Abstract
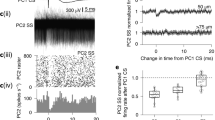
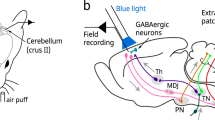
A persistent change in neuronal activity after brief stimuli is a common feature of many neuronal microcircuits. This persistent activity can be sustained by ongoing reverberant network activity or by the intrinsic biophysical properties of individual cells. Here we demonstrate that rat and guinea pig cerebellar Purkinje cells in vivo show bistability of membrane potential and spike output on the time scale of seconds. The transition between membrane potential states can be bidirectionally triggered by the same brief current pulses. We also show that sensory activation of the climbing fiber input can switch Purkinje cells between the two states. The intrinsic nature of Purkinje cell bistability and its control by sensory input can be explained by a simple biophysical model. Purkinje cell bistability may have a key role in the short-term processing and storage of sensory information in the cerebellar cortex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Fuster, J.M. Unit activity in prefrontal cortex during delayed-response performance: neuronal correlates of transient memory. J. Neurophysiol. 36, 61–78 (1973).
McCormick, D.A. et al. Persistent cortical activity: mechanisms of generation and effects on neuronal excitability. Cereb. Cortex 13, 1219–1231 (2003).
Shu, Y., Hasenstaub, A. & McCormick, D.A. Turning on and off recurrent balanced cortical activity. Nature 423, 288–293 (2003).
Camperi, M. & Wang, X.J. A model of visuospatial working memory in prefrontal cortex: recurrent network and cellular bistability. J. Comput. Neurosci. 5, 383–405 (1998).
Marder, E., Abbott, L.F., Turrigiano, G.G., Liu, Z. & Golowasch, J. Memory from the dynamics of intrinsic membrane currents. Proc. Natl. Acad. Sci. USA 93, 13481–13486 (1996).
Llinas, R. & Sugimori, M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J. Physiol. (Lond.) 305, 171–195 (1980).
Heyward, P., Ennis, M., Keller, A. & Shipley, M.T. Membrane bistability in olfactory bulb mitral cells. J. Neurosci. 21, 5311–5320 (2001).
Lee, R.H. & Heckman, C.J. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J. Neurophysiol. 80, 583–593 (1998).
Egorov, A.V., Hamam, B.N., Fransen, E., Hasselmo, M.E. & Alonso, A.A. Graded persistent activity in entorhinal cortex neurons. Nature 420, 173–178 (2002).
Koulakov, A.A., Raghavachari, S., Kepecs, A. & Lisman, J.E. Model for a robust neural integrator. Nat. Neurosci. 5, 775–782 (2002).
Goldman, M.S., Levine, J.H., Major, G., Tank, D.W. & Seung, H.S. Robust persistent neural activity in a model integrator with multiple hysteretic dendrites per neuron. Cereb. Cortex 13, 1185–1195 (2003).
Loewenstein, Y. & Sompolinsky, H. Temporal integration by calcium dynamics in a model neuron. Nat. Neurosci. 6, 961–967 (2003).
Häusser, M. & Clark, B.A. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron 19, 665–678 (1997).
Ito, M. The Cerebellum and Neural Control (Raven, New York, 1984).
Williams, S.R., Christensen, S.R. Stuart, G.J. & Häusser, M. Membrane potential bistability is controlled by the hyperpolarization- activated current I(H) in rat cerebellar Purkinje neurons in vitro. J. Physiol. (Lond.) 539, 469–483 (2002).
Hartigan, J.A. & Hartigan, P.M. The dip test of unimodality. Ann. Stat. 13, 70–84 (1985).
Chadderton, P., Margrie, T.W. & Häusser, M. Integration of quanta in cerebellar granule cells during sensory processing. Nature 428, 856–860 (2004).
Armstrong, D.M. & Rawson, J.A. Activity patterns of cerebellar cortical neurones and climbing fibre afferents in the awake cat. J. Physiol. (Lond.) 289, 425–448 (1979).
Jorntell, H. & Ekerot, C.F. Receptive field plasticity profoundly alters the cutaneous parallel fiber synaptic input to cerebellar interneurons in vivo. J. Neurosci. 23, 9620–9631 (2003).
Brown, I.E. & Bower, J.M. Congruence of mossy fiber and climbing fiber tactile projections in the lateral hemispheres of the rat cerebellum. J. Comp. Neurol. 429, 59–70 (2001).
Rapp, M., Segev, I. & Yarom, Y. Physiology, morphology and detailed passive models of guinea-pig cerebellar Purkinje cells. J. Physiol. (Lond.) 474, 101–118 (1994).
Chang, W., Strahlendorf, J.C. & Strahlendorf, H.K. Ionic contributions to the oscillatory firing activity of rat Purkinje cells in vitro. Brain Res. 614, 335–341 (1993).
Hounsgaard, J. & Midtgaard, J. Intrinsic determinants of firing pattern in Purkinje cells of the turtle cerebellum in vitro. J. Physiol. (Lond.) 402, 731–749 (1988).
Bell, C.C. & Grimm, R.J. Discharge properties of Purkinje cells recorded on single and double microelectrodes. J. Neurophysiol. 32, 1044–1055 (1969).
Brookhart, J.M., Moruzzi, G. & Snider, R.S. Spike discharges of single units in the cerebellar cortex. J. Neurophysiol. 13, 465–486 (1950).
McDevitt, C.J., Ebner, T.J. & Bloedel, J.R. The changes in Purkinje cell simple spike activity following spontaneous climbing fiber inputs. Brain Res. 237, 484–491 (1982).
Granit, R. & Phillips, C.G. Excitatory and inhibitory processes acting upon individual Purkinje cells of the cerebellum in cats. J. Physiol. (Lond.) 133, 520–547 (1956).
Nacimiento, R.C. Spontaneous and evoked discharges of cerebellar Purkinje cells in the frog. in Neurobiology of Cerebellar Evolution and Development (ed. Llinas, R.) 373–395 (American Medical Assn., Chicago, 1969).
McCarley, R.W. & Hobson, J.A. Simple spike firing patterns of cat cerebellar Purkinje cells in sleep and waking. Electroencephalogr. Clin. Neurophysiol. 33, 471–483 (1972).
Edgley, S.A. & Lidierth, M. Step-related discharges of Purkinje cells in the paravermal cortex of the cerebellar anterior lobe in the cat. J. Physiol. (Lond.) 401, 399–415 (1988).
Hirata, Y. & Highstein, S.M. Analysis of the discharge pattern of floccular Purkinje cells in relation to vertical head and eye movement in the squirrel monkey. Prog. Brain Res. 124, 221–232 (2000).
Bauswein, E., Kolb, F.P. & Rubia, F.J. Cerebellar feedback signals of a passive hand movement in the awake monkey. Pflugers Arch. 402, 292–299 (1984).
Kobayashi, Y. et al. Temporal firing patterns of Purkinje cells in the cerebellar ventral paraflocculus during ocular following responses in monkeys II. Complex spikes. J. Neurophysiol. 80, 832–848 (1998).
Williams, S.R., Toth, T.I., Turner, J.P., Hughes, S.W. & Crunelli, V. The 'window' component of the low threshold Ca2+ current produces input signal amplification and bistability in cat and rat thalamocortical neurones. J. Physiol. (Lond.) 505, 689–705 (1997).
Rawson, J.A. & Tilokskulchai, K. Repetitive firing of cerebellar Purkinje cells in response to impulse in climbing fibre afferents. Neurosci. Lett. 25, 131–135 (1981).
Sato, Y., Miura, A., Fushiki, H. & Kawasaki, T. Short-term modulation of cerebellar Purkinje cell activity after spontaneous climbing fiber input. J. Neurophysiol. 68, 2051–2062 (1992).
Rawson, J.A. & Tilokskulchai, K. Suppression of simple spike discharges of cerebellar Purkinje cells by impulses in climbing fibre afferents. Neurosci. Lett. 25, 125–130 (1981).
Ekerot, C.F. & Kano, M. Long-term depression of parallel fibre synapses following stimulation of climbing fibres. Brain Res. 342, 357–360 (1985).
Mahon, S., Deniau, J.M. & Charpier, S. Relationship between EEG potentials and intracellular activity of striatal and cortico-striatal neurons: an in vivo study under different anesthetics. Cereb. Cortex 11, 360–373 (2001).
Rinzel, J. & Ermentrout, B. Analysis of Neural Excitability and Oscillations in Methods of Neuronal Modeling (eds. Koch, C. & Segev, I.) 251–291 (MIT Press, Cambridge, 1998).
Yuen, G.L., Hockberger, P.E. & Houk, J.C. Bistability in cerebellar Purkinje cell dendrites modelled with high- threshold calcium and delayed-rectifier potassium channels. Biol. Cybern. 73, 375–388 (1995).
Genet, S. & Delord, B. A biophysical model of nonlinear dynamics underlying plateau potentials and calcium spikes in Purkinje cell dendrites. J. Neurophysiol. 88, 2430–2444 (2002).
Llinas, R. & Sugimori, M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J. Physiol. (Lond.) 305, 197–213 (1980).
Ebner, T.J. & Bloedel, J.R. Role of climbing fiber afferent input in determining responsiveness of Purkinje cells to mossy fiber inputs. J. Neurophysiol. 45, 962–971 (1981).
Midtgaard, J., Lasser-Ross, N. & Ross, W. Spatial distribution of Ca2+ influx in turtle Purkinje cell dendrites in vitro: role of a transient outward current. J. Neurophysiol. 70, 2455–2469 (1993).
Wang, S.S., Denk, W. & Häusser, M. Coincidence detection in single dendritic spines mediated by calcium release. Nat. Neurosci. 3, 1266–1273 (2000).
Margrie, T.W., Brecht, M. & Sakmann, B. In vivo, low-resistance, whole-cell recordings from neurons in the anaesthetized and awake mammalian brain. Pflugers Arch. 444, 491–498 (2002).
Mann-Metzer, P. & Yarom, Y. Electrotonic coupling interacts with intrinsic properties to generate synchronized activity in cerebellar networks of inhibitory interneurons. J. Neurosci. 19, 3298–3306 (1999).
Acknowledgements
We thank H. Meiri, E. Chorev and P. Mann-Metzer for excellent technical assistance, J.T. Davie for help with programming, and J.I. Simpson and T. Margrie for encouragement and helpful discussions. This work was supported by grants from the European Commission (M.H. and Y.Y.), Wellcome Trust (M.H and S.M), Gatsby Foundation (M.H), JSPS (K.K.), US-Israel BSF (Y.Y.), the Israel Science Foundation (Y.Y.), the Israel Science Foundation Center of Excellence 8006-00 (H.S.) and the Yeshaya Horowitz Association (Y.L.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Activation of h-current in cerebellar Purkinje cells in vivo. (PDF 520 kb)
Supplementary Fig. 2
A model of bistability demonstrates CF-evoked and spontaneous transitions. (PDF 263 kb)
Rights and permissions
About this article
Cite this article
Loewenstein, Y., Mahon, S., Chadderton, P. et al. Bistability of cerebellar Purkinje cells modulated by sensory stimulation. Nat Neurosci 8, 202–211 (2005). https://doi.org/10.1038/nn1393
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1393
This article is cited by
-
Development, circuitry, and function of the zebrafish cerebellum
Cellular and Molecular Life Sciences (2023)
-
Bistability at the onset of neuronal oscillations
Biological Cybernetics (2023)
-
Controlling switching between birhythmic states in a new conductance-based bursting neuronal model
Nonlinear Dynamics (2022)
-
Coexistence of Attractors and Its Control with Selection of a Desired Attractor in a Model of Extended Hindmarsh–Rose Neuron with Nonlinear Smooth Fitting Function: Microcontroller Implementation
Journal of Vibration Engineering & Technologies (2022)