral tegmental area projecting to the medial prefrontal cortex and shell of nucleus accumbens
Brain Structure and Function (2022)
-
Subcellular localization of D2 receptors in the murine substantia nigra
Brain Structure and Function (2022)
-
Oxycodone in the Opioid Epidemic: High ‘Liking’, ‘Wanting’, and Abuse Liability
Cellular and Molecular Neurobiology (2021)
-
Neurophysiological signature of gamma-hydroxybutyrate augmented sleep in male healthy volunteers may reflect biomimetic sleep enhancement: a randomized controlled trial
Neuropsychopharmacology (2019)
-
Regulator of G protein signaling 14 (RGS14) is expressed pre- and postsynaptically in neurons of hippocampus, basal ganglia, and amygdala of monkey and human brain
Brain Structure and Function (2018)