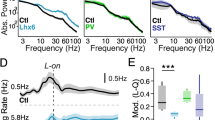
Abstract
Hippocampal network activity is generated by a complex interplay between excitatory pyramidal cells and inhibitory interneurons. Although much is known about the molecular properties of excitatory synapses on pyramidal cells, comparatively little is known about excitatory synapses on interneurons. Here we show that conditional deletion of the postsynaptic cell adhesion molecule neuroligin-3 in parvalbumin interneurons causes a decrease in NMDA-receptor-mediated postsynaptic currents and an increase in presynaptic glutamate release probability by selectively impairing the inhibition of glutamate release by presynaptic Group III metabotropic glutamate receptors. As a result, the neuroligin-3 deletion altered network activity by reducing gamma oscillations and sharp wave ripples, changes associated with a decrease in extinction of contextual fear memories. These results demonstrate that neuroligin-3 specifies the properties of excitatory synapses on parvalbumin-containing interneurons by a retrograde trans-synaptic mechanism and suggest a molecular pathway whereby neuroligin-3 mutations contribute to neuropsychiatric disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Buzsáki, G., Anastassiou, C.A. & Koch, C. The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 13, 407–420 (2012).
Gulyás, A.I. & Freund, T.T. Generation of physiological and pathological high frequency oscillations: the role of perisomatic inhibition in sharp-wave ripple and interictal spike generation. Curr. Opin. Neurobiol. 31, 26–32 (2015).
Korotkova, T., Fuchs, E.C., Ponomarenko, A., von Engelhardt, J. & Monyer, H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron 68, 557–569 (2010).
Marín, O. Interneuron dysfunction in psychiatric disorders. Nat. Rev. Neurosci. 13, 107–120 (2012).
Sah, P., Hestrin, S. & Nicoll, R.A. Properties of excitatory postsynaptic currents recorded in vitro from rat hippocampal interneurones. J. Physiol. (Lond.) 430, 605–616 (1990).
Geiger, J.R. et al. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 15, 193–204 (1995).
Lamsa, K.P., Heeroma, J.H., Somogyi, P., Rusakov, D.A. & Kullmann, D.M. Anti-Hebbian long-term potentiation in the hippocampal feedback inhibitory circuit. Science 315, 1262–1266 (2007).
Jamain, S. et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 34, 27–29 (2003).
Südhof, T.C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455, 903–911 (2008).
Rothwell, P.E. et al. Autism-associated neuroligin-3 mutations commonly impair striatal circuits to boost repetitive behaviors. Cell 158, 198–212 (2014).
Isaac, J.T., Ashby, M.C. & McBain, C.J. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 54, 859–871 (2007).
Nyíri, G., Stephenson, F.A., Freund, T.F. & Somogyi, P. Large variability in synaptic N-methyl-D-aspartate receptor density on interneurons and a comparison with pyramidal-cell spines in the rat hippocampus. Neuroscience 119, 347–363 (2003).
Zucker, R.S. & Regehr, W.G. Short-term synaptic plasticity. Annu. Rev. Physiol. 64, 355–405 (2002).
Kavalali, E.T. The mechanisms and functions of spontaneous neurotransmitter release. Nat. Rev. Neurosci. 16, 5–16 (2015).
Hessler, N.A., Shirke, A.M. & Malinow, R. The probability of transmitter release at a mammalian central synapse. Nature 366, 569–572 (1993).
Földy, C., Malenka, R.C. & Südhof, T.C. Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling. Neuron 78, 498–509 2013).
Anderson, G.R. et al. β-Neurexins control neural circuits by regulating synaptic endocannabinoid signaling. Cell 162, 593–606 (2015).
Gibson, H.E., Edwards, J.G., Page, R.S., Van Hook, M.J. & Kauer, J.A. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron 57, 746–759 (2008).
Thompson, S.M., Capogna, M. & Scanziani, M. Presynaptic inhibition in the hippocampus. Trends Neurosci. 16, 222–227 (1993).
Shigemoto, R. et al. Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zone. Nature 381, 523–525 (1996).
Allen, K. & Monyer, H. Interneuron control of hippocampal oscillations. Curr. Opin. Neurobiol. 31, 81–87 (2015).
Stark, E. et al. Pyramidal cell-interneuron interactions underlie hippocampal ripple oscillations. Neuron 83, 467–480 (2014).
Jadhav, S.P., Kemere, C., German, P.W. & Frank, L.M. Awake hippocampal sharp-wave ripples support spatial memory. Science 336, 1454–1458 (2012).
Girardeau, G., Benchenane, K., Wiener, S.I., Buzsáki, G. & Zugaro, M.B. Selective suppression of hippocampal ripples impairs spatial memory. Nat. Neurosci. 12, 1222–1223 (2009).
Dzhala, V.I. & Staley, K.J. Mechanisms of fast ripples in the hippocampus. J. Neurosci. 24, 8896–8906 (2004).
Donato, F., Rompani, S.B. & Caroni, P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature 504, 272–276 (2013).
Xu, W. et al. Distinct neuronal coding schemes in memory revealed by selective erasure of fast synchronous synaptic transmission. Neuron 73, 990–1001 (2012).
Fowler, S.C. et al. A force-plate actometer for quantitating rodent behaviors: illustrative data on locomotion, rotation, spatial patterning, stereotypies, and tremor. J. Neurosci. Methods 107, 107–124 (2001).
Chubykin, A.A. et al. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron 54, 919–931 (2007).
Blundell, J. et al. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J. Neurosci. 30, 2115–2129 (2010).
Jung, S.Y. et al. Input-specific synaptic plasticity in the amygdala is regulated by neuroligin-1 via postsynaptic NMDA receptors. Proc. Natl. Acad. Sci. USA 107, 4710–4715 (2010).
Losonczy, A., Somogyi, P. & Nusser, Z. Reduction of excitatory postsynaptic responses by persistently active metabotropic glutamate receptors in the hippocampus. J. Neurophysiol. 89, 1910–1919 (2003).
Scanziani, M., Gähwiler, B.H. & Charpak, S. Target cell-specific modulation of transmitter release at terminals from a single axon. Proc. Natl. Acad. Sci. USA 95, 12004–12009 (1998).
Losonczy, A., Zhang, L., Shigemoto, R., Somogyi, P. & Nusser, Z. Cell type dependence and variability in the short-term plasticity of EPSCs in identified mouse hippocampal interneurones. J. Physiol. (Lond.) 542, 193–210 (2002).
Ferraguti, F. et al. Metabotropic glutamate receptor 8-expressing nerve terminals target subsets of GABAergic neurons in the hippocampus. J. Neurosci. 25, 10520–10536 (2005).
Pelkey, K.A., Topolnik, L., Yuan, X.Q., Lacaille, J.C. & McBain, C.J. State-dependent cAMP sensitivity of presynaptic function underlies metaplasticity in a hippocampal feedforward inhibitory circuit. Neuron 60, 980–987 (2008).
Suh, Y.H. et al. Corequirement of PICK1 binding and PKC phosphorylation for stable surface expression of the metabotropic glutamate receptor mGluR7. Neuron 58, 736–748 (2008).
Zhang, C.S. et al. Knock-in mice lacking the PDZ-ligand motif of mGluR7a show impaired PKC-dependent autoinhibition of glutamate release, spatial working memory deficits, and increased susceptibility to pentylenetetrazol. J. Neurosci. 28, 8604–8614 (2008).
Schlingloff, D., Káli, S., Freund, T.F., Hájos, N. & Gulyás, A.I. Mechanisms of sharp wave initiation and ripple generation. J. Neurosci. 34, 11385–11398 (2014).
Forro, T., Valenti, O., Lasztoczi, B. & Klausberger, T. Temporal organization of GABAergic interneurons in the intermediate CA1 hippocampus during network oscillations. Cereb. Cortex 25, 1228–1240 (2015).
Carlén, M. et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol. Psychiatry 17, 537–548 (2012).
Zhang, B. et al. Neuroligins sculpt cerebellar purkinje-cell circuits by differential control of distinct classes of synapses. Neuron 87, 781–796 (2015).
Aoto, J., Földy, C., Ilcus, S.M., Tabuchi, K. & Südhof, T.C. Distinct circuit-dependent functions of presynaptic neurexin-3 at GABAergic and glutamatergic synapses. Nat. Neurosci. 18, 997–1007 (2015).
Liang, J. et al. Conditional neuroligin-2 knockout in adult medial prefrontal cortex links chronic changes in synaptic inhibition to cognitive impairments. Mol. Psychiatry 20, 850–859 (2015).
Etherton, M. et al. Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function. Proc. Natl. Acad. Sci. USA 108, 13764–13769 (2011).
Tabuchi, K. et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science 318, 71–76 (2007).
Jiang, M. et al. Conditional ablation of neuroligin-1 in CA1 pyramidal neurons blocks LTP by a cell-autonomous NMDA receptor-independent mechanism. Mol. Psychiatry http://dx.doi.org/10.1038/mp.2016.80 (2016).
Clem, R.L. & Huganir, R.L. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science 330, 1108–1112 (2010).
Acknowledgements
We thank S. Maxeiner for sharing NL3fl mice, S. Fang and S. Atiyeh Afjei for help with stereotaxic injections, K. Lee for help with in vivo recording experiments and S. Botelho for help with biochemical assays. This work was supported by grants from NIH (P50MH086403 to R.C.M. and T.C.S.) and the Simons Foundation Autism Research Initiative Award 307762 (to T.C.S.).
Author information
Authors and Affiliations
Contributions
J.S.P., T.C.S. and R.C.M. conceived the project, designed the experiments, and wrote and edited the manuscript. H.W. performed the in vivo electrophysiology experiments with input from C.H.H., and D.G. performed the immunohistochemistry experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Generation of NL3fl/PV-Cre mice and quantification of PV interneuron distribution in the hippocampus
a. Schematic representation of the mouse breeding strategy to generate NL3fl/PV-Cremice. b. Distribution of PV interneurons in the hippocampus. Representative coronal sections of the hippocampus from PV-Cre (top) and NL3fl/PV-Cre (bottom) mice showing PV-positive cells. c. Bar graph showing distribution of PV immunopositive cells in the hippocampus of PV-Cre (41 ± 3 cells/mm2, n = 3 mice) and NL3fl/PV-Cre mice (45 ± 3 cells/mm2, n = 3 mice).
Supplementary Figure 2 AAV injection at P21
a. Schematic representation of stereotaxic injection of AAV-hSyn-DIO-eGFP in P21 mice. b. Image of GFP expressing PV interneurons in the hippocampus of PV-Cre mice 10 days following AAV injection
Supplementary Figure 3 Inhibitory synaptic transmission onto PV interneurons is not changed by NL3 deletion.
a-b. (top) sample traces showing mIPSCs and (bottom) cumulative distribution plot for GABAA-R mediated mIPSC amplitude (a) and mIPSC frequency (b) from PV-Cre and NL3fl/PV-Cre cells showing no change due to NL3 deletion (p > 0.05, KS test for amplitude and frequency). Inset shows average mIPSC amplitude (PV-Cre, 22 ± 2 pA, n = 9 cells; NL3fl/PV-Cre, 21 ± 1 pA, n = 11 cells; p > 0.05) and mIPSC frequency (PV-Cre, 13 ± 2 Hz; NL3fl/PV-Cre 15 ± 2 Hz; p > 0.05).
Supplementary Figure 4 Summary plots of raw data showing the effects of various drugs on EPSCs and PPRs in PV interneurons.
a- before and after the application of CB1 receptor agonist WIN 55212-2 (5 μM) i, EPSCs; ii, PPRs in PV-Cre (black) and NL3fl/PV-Cre (orange) mice. b, same as in ‘a’ but for the application of CB1 receptor antagonist AM-251 (10 μM). c, same as in ‘a’ but for the application of Gr-III mGluR agonist L-AP4 (10 μM). d, same as in ‘a’ but for the application of Gr-III mGluR antagonist LY-341495 (1 μM). e, same as in ‘a’ but for the application of GABAB receptor agonist baclofen (10 μM). f, same as in ‘a’ but for the application of A1 receptor agonist N6-CPA (1 μM).
Supplementary Figure 5 GABAB-receptor- and A1-receptor-mediated presynaptic inhibition of excitatory synaptic transmission in PV interneurons are not affected by NL3 deletion
a-d, The depression of excitatory synaptic transmission in PV interneurons by the GABAB receptor agonist baclofen (10 μM) is not affected by NL3 deletion. Time course of normalized AMPAR EPSCs during application of baclofen (a) and sample EPSCs in response to paired pulse stimulation before and after baclofen application (b) in PV-Cre (before-black, after-blue) and NL3fl/PV-Cre (before-orange, after-green) neurons. Summary plot of normalized EPSC changes due to baclofen (c) and normalized PPR changes (d) in individual cells (PV-Cre, 0.4 ± 0.1, 1.3 ± 0.1, n = 4; NL3fl/PV-Cre, 0.4 ± 0.1, 1.3 ± 0.04, n = 6) e-h, Same as a-d but for the application of A1-R agonist N6-CPA (1 μM). Representative EPSCs (f) before and after N6-CPA for PV-Cre (before-black, after-blue) and NL3fl/PV-Cre (before-orange, after-green). Individual normalized EPSC (g) and PPR (h) changes caused after N6-CPA (PV-Cre, 0.3 ± 0.05, 1.1 ± 0.1, n = 6; NL3fl/PV-Cre, 0.3 ± 0.03, 1.0 ± 0.02, n = 4).
Supplementary Figure 6 Frequency-dependent attenuation of EPSCs in PV interneurons in PV-Cre and NL3fl/PV-Cre mice.
a-b, EPSC amplitudes from individual cells in response to stimulation trains at increasing frequencies. Responses in PV-Cre (black) and NL3fl/PV-Cre (orange) cells for 2 Hz, 5 Hz, 10 Hz and 20 Hz stimulation (a) and for 30 Hz, 50 Hz, 100 Hz, and 200 Hz stimulation (b). c, Logarithmic graph showing the summary data from Fig. 4a, b, presented as mean ± SEM of synaptic summation (of EPSCs) as a function of the stimulation frequency (2-200 Hz) for stimuli 1-10 (left) and 10-20 (right). NL3fl/PV-Cre cells show a low pass filtering in the frequency dependent summation of EPSCs from 30 Hz upwards.
Supplementary Figure 7 In vivo hippocampal CA1 LFPs show differences between PV-Cre and NL3fl/PV-Cre mice
a, Representative LFP traces (from left to right: unfiltered, 3-12 Hz band pass filtered, 35-85 Hz band pass filtered and 100-250 Hz band pass filtered) from the 4 recording channels from an individual PV-Cre mouse. b, Ratio of gamma normalized to theta is reduced in NL3fl/PV-Cre mice (PV-Cre, 0.19 ± 0.03, n = 5; NL3fl/PV-Cre 0.06 ± 0.01, n = 4; * p < 0.05). c, Combined normalized average of all captured, peak aligned SWRs from PV-Cre (black, n = 182) and NL3fl/PV-Cre (orange, n = 352).
Supplementary Figure 8 Virally injected DIO-NL3-Venus infects the majority of PV cells in the hippocampus.
a, Images of sections from AAV injected hippocampus from a NL3fl/PV-Cre mice showing colocalization of NL3-Venus (green) and anti-PV antibody (red). b, high resolution images from a section stained for PV (top), GFP (middle) and their colocalization (bottom, yellow). c, 86 ± 4% of PV-positive cells show AAV infection (n = 2 mice).
Supplementary Figure 9 Rescue of synaptic changes in NL3fl/PV-Cre cells by selective re-expression of NL3.
a, Representative traces of AMPAR EPSCs recorded at -60 mV and dual component EPSC at +40 mV (left). NMDAR/AMPAR ratio (right) is rescued by expression of NL3 in NL3fl/PV-Cre cells (NL3fl/PV-Cre+Rescue, 0.17 ± 0.02, n = 8 cells; p > 0.1 vs PV-Cre, *p < 0.05 vs NL3fl/PV-Cre). (PV-Cre and NL3fl/PV-Cre data from Fig. 1g.) b, Representative average traces (left) and summary graph (right) show rescue of paired-pulse ratios comparable to PV-Cre cells in NL3fl/PV-Cre+Rescue cells at inter-stimulus intervals of 20, 50, 100 and 200 ms (PV-Cre, 2.5 ± 0.1, 2.2 ± 0.1, 1.6 ± 0.1, 1.4 ± 0.1, n = 11 cells; NL3fl/PV-Cre + Rescue, 2.13 ± 0.17, 1.9 ± 0.17, 1.6 ± 0.1, 1.3 ± 0.1, n = 8 cells; p > 0.05 rescue vs control for all ISI). (PV-Cre and NL3fl/PV-Cre data from Fig 2b.) c-e, Inhibition of excitatory synaptic transmission by the Gr-III mGluR agonist L-AP4 is rescued by the expression of NL3 in NL3fl/PV-Cre cells. c, (left) time course of normalized AMPAR EPSCs during application of L-AP4 and sample EPSCs in response to paired-pulse stimulation before and after L-AP4 application (right) in PV-Cre (before-black, after-blue), NL3fl/PV-Cre (before-orange, after-green) and NL3fl/PV-Cre-DIO-NL3 (rescue: before-brown, after-green). d, Normalized summary plot (i) and EPSC amplitudes (ii) (NL3fl/PV-Cre-DIO-NL3: 0.58 ± 0.1, n = 6, p > 0.1), e, Normalized PPR changes (NL3fl/PV-Cre-DIO-NL3: 1.3 ± 0.1, n = 7, p > 0.1 vs PV-Cre) (i) and PPR (ii) values in response to L-AP4 application in NL3fl/PV-Cre-DIO-NL3 cells. (PV-Cre and NL3fl/PV-Cre data from Fig. 3i-l.)
Supplementary Figure 10 Performance in a reward alternation task and locomotor activity are not affected by deletion of NL3 from PV cells.
a. Schematic representation of a reward alternation task in a T maze (left). PV-Cre and NL3fl/PV-Cre mice make similar %correct choices after 6 days of training in the T maze (PV-Cre, 78 ± 4% n = 7; NL3fl/PV-Cre, 74 ± 4, n = 7). b, total distance travelled on the force plate in 20 min (84.9 ± 4.2 m for NL3PVcre+/+, n = 5 and 77.7 ± 2.1 m for NL3fl/PV-Cre, n = 7). c, number of low motion bouts in a 20 min session (14.6 ± 2.1 for NL3PVcre+/+, n = 5 and 21.8 ± 3.8 for NL3fl/PV-Cre, n = 7). d, average stereotypic score (1683 ± 242 for NL3PVcre+/+, n = 5 and 1430 ± 133 for NL3fl/PV-Cre, n = 7).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 1278 kb)
Rights and permissions
About this article
Cite this article
Polepalli, J., Wu, H., Goswami, D. et al. Modulation of excitation on parvalbumin interneurons by neuroligin-3 regulates the hippocampal network. Nat Neurosci 20, 219–229 (2017). https://doi.org/10.1038/nn.4471
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4471
This article is cited by
-
Differential contribution of canonical and noncanonical NLGN3 pathways to early social development and memory performance
Molecular Brain (2024)
-
Combinatorial expression of neurexins and LAR-type phosphotyrosine phosphatase receptors instructs assembly of a cerebellar circuit
Nature Communications (2023)
-
BACE1 in PV interneuron tunes hippocampal CA1 local circuits and resets priming of fear memory extinction
Molecular Psychiatry (2023)
-
Neuroligin-3 Regulates Excitatory Synaptic Transmission and EPSP-Spike Coupling in the Dentate Gyrus In Vivo
Molecular Neurobiology (2022)
-
Imbalance of flight–freeze responses and their cellular correlates in the Nlgn3−/y rat model of autism
Molecular Autism (2022)