Abstract
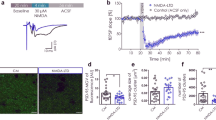
Calcium (Ca2+) influx through NMDA receptors (NMDARs) is essential for synaptogenesis, experience-dependent synaptic remodeling and plasticity. The NMDAR-mediated rise in postsynaptic Ca2+ activates a network of kinases and phosphatases that promote persistent changes in synaptic strength, such as long-term potentiation (LTP). Here we show that the Ca2+ permeability of neuronal NMDARs is under the control of the cyclic AMP–protein kinase A (cAMP-PKA) signaling cascade. PKA blockers reduced the relative fractional Ca2+ influx through NMDARs as determined by reversal potential shift analysis and by a combination of electrical recording and Ca2+ influx measurements in rat hippocampal neurons in culture and hippocampal slices from mice. In slices, PKA blockers markedly inhibited NMDAR-mediated Ca2+ rises in activated dendritic spines, with no significant effect on synaptic current. Consistent with this, PKA blockers depressed the early phase of NMDAR-dependent LTP at hippocampal Schaffer collateral–CA1 (Sch-CA1) synapses. Our data link PKA-dependent synaptic plasticity to Ca2+ signaling in spines and thus provide a new mechanism whereby PKA regulates the induction of LTP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Cull-Candy, S.G., Brickley, S. & Farrant, M. NMDA receptor subunits: diversity, development and disease. Curr. Opin. Neurobiol. 11, 327–335 (2001).
Carroll, R.C. & Zukin, R.S. NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci. 25, 571–577 (2002).
Cull-Candy, S.G. & Leszkiewicz, D.N. Role of distinct NMDA receptor subtypes at central synapses. Sci. STKE 2004, re16 (2004).
Mayer, M.L. & Westbrook, G.L. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J. Physiol. (Lond.) 394, 501–527 (1987).
Schneggenburger, R., Zhou, Z., Konnerth, A. & Neher, E. Fractional contribution of calcium to the cation current through glutamate receptor channels. Neuron 11, 133–143 (1993).
Burnashev, N., Zhou, Z., Neher, E. & Sakmann, B. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J. Physiol. (Lond.) 485, 403–418 (1995).
Rogers, M. & Dani, J.A. Comparison of quantitative calcium flux through NMDA, ATP, and ACh receptor channels. Biophys. J. 68, 501–506 (1995).
Garaschuk, O., Schneggenburger, R., Schirra, C., Tempia, F. & Konnerth, A. Fractional Ca2+ currents through somatic and dendritic glutamate receptor channels of rat hippocampal CA1 pyramidal neurones. J. Physiol. (Lond.) 491, 757–772 (1996).
Malenka, R.C. & Bear, M.F. LTP and LTD: an embarrassment of riches. Neuron 44, 5–21 (2004).
Scannevin, R.H. & Huganir, R.L. Postsynaptic organization and regulation of excitatory synapses. Nat. Rev. Neurosci. 1, 133–141 (2000).
Sheng, M. Molecular organization of the postsynaptic specialization. Proc. Natl. Acad. Sci. USA 98, 7058–7061 (2001).
Winder, D.G. & Sweatt, J.D. Roles of serine/threonine phosphatases in hippocampal synaptic plasticity. Nat. Rev. Neurosci. 2, 461–474 (2001).
Westphal, R.S. et al. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science 285, 93–96 (1999).
Leonard, A.S. & Hell, J.W. Cyclic AMP-dependent protein kinase and protein kinase C phosphorylate N-methyl-D-aspartate receptors at different sites. J. Biol. Chem. 272, 12107–12115 (1997).
Tingley, W.G. et al. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J. Biol. Chem. 272, 5157–5166 (1997).
Wang, L.Y., Orser, B.A., Brautigan, D.L. & MacDonald, J.F. Regulation of NMDA receptors in cultured hippocampal neurons by protein phosphatases 1 and 2A. Nature 369, 230–232 (1994).
Raman, I.M., Tong, G. & Jahr, C.E. Beta-adrenergic regulation of synaptic NMDA receptors by cAMP-dependent protein kinase. Neuron 16, 415–421 (1996).
Blank, T. et al. The phosphoprotein DARPP-32 mediates cAMP-dependent potentiation of striatal N-methyl-D-aspartate responses. Proc. Natl. Acad. Sci. USA 94, 14859–14864 (1997).
Snyder, G.L., Fienberg, A.A., Huganir, R.L. & Greengard, P. A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. J. Neurosci. 18, 10297–10303 (1998).
Otmakhova, N.A., Otmakhov, N., Mortenson, L.H. & Lisman, J.E. Inhibition of the cAMP pathway decreases early long-term potentiation at CA1 hippocampal synapses. J. Neurosci. 20, 4446–4451 (2000).
Yasuda, H., Barth, A.L., Stellwagen, D. & Malenka, R.C. A developmental switch in the signaling cascades for LTP induction. Nat. Neurosci. 6, 15–16 (2003).
Blitzer, R.D. et al. Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science 280, 1940–1942 (1998).
Huang, Y.Y. & Kandel, E.R. Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learn. Mem. 1, 74–82 (1994).
Qi, M. et al. Impaired hippocampal plasticity in mice lacking the Cbeta1 catalytic subunit of cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 93, 1571–1576 (1996).
Abel, T. et al. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88, 615–626 (1997).
Wong, S.T. et al. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron 23, 787–798 (1999).
Zorumski, C.F., Yang, J. & Fischbach, G.D. Calcium-dependent, slow desensitization distinguishes different types of glutamate receptors. Cell. Mol. Neurobiol. 9, 95–104 (1989).
Legendre, P., Rosenmund, C. & Westbrook, G.L. Inactivation of NMDA channels in cultured hippocampal neurons by intracellular calcium. J. Neurosci. 13, 674–684 (1993).
Tong, G., Shepherd, D. & Jahr, C.E. Synaptic desensitization of NMDA receptors by calcineurin. Science 267, 1510–1512 (1995).
Kaczmarek, L.K., Jennings, K.R. & Strumwasser, F. An early sodium and a late calcium phase in the afterdischarge of peptide-secreting neurons of Aplysia. Brain Res. 238, 105–115 (1982).
Lan, J.Y. et al. Protein kinase C modulates NMDA receptor trafficking and gating. Nat. Neurosci. 4, 382–390 (2001).
Quinlan, E.M., Philpot, B.D., Huganir, R.L. & Bear, M.F. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat. Neurosci. 2, 352–357 (1999).
Beaver, C.J., Ji, Q., Fischer, Q.S. & Daw, N.W. Cyclic AMP-dependent protein kinase mediates ocular dominance shifts in cat visual cortex. Nat. Neurosci. 4, 159–163 (2001).
Hille, B. Ion Channels of Excitable Membranes 3rd edn. (Sinauer Associates, Sunderland, Massachusetts, 2001).
Schneggenburger, R. Simultaneous measurement of Ca2+ influx and reversal potentials in recombinant N-methyl-D-aspartate receptor channels. Biophys. J. 70, 2165–2174 (1996).
Hoffmann, H., Gremme, T., Hatt, H. & Gottmann, K. Synaptic activity-dependent developmental regulation of NMDA receptor subunit expression in cultured neocortical neurons. J. Neurochem. 75, 1590–1599 (2000).
Zhong, J., Russell, S.L., Pritchett, D.B., Molinoff, P.B. & Williams, K. Expression of mRNAs encoding subunits of the N-methyl-D-aspartate receptor in cultured cortical neurons. Mol. Pharmacol. 45, 846–853 (1994).
Yuste, R., Majewska, A., Cash, S.S. & Denk, W. Mechanisms of calcium influx into hippocampal spines: heterogeneity among spines, coincidence detection by NMDA receptors, and optical quantal analysis. J. Neurosci. 19, 1976–1987 (1999).
Yuste, R. & Denk, W. Dendritic spines as basic functional units of neuronal integration. Nature 375, 682–684 (1995).
Malenka, R.C. Postsynaptic factors control the duration of synaptic enhancement in area CA1 of the hippocampus. Neuron 6, 53–60 (1991).
Cummings, J.A., Mulkey, R.M., Nicoll, R.A. & Malenka, R.C. Ca2+ signaling requirements for long-term depression in the hippocampus. Neuron 16, 825–833 (1996).
Sobczyk, A., Scheuss, V. & Svoboda, K. NMDA receptor subunit-dependent [Ca2+] signaling in individual hippocampal dendritic spines. J. Neurosci. 25, 6037–6046 (2005).
Thomas, M.J., Moody, T.D., Makhinson, M. & O'Dell, T.J. Activity-dependent beta-adrenergic modulation of low frequency stimulation induced LTP in the hippocampal CA1 region. Neuron 17, 475–482 (1996).
Otmakhova, N.A. & Lisman, J.E. D1/D5 dopamine receptor activation increases the magnitude of early long-term potentiation at CA1 hippocampal synapses. J. Neurosci. 16, 7478–7486 (1996).
Lieberman, D.N. & Mody, I. Regulation of NMDA channel function by endogenous Ca(2+)-dependent phosphatase. Nature 369, 235–239 (1994).
Scemes, E., Suadicani, S.O. & Spray, D.C. Intercellular communication in spinal cord astrocytes: fine tuning between gap junctions and P2 nucleotide receptors in calcium wave propagation. J. Neurosci. 20, 1435–1445 (2000).
Matsuzaki, M. et al. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat. Neurosci. 4, 1086–1092 (2001).
Wang, S.S. & Augustine, G.J. Confocal imaging and local photolysis of caged compounds: dual probes of synaptic function. Neuron 15, 755–760 (1995).
Lin, Y., Skeberdis, V.A., Francesconi, A., Bennett, M.V. & Zukin, R.S. Postsynaptic density protein-95 regulates NMDA channel gating and surface expression. J. Neurosci. 24, 10138–10148 (2004).
Majewska, A., Yiu, G. & Yuste, R. A custom-made two-photon microscope and deconvolution system. Pflugers Arch. 441, 398–408 (2000).
Acknowledgements
The authors thank K. Svoboda for helpful scientific discussions, G. Bassell for neuronal cultures, G. Hjelmstad for the IgorPro procedures for data acquisition and L. Formisano for technical assistance. This work was supported by the US National Institutes of Health (grants NS20752 to R.S.Z., DA17392 to P.E.C., MH65495 supporting S.O.S. and NS45287 to M.V.L.B.). P.E.C. is a Pew Biomedical Scholar. M.V.L.B. is the Sylvia and Robert S. Olnick Professor of Neuroscience and Distinguished Professor at the Albert Einstein College of Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
PKA inhibitors and activators do not alter NMDA-elicited currents in Xenopus oocytes expressing recombinant NMDARs. (PDF 159 kb)
Supplementary Fig. 2
PKA does not modulate NMDAR surface expression in cortical neurons. (PDF 103 kb)
Supplementary Fig. 3
PKA inhibitors and activators modulate Ca2+ influx through NMDARs. (PDF 97 kb)
Supplementary Fig. 4
PKA does not act presynaptically to inhibit LTP at the Schaffer collateral-CA1 synapse. (PDF 102 kb)
Rights and permissions
About this article
Cite this article
Skeberdis, V., Chevaleyre, V., Lau, C. et al. Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci 9, 501–510 (2006). https://doi.org/10.1038/nn1664
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1664
This article is cited by
-
Action of GABAB receptor on local network oscillation in somatosensory cortex of oral part: focusing on NMDA receptor
The Journal of Physiological Sciences (2024)
-
Neuromodulation of prefrontal cortex cognitive function in primates: the powerful roles of monoamines and acetylcholine
Neuropsychopharmacology (2022)
-
The genie in the bottle-magnified calcium signaling in dorsolateral prefrontal cortex
Molecular Psychiatry (2021)
-
Cell-type-specific asynchronous modulation of PKA by dopamine in learning
Nature (2021)
-
Protopanaxadiol ginsenoside Rd protects against NMDA receptor-mediated excitotoxicity by attenuating calcineurin-regulated DAPK1 activity
Scientific Reports (2020)