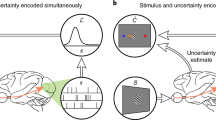
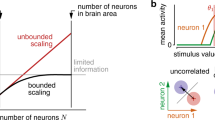
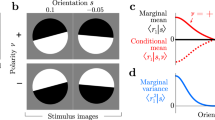
Abstract
Recent psychophysical experiments indicate that humans perform near-optimal Bayesian inference in a wide variety of tasks, ranging from cue integration to decision making to motor control. This implies that neurons both represent probability distributions and combine those distributions according to a close approximation to Bayes' rule. At first sight, it would seem that the high variability in the responses of cortical neurons would make it difficult to implement such optimal statistical inference in cortical circuits. We argue that, in fact, this variability implies that populations of neurons automatically represent probability distributions over the stimulus, a type of code we call probabilistic population codes. Moreover, we demonstrate that the Poisson-like variability observed in cortex reduces a broad class of Bayesian inference to simple linear combinations of populations of neural activity. These results hold for arbitrary probability distributions over the stimulus, for tuning curves of arbitrary shape and for realistic neuronal variability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Knill, D.C. & Richards, W. Perception as Bayesian Inference (Cambridge Univ. Press, New York, 1996).
van Beers, R.J., Sittig, A.C. & Gon, J.J. Integration of proprioceptive and visual position-information: an experimentally supported model. J. Neurophysiol. 81, 1355–1364 (1999).
Ernst, M.O. & Banks, M.S. Humans integrate visual and haptic information in a statistically optimal fashion. Nature 415, 429–433 (2002).
Kording, K.P. & Wolpert, D.M. Bayesian integration in sensorimotor learning. Nature 427, 244–247 (2004).
Stocker, A.A. & Simoncelli, E.P. Noise characteristics and prior expectations in human visual speed perception. Nat. Neurosci. 9, 578–585 (2006).
Tolhurst, D., Movshon, J. & Dean, A. The statistical reliability of signals in single neurons in cat and monkey visual cortex. Vision Res. 23, 775–785 (1982).
Stevens, C.F. Neurotransmitter release at central synapses. Neuron 40, 381–388 (2003).
Foldiak, P. in Computation and Neural Systems (eds. Eeckman, F. & Bower, J.) 55–60 (Kluwer Academic Publishers, Norwell, Massachusetts, 1993).
Sanger, T. Probability density estimation for the interpretation of neural population codes. J. Neurophysiol. 76, 2790–2793 (1996).
Salinas, E. & Abbot, L. Vector reconstruction from firing rate. J. Comput. Neurosci. 1, 89–107 (1994).
Zemel, R., Dayan, P. & Pouget, A. Probabilistic interpretation of population code. Neural Comput. 10, 403–430 (1998).
Anderson, C. in Computational Intelligence Imitating Life (eds. Zurada, J.M., Marks, R.J., II & Robinson, C.J.) 213–222 (IEEE Press, New York, 1994).
Seung, H. & Sompolinsky, H. Simple model for reading neuronal population codes. Proc. Natl. Acad. Sci. USA 90, 10749–10753 (1993).
Snippe, H.P. Parameter extraction from population codes: a critical assessment. Neural Comput. 8, 511–529 (1996).
Wu, S., Nakahara, H. & Amari, S. Population coding with correlation and an unfaithful model. Neural Comput. 13, 775–797 (2001).
Hinton, G.E. in Proceedings of the Ninth International Conference on Artificial Neural Network 1–6 (IEEE, London, England, 1999).
Clark, J.J. & Yuille, A.L. Data Fusion for Sensory Information Processing Systems (Kluwer Academic, Boston, 1990).
Knill, D.C. Discrimination of planar surface slant from texture: human and ideal observers compared. Vision Res. 38, 1683–1711 (1998).
Gepshtein, S. & Banks, M.S. Viewing geometry determines how vision and haptics combine in size perception. Curr. Biol. 13, 483–488 (2003).
Gur, M. & Snodderly, D.M. High response reliability of neurons in primary visual cortex (V1) of alert, trained monkeys. Cereb Cortex 16, 888–895 (2006).
Platt, M.L. & Glimcher, P.W. Neural correlates of decision variables in parietal cortex. Nature 400, 233–238 (1999).
Basso, M.A. & Wurtz, R.H. Modulation of neuronal activity by target uncertainty. Nature 389, 66–69 (1997).
Series, P., Latham, P. & Pouget, A. Tuning curve sharpening for orientation selectivity: coding efficiency and the impact of correlations. Nat. Neurosci. 7, 1129–1135 (2004).
Stein, B.E. & Meredith, M.A. The Merging of the Senses (MIT Press, Cambridge, Massachusetts, 1993).
Stanford, T.R., Quessy, S. & Stein, B.E. Evaluating the operations underlying multisensory integration in the cat superior colliculus. J. Neurosci. 25, 6499–6508 (2005).
Perrault, T.J., Jr., Vaughan, J.W., Stein, B.E. & Wallace, M.T. Superior colliculus neurons use distinct operational modes in the integration of multisensory stimuli. J. Neurophysiol. 93, 2575–2586 (2005).
Shadlen, M.N. & Newsome, W.T. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J. Neurophysiol. 86, 1916–1936 (2001).
Gold, J.I. & Shadlen, M.N. Neural computations that underlie decisions about sensory stimuli. Trends Cogn. Sci. 5, 10–16 (2001).
Britten, K.H., Shadlen, M.N., Newsome, W.T. & Movshon, J.A. Responses of neurons in macaque MT to stochastic motion signals. Vis. Neurosci. 10, 1157–1169 (1993).
Weiss, Y. & Fleet, D.J. in Probabilistic Models of the Brain: Perception and Neural Function (eds. Rao, R., Olshausen, B. & Lewicki, M.S.) 77–96 (MIT Press, Cambridge, Massachusetts, 2002).
Anderson, J.S., Lampl, I., Gillespie, D.C. & Ferster, D. The contribution of noise to contrast invariance of orientation tuning in cat visual cortex. Science 290, 1968–1972 (2000).
Sclar, G. & Freeman, R. Orientation selectivity in the cat's striate cortex is invariant with stimulus contrast. Exp. Brain Res. 46, 457–461 (1982).
Deneve, S., Latham, P. & Pouget, A. Reading population codes: a neural implementation of ideal observers. Nat. Neurosci. 2, 740–745 (1999).
Deneve, S., Latham, P. & Pouget, A. Efficient computation and cue integration with noisy population codes. Nat. Neurosci. 4, 826–831 (2001).
Barlow, H.B. Pattern recognition and the responses of sensory neurons. Ann. NY Acad. Sci. 156, 872–881 (1969).
Simoncelli, E., Adelson, E. & Heeger, D. in Proceedings 1991 IEEE Computer Society Conference on Computer Vision and Pattern Recognition 310–315 (1991).
Koechlin, E., Anton, J.L. & Burnod, Y. Bayesian inference in populations of cortical neurons: a model of motion integration and segmentation in area MT. Biol. Cybern. 80, 25–44 (1999).
Anastasio, T.J., Patton, P.E. & Belkacem-Boussaid, K. Using Bayes' rule to model multisensory enhancement in the superior colliculus. Neural Comput. 12, 1165–1187 (2000).
Hoyer, P.O. & Hyvarinen, A. in Neural Information Processing Systems 277–284 (MIT Press, Cambridge, Massachusetts, 2003).
Sahani, M. & Dayan, P. Doubly distributional population codes: simultaneous representation of uncertainty and multiplicity. Neural Comput. 15, 2255–2279 (2003).
Rao, R.P. Bayesian computation in recurrent neural circuits. Neural Comput. 16, 1–38 (2004).
Deneve, S. in Neural Information Processing Systems 353–360 (MIT Press, Cambridge, Massachusetts, 2005).
Jazayeri, M. & Movshon, J.A. Optimal representation of sensory information by neural populations. Nat. Neurosci. 9, 690–696 (2006).
Poggio, T. A theory of how the brain might work. Cold Spring Harb. Symp. Quant. Biol. 55, 899–910 (1990).
Douglas, R.J. & Martin, K.A. A functional microcircuit for cat visual cortex. J. Physiol. (Lond.) 440, 735–769 (1991).
Heeger, D.J. Normalization of cell responses in cat striate cortex. Vis. Neurosci. 9, 181–197 (1992).
Nelson, J.I., Salin, P.A., Munk, M.H., Arzi, M. & Bullier, J. Spatial and temporal coherence in cortico-cortical connections: a cross-correlation study in areas 17 and 18 in the cat. Vis. Neurosci. 9, 21–37 (1992).
Huys, Q., Zemel, R.S., Natarajan, R. & Dayan, P. Fast population coding. Neural Comput. (in the press).
Acknowledgements
W.J.M. was supported by a grant from the Schmitt foundation, J.B. by grants from the US National Institutes of Health (NEI 5 T32 MH019942) and the National Institute of Mental Health (T32 MH19942), P.E.L. by the Gatsby Charitable Foundation and National Institute of Mental Health (grant R01 MH62447) and A.P. by the National Science Foundation (grants BCS0346785 and BCS0446730) and by a research grant from the James S. McDonnell Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Ma, W., Beck, J., Latham, P. et al. Bayesian inference with probabilistic population codes. Nat Neurosci 9, 1432–1438 (2006). https://doi.org/10.1038/nn1790
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1790
This article is cited by
-
A unifying theory explains seemingly contradictory biases in perceptual estimation
Nature Neuroscience (2024)
-
Multivariate multiscale entropy (mMSE) as a tool for understanding the resting-state EEG signal dynamics: the spatial distribution and sex/gender-related differences
Behavioral and Brain Functions (2023)
-
Studying the neural representations of uncertainty
Nature Neuroscience (2023)
-
Sampling-based Bayesian inference in recurrent circuits of stochastic spiking neurons
Nature Communications (2023)
-
Bayesian encoding and decoding as distinct perspectives on neural coding
Nature Neuroscience (2023)