Abstract
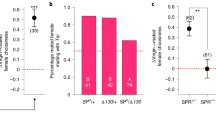
The regulation of female reproductive behaviors may involve memories of male pheromone signatures, formed in part by neural circuitry involving the olfactory bulb and hippocampus. These neural structures are the principal sites of adult neurogenesis; however, previous studies point to their independent regulation by sensory and physiological stimuli. Here we report that the pheromones of dominant (but not subordinate) males stimulate neuronal production in both the olfactory bulb and hippocampus of female mice, which are independently mediated by prolactin and luteinizing hormone, respectively. Neurogenesis induced by dominant-male pheromones correlates with a female preference for dominant males over subordinate males, whereas blocking neurogenesis with the mitotic inhibitor cytosine arabinoside eliminated this preference. These results suggest that male pheromones are involved in regulating neurogenesis in both the olfactory bulb and hippocampus, which may be important for female reproductive success.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
12 February 2008
The Supplementary Methods section to this article was not posted at the time the paper was published. The error has been corrected.
References
Bakker, J. Sexual differentiation of the neuroendocrine mechanisms regulating mate recognition in mammals. J. Neuroendocrinol. 15, 615–621 (2003).
Brennan, P.A. The nose knows who's who: chemosensory individuality and mate recognition in mice. Horm. Behav. 46, 231–240 (2004).
Brennan, P. & Keverne, E.B. Something in the air? New insights into mammalian pheromones. Curr. Biol. 14, R81–R89 (2004).
Dulac, C. & Torello, A.T. Molecular detection of pheromone signals in mammals: from genes to behavior. Nat. Rev. Neurosci. 4, 551–562 (2003).
Ganem, G., Ginane, C., Ostrowski, M. & Orth, A. Assessment of mate preference in the house mouse with reference to investigations on assortative mating. Biol. J. Linn. Soc. 84, 461–471 (2005).
Kavaliers, M., Choleris, E. & Pfaff, D.W. Recognition and avoidance of the odors of parasitized conspecifics and predators: differential genomic correlates. Neurosci. Biobehav. Rev. 29, 1347–1359 (2005).
Liberles, S.D. & Buck, L.B. A second class of chemosensory receptors in the olfactory epithelium. Nature 442, 645–650 (2006).
Slotnick, B. Animal cognition and the rat olfactory system. Trends Cogn. Sci. 5, 216–222 (2001).
Simerly, R.B. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu. Rev. Neurosci. 25, 507–536 (2002).
Davis, R.L. Olfactory learning. Neuron 44, 31–48 (2004).
Doetsch, F. & Hen, R. Young and excitable: the function of new neurons in the adult mammalian brain. Curr. Opin. Neurobiol. 15, 121–128 (2005).
Ming, G.L. & Song, H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 28, 223–250 (2005).
Lois, C. & Alvarez-Buylla, A. Long-distance neuronal migration in the adult mammalian brain. Science 264, 1145–1148 (1994).
Enwere, E. et al. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J. Neurosci. 24, 8354–8365 (2004).
Rochefort, C., Gheusi, G., Vincent, J.D. & Lledo, P.M. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J. Neurosci. 22, 2679–2689 (2002).
Meshi, D. et al. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat. Neurosci. 9, 729–731 (2006).
Shors, T.J. et al. Neurogenesis in the adult is involved in the formation of trace memories. Nature 410, 372–376 (2001).
Santarelli, L. et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809 (2003).
van Praag, H., Kempermann, G. & Gage, F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 2, 266–270 (1999).
Kempermann, G., Kuhn, H.G. & Gage, F.H. More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495 (1997).
Gould, E., Beylin, A., Tanapat, P., Reeves, A. & Shors, T.J. Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 2, 260–265 (1999).
Brown, J. et al. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur. J. Neurosci. 17, 2042–2046 (2003).
Shingo, T. et al. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science 299, 117–120 (2003).
Boehm, U., Zou, Z. & Buck, L.B. Feedback loops link odor and pheromone signaling with reproduction. Cell 123, 683–695 (2005).
Yoon, H., Enquist, L.W. & Dulac, C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell 123, 669–682 (2005).
Mucignat-Caretta, C. & Caretta, A. Chemical signals in male house mice urine: protein-bound molecules modulate interactions between sexes. Behaviour 136, 331–343 (1999).
Keller, M., Douhard, Q., Baum, M.J. & Bakker, J. Destruction of the main olfactory epithelium reduces female sexual behavior and olfactory investigation in female mice. Chem. Senses 31, 315–323 (2006).
Mossman, C.A. & Drickamer, L.C. Odor preferences of female house mice (Mus domesticus) in seminatural enclosures. J. Comp. Psychol. 110, 131–138 (1996).
Drickamer, L.C., Gowaty, P.A. & Holmes, C.M. Free female mate choice in house mice affects reproductive success and offspring viability and performance. Anim. Behav. 59, 371–378 (2000).
Lledo, P.M., Gheusi, G. & Vincent, J.D. Information processing in the mammalian olfactory system. Physiol. Rev. 85, 281–317 (2005).
Rao, M.S. & Shetty, A.K. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur. J. Neurosci. 19, 234–246 (2004).
Becker, J.B. et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146, 1650–1673 (2005).
Zhang, F.P., Poutanen, M., Wilbertz, J. & Huhtaniemi, I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol. Endocrinol. 15, 172–183 (2001).
Huhtaniemi, I., Zhang, F.P., Kero, J., Hamalainen, T. & Poutanen, M. Transgenic and knockout mouse models for the study of luteinizing hormone and luteinizing hormone receptor function. Mol. Cell. Endocrinol. 187, 49–56 (2002).
Doetsch, F., Garcia-Verdugo, J.M. & Alvarez-Buylla, A. Regeneration of a germinal layer in the adult mammalian brain. Proc. Natl. Acad. Sci. USA 96, 11619–11624 (1999).
Kokoeva, M.V., Yin, H. & Flier, J.S. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science 310, 679–683 (2005).
Trinh, K. & Storm, D.R. Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nat. Neurosci. 6, 519–525 (2003).
Bole-Feysot, C., Goffin, V., Edery, M., Binart, N. & Kelly, P.A. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev. 19, 225–268 (1998).
Rao, C.V. & Lei, Z.M. Consequences of targeted inactivation of LH receptors. Mol. Cell. Endocrinol. 187, 57–67 (2002).
Knobil, E. & Neil, J.D. The Physiology of Reproduction (Raven Press, Ltd, New York, 1994).
Dupret, D. et al. Methylazoxymethanol acetate does not fully block cell genesis in the young and aged dentate gyrus. Eur. J. Neurosci. 22, 778–783 (2005).
Saxe, M.D. et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. USA 103, 17501–17506 (2006).
Wojtowicz, J.M. Irradiation as an experimental tool in studies of adult neurogenesis. Hippocampus 16, 261–266 (2006).
Grammer, K., Fink, B. & Neave, N. Human pheromones and sexual attraction. Eur. J. Obstet. Gynecol. Reprod. Biol. 118, 135–142 (2005).
Savic, I., Berglund, H., Gulyas, B. & Roland, P. Smelling of odorous sex hormone–like compounds causes sex-differentiated hypothalamic activations in humans. Neuron 31, 661–668 (2001).
Bigiani, A., Mucignat-Caretta, C., Montani, G. & Tirindelli, R. Pheromone reception in mammals. Rev. Physiol. Biochem. Pharmacol. 154, 1–35 (2005).
Bedard, A. & Parent, A. Evidence of newly generated neurons in the human olfactory bulb. Brain Res. Dev. Brain Res. 151, 159–168 (2004).
Grant, E.C. & Mackintosh, J.H. A comparison of the social postures of some laboratory rodents. Behaviour 21, 246–259 (1963).
Kero, J. et al. Elevated luteinizing hormone induces expression of its receptor and promotes steroidogenesis in the adrenal cortex. J. Clin. Invest. 105, 633–641 (2000).
Ling, C. et al. Prolactin (PRL) receptor gene expression in mouse adipose tissue: increases during lactation and in PRL-transgenic mice. Endocrinology 141, 3564–3572 (2000).
Acknowledgements
We are grateful to K. Markham for providing helpful instruction on the molecular techniques used in this study. We thank A. Chojnacki, B. Kolb, K. Lukowiak, C. Morshead and Q. Pittman for comments on earlier versions of this manuscript. This work was supported by the Canadian Institutes for Health Research and the Academy of Finland. G.K.M. and C.G. were recipients of studentships from the Stem Cell Network of the Canadian Network of Centres of Excellence and the Alberta Heritage Foundation for Medical Research (AHFMR), respectively. S.W. is an AHFMR Scientist.
Author information
Authors and Affiliations
Contributions
G.K.M. conducted all of the experiments and co-authored the manuscript. E.E.K. and C.G. contributed to the planning and interpretation of the pheromone- and hormone-stimulated neurogenesis studies, T.P, M.P. and I.H. collaborated on studies of LH actions on neurogenesis, LHR immunochemistry and pheromone-stimulated neurogenesis in Lhcgr mice, and S.W. supervised the project and co-authored the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Paradigm for male pheromone exposure. (PDF 116 kb)
Supplementary Fig. 2
Male pheromones simultaneously increase cell proliferation in the SVZ and DG. (PDF 1984 kb)
Supplementary Fig. 3
Females exposed to novel odors do not have increased proliferation in the SVZ. or DG compared with females exposed to male pheromones. (PDF 267 kb)
Supplementary Fig. 4
Male pheromones simultaneously increase neurogenesis in the dorsolateral corner (DLC) of the SVZ and DG. (PDF 2466 kb)
Supplementary Fig. 5
Short exposure to dominant male pheromones, which result in a lack of increased adult female neurogenesis, also result in a lack of preference for dominant males. (PDF 234 kb)
Supplementary Fig. 6
Plrl?/? females exposed to dominant male pheromones for 7 d do not show a subsequent preference for dominant males. AraC infusions attenuate OB and DG neurogenesis. (PDF 173 kb)
Supplementary Fig. 7
AraC infusions attenuate OB and DG neurogenesis. (PDF 4534 kb)
Supplementary Fig. 8
Females with attenuated dominant male pheromone?induced neurogenesis in the OB and DG do not show a preference for a subordinate or dominant male. (PDF 279 kb)
Supplementary Fig. 9
Assay to determine novel odor detection and habituation. (PDF 314 kb)
Rights and permissions
About this article
Cite this article
Mak, G., Enwere, E., Gregg, C. et al. Male pheromone–stimulated neurogenesis in the adult female brain: possible role in mating behavior.. Nat Neurosci 10, 1003–1011 (2007). https://doi.org/10.1038/nn1928
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1928
This article is cited by
-
Increased proliferation and neuronal fate in prairie vole brain progenitor cells cultured in vitro: effects by social exposure and sexual dimorphism
Biology of Sex Differences (2023)
-
Is abnormal metabolism in the olfactory bulb and amygdala associated with bipolar disorder?
Journal of Neural Transmission (2023)
-
Home sweet home: the neural stem cell niche throughout development and after injury
Cell and Tissue Research (2018)
-
Genome-wide differential mRNA expression profiles in follicles of two breeds and at two stages of estrus cycle of gilts
Scientific Reports (2017)
-
Developmentally defined forebrain circuits regulate appetitive and aversive olfactory learning
Nature Neuroscience (2017)