Abstract
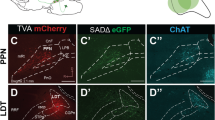
Dopamine neurons in the ventral tegmental area (VTA) receive cholinergic innervation from brainstem structures that are associated with either movement or reward. Whereas cholinergic neurons of the pedunculopontine nucleus (PPN) carry an associative/motor signal, those of the laterodorsal tegmental nucleus (LDT) convey limbic information. We used optogenetics and in vivo juxtacellular recording and labeling to examine the influence of brainstem cholinergic innervation of distinct neuronal subpopulations in the VTA. We found that LDT cholinergic axons selectively enhanced the bursting activity of mesolimbic dopamine neurons that were excited by aversive stimulation. In contrast, PPN cholinergic axons activated and changed the discharge properties of VTA neurons that were integrated in distinct functional circuits and were inhibited by aversive stimulation. Although both structures conveyed a reinforcing signal, they had opposite roles in locomotion. Our results demonstrate that two modes of cholinergic transmission operate in the VTA and segregate the neurons involved in different reward circuits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Wise, R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 5, 483–494 (2004).
Montague, P.R., Hyman, S.E. & Cohen, J.D. Computational roles for dopamine in behavioural control. Nature 431, 760–767 (2004).
Joshua, M. et al. Synchronization of midbrain dopaminergic neurons is enhanced by rewarding events. Neuron 62, 695–704 (2009).
Schultz, W. Updating dopamine reward signals. Curr. Opin. Neurobiol. 23, 229–238 (2013).
Tsai, H.C. et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324, 1080–1084 (2009).
Sesack, S.R. & Grace, A.A. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology 35, 27–47 (2010).
Mena-Segovia, J., Winn, P. & Bolam, J.P. Cholinergic modulation of midbrain dopaminergic systems. Brain Res. Rev. 58, 265–271 (2008).
Floresco, S.B., West, A.R., Ash, B., Moore, H. & Grace, A.A. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat. Neurosci. 6, 968–973 (2003).
Lokwan, S.J., Overton, P.G., Berry, M.S. & Clark, D. Stimulation of the pedunculopontine tegmental nucleus in the rat produces burst firing in A9 dopaminergic neurons. Neuroscience 92, 245–254 (1999).
Lodge, D.J. & Grace, A.A. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc. Natl. Acad. Sci. USA 103, 5167–5172 (2006).
Lammel, S. et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature 491, 212–217 (2012).
Clarke, P.B. & Pert, A. Autoradiographic evidence for nicotine receptors on nigrostriatal and mesolimbic dopaminergic neurons. Brain Res. 348, 355–358 (1985).
Calabresi, P., Lacey, M.G. & North, R.A. Nicotinic excitation of rat ventral tegmental neurones in vitro studied by intracellular recording. Br. J. Pharmacol. 98, 135–140 (1989).
Lacey, M.G., Calabresi, P. & North, R.A. Muscarine depolarizes rat substantia nigra zona compacta and ventral tegmental neurons in vitro through M1-like receptors. J. Pharmacol. Exp. Ther. 253, 395–400 (1990).
Zhang, L., Liu, Y. & Chen, X. Carbachol induces burst firing of dopamine cells in the ventral tegmental area by promoting calcium entry through L-type channels in the rat. J. Physiol. (Lond.) 568, 469–481 (2005).
Yeomans, J. & Baptista, M. Both nicotinic and muscarinic receptors in ventral tegmental area contribute to brain-stimulation reward. Pharmacol. Biochem. Behav. 57, 915–921 (1997).
Miller, A.D. & Blaha, C.D. Midbrain muscarinic receptor mechanisms underlying regulation of mesoaccumbens and nigrostriatal dopaminergic transmission in the rat. Eur. J. Neurosci. 21, 1837–1846 (2005).
Semba, K. & Fibiger, H.C. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. J. Comp. Neurol. 323, 387–410 (1992).
Steriade, M. Arousal: revisiting the reticular activating system. Science 272, 225–226 (1996).
Cornwall, J., Cooper, J.D. & Phillipson, O.T. Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain Res. Bull. 25, 271–284 (1990).
Lammel, S. et al. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron 57, 760–773 (2008).
Matsumoto, M. & Hikosaka, O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature 459, 837–841 (2009).
Brischoux, F., Chakraborty, S., Brierley, D.I. & Ungless, M.A. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci. USA 106, 4894–4899 (2009).
Roeper, J. Dissecting the diversity of midbrain dopamine neurons. Trends Neurosci. 36, 336–342 (2013).
Ikemoto, S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res. Rev. 56, 27–78 (2007).
Bromberg-Martin, E.S., Matsumoto, M. & Hikosaka, O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68, 815–834 (2010).
Witten, I.B. et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron 72, 721–733 (2011).
Scarnati, E., Proia, A., Campana, E. & Pacitti, C. A microiontophoretic study on the nature of the putative synaptic neurotransmitter involved in the pedunculopontine-substantia nigra pars compacta excitatory pathway of the rat. Exp. Brain Res. 62, 470–478 (1986).
Futami, T., Takakusaki, K. & Kitai, S.T. Glutamatergic and cholinergic inputs from the pedunculopontine tegmental nucleus to dopamine neurons in the substantia nigra pars compacta. Neurosci. Res. 21, 331–342 (1995).
Mantz, J., Thierry, A.M. & Glowinski, J. Effect of noxious tail pinch on the discharge rate of mesocortical and mesolimbic dopamine neurons: selective activation of the mesocortical system. Brain Res. 476, 377–381 (1989).
Coizet, V., Dommett, E.J., Redgrave, P. & Overton, P.G. Nociceptive responses of midbrain dopaminergic neurones are modulated by the superior colliculus in the rat. Neuroscience 139, 1479–1493 (2006).
Ungless, M.A., Magill, P.J. & Bolam, J.P. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science 303, 2040–2042 (2004).
Dormont, J.F., Condé, H. & Farin, D. The role of the pedunculopontine tegmental nucleus in relation to conditioned motor performance in the cat. I. Context-dependent and reinforcement-related single unit activity. Exp. Brain Res. 121, 401–410 (1998).
Okada, K., Toyama, K., Inoue, Y., Isa, T. & Kobayashi, Y. Different pedunculopontine tegmental neurons signal predicted and actual task rewards. J. Neurosci. 29, 4858–4870 (2009).
Hong, S. & Hikosaka, O. Pedunculopontine tegmental nucleus neurons provide reward, sensorimotor, and alerting signals to midbrain dopamine neurons. Neuroscience 282C, 139–155 (2014).
Johnson, K., Churchill, L., Klitenick, M.A., Hooks, M.S. & Kalivas, P.W. Involvement of the ventral tegmental area in locomotion elicited from the nucleus accumbens or ventral pallidum. J. Pharmacol. Exp. Ther. 277, 1122–1131 (1996).
Bevan, M.D. & Bolam, J.P. Cholinergic, GABAergic, and glutamate-enriched inputs from the mesopontine tegmentum to the subthalamic nucleus in the rat. J. Neurosci. 15, 7105–7120 (1995).
Charara, A., Smith, Y. & Parent, A. Glutamatergic inputs from the pedunculopontine nucleus to midbrain dopaminergic neurons in primates: Phaseolus vulgaris-leucoagglutinin anterograde labeling combined with postembedding glutamate and GABA immunohistochemistry. J. Comp. Neurol. 364, 254–266 (1996).
Omelchenko, N. & Sesack, S.R. Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area. J. Comp. Neurol. 483, 217–235 (2005).
Wang, H.L. & Morales, M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur. J. Neurosci. 29, 340–358 (2009).
Woolf, N.J. & Butcher, L.L. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res. Bull. 16, 603–637 (1986).
Mena-Segovia, J., Bolam, J.P. & Magill, P.J. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci. 27, 585–588 (2004).
Bolton, R.F., Cornwall, J. & Phillipson, O.T. Collateral axons of cholinergic pontine neurones projecting to midline, mediodorsal and parafascicular thalamic nuclei in the rat. J. Chem. Neuroanat. 6, 101–114 (1993).
Dautan, D. et al. A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem. J. Neurosci. 34, 4509–4518 (2014).
Brown, M.T. et al. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature 492, 452–456 (2012).
Petzold, A., Valencia, M., Pál, B. & Mena-Segovia, J. Decoding brain state transitions in the pedunculopontine nucleus: cooperative phasic and tonic mechanisms. Front. Neural Circuits 9, 68 (2015).
Munk, M.H., Roelfsema, P.R., König, P., Engel, A.K. & Singer, W. Role of reticular activation in the modulation of intracortical synchronization. Science 272, 271–274 (1996).
Pan, W.X. & Hyland, B.I. Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. J. Neurosci. 25, 4725–4732 (2005).
Good, C.H. & Lupica, C.R. Properties of distinct ventral tegmental area synapses activated via pedunculopontine or ventral tegmental area stimulation in vitro. J. Physiol. (Lond.) 587, 1233–1247 (2009).
Boucetta, S., Cissé, Y., Mainville, L., Morales, M. & Jones, B.E. Discharge profiles across the sleep-waking cycle of identified cholinergic, GABAergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J. Neurosci. 34, 4708–4727 (2014).
Mena-Segovia, J., Sims, H.M., Magill, P.J. & Bolam, J.P. Cholinergic brainstem neurons modulate cortical gamma activity during slow oscillations. J. Physiol. (Lond.) 586, 2947–2960 (2008).
Pinault, D. A novel single-cell staining procedure performed in vivo under electrophysiological control: morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or Neurobiotin. J. Neurosci. Methods 65, 113–136 (1996).
Thiele, A., Delicato, L.S., Roberts, M.J. & Gieselmann, M.A. A novel electrode-pipette design for simultaneous recording of extracellular spikes and iontophoretic drug application in awake behaving monkeys. J. Neurosci. Methods 158, 207–211 (2006).
Herrero, J.L. et al. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature 454, 1110–1114 (2008).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates (Academic Press, San Diego, 1986).
Mena-Segovia, J., Micklem, B.R., Nair-Roberts, R.G., Ungless, M.A. & Bolam, J.P. GABAergic neuron distribution in the pedunculopontine nucleus defines functional subterritories. J. Comp. Neurol. 515, 397–408 (2009).
Dautan, D., Haciogˇlu Bay, H., Bolam, J.P., Gerdjikov, T.V. & Mena-Segovia, J. Extrinsic sources of cholinergic innervation of the striatal complex: a whole-brain mapping analysis. Front. Neuroanat. 10, 1 (2016).
Blejec, A. Statistical method for detection of firing rate changes in spontaneously active neurons. Neurocomputing 65, 557–563 (2005).
Maris, E. & Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164, 177–190 (2007).
Grace, A.A. & Bunney, B.S. The control of firing pattern in nigral dopamine neurons: single spike firing. J. Neurosci. 4, 2866–2876 (1984).
Swanson, L.W. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res. Bull. 9, 321–353 (1982).
Mena-Segovia, J. Structural and functional considerations of the cholinergic brainstem. J. Neural Transm. (Vienna) (in the press) (2016).
Garzón, M. & Pickel, V.M. Subcellular distribution of M2 muscarinic receptors in relation to dopaminergic neurons of the rat ventral tegmental area. J. Comp. Neurol. 498, 821–839 (2006).
Garzón, M. & Pickel, V.M. Somatodendritic targeting of M5 muscarinic receptor in the rat ventral tegmental area: implications for mesolimbic dopamine transmission. J. Comp. Neurol. 521, 2927–2946 (2013).
Azam, L., Winzer-Serhan, U.H., Chen, Y. & Leslie, F.M. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J. Comp. Neurol. 444, 260–274 (2002).
Faure, P., Tolu, S., Valverde, S. & Naudé, J. Role of nicotinic acetylcholine receptors in regulating dopamine neuron activity. Neuroscience 282C, 86–100 (2014).
Acknowledgements
We thank M. Ungless for discussions on a previous version of this manuscript, A. Asif-Malik for training rats in the operant task, and E. Norman, L. Conyers and L. Black for their technical assistance. This work was supported by the Medical Research Council UK and by a Leverhulme Trust grant to J.M.-S. and T.V.G. (RPG-2012-690). D.D. was funded by a University of Leicester PhD studentship. A.S.S. was supported by People Marie Curie Actions: Latin America and Europe Liaison and Universidade Federal de Mato Grosso do Sul.
Author information
Authors and Affiliations
Contributions
J.M.-S. conceived and supervised the project. D.D. and A.S.S. performed in vivo experiments and anatomical tracing. I.H.-O. performed the electron microscopy and analyzed the data. M.A. performed the in vitro experiments under the supervision of J.M.T. D.D. performed the behavioral experiments under the supervision of T.V.G. M.V., D.D., J.P.B., I.H.-O., T.V.G. and J.M.-S. analyzed the data. I.B.W. and K.D. provided the transgenic rats. D.D., J.P.B. and J.M.-S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Cholinergic neurons projecting to the VTA are concentrated in the caudal mesopontine region.
(a) Schematic showing the sites of retrograde tracer injections in the VTA: cholera toxin b (CTb) was injected in the rostral VTA and red retrobeads (RB-R) in the caudal VTA to identify the topography of the projections from the PPN and LDT. (b) ChAT-immunopositive neurons contained CTb and/or RB-R in both PPN and LDT (arrows; the PPN neuron is triple-labeled and the LDT neuron in double-labeled). (c) Pattern of retrograde labeling in relation to the expression of ChAT at three mediolateral levels of the PPN/LDT in a representative animal. (d) Similar numbers of VTA-projecting cholinergic neurons were detected at different mediolateral levels. A larger proportion of non-cholinergic VTA-projecting neurons was observed in the most lateral sections of the PPN. (e) The number of cholinergic neurons containing one of the tracers was normalized against the total number of cholinergic neurons and expressed as a percentage of the total. This was quantified at three rostrocaudal 900 μm-segments of the PPN and in the LDT. The number of cholinergic neurons projecting to the VTA is higher in the caudal PPN segments and the LDT. This contrasts with the low number of ChAT+/RB-R+ neurons projecting to the caudal VTA located in the rostral PPN. Bars represent SEM (n = 4 rats).
Supplementary Figure 2 Specificity of transduction for cholinergic neurons in different brain areas.
We tested the specificity of the transduction by injecting AAV2-EF1a-DIO-YFP in other brain regions in a different set of animals (n=3 rats), including the cerebellum, ventromedial thalamus, superior colliculus and striatum as well as the PPN and LDT (n = 5 rats). Positive (i.e. YFP-expressing) somata were only observed in the striatum, the PPN and LDT, and no retrograde transduction of cholinergic neurons was detected. The presence of two fluorescent markers in the cholinergic neurons of the PPN (AAV2-EF1a-DIO-YFP) and LDT (AAV5-EF1a-DIO-mCherry) of ChAT::Cre+ rats was used to evaluate the specificity for the transduction, in combination with immunofluorescent detection of ChAT. See Supplementary Table 2 for values.
Supplementary Figure 3 Lack of forebrain cholinergic innervation of the VTA.
Coronal (a-e) or sagittal (f) sections showing the VTA following injections of AAV2-DIO-EF1a-eYFP into the medial septum (a), vertical limb of the diagonal band of Broca (b), horizontal limb of the diagonal band of Broca (c), nucleus basalis of Meynert (d), medial habenula (e) and the parabigeminal nucleus (f) (n = 3 per group). None of the transductions in these areas produced cholinergic axonal labeling in the VTA. The borders of the VTA were delimited by TH immunostaining (in red). Scale bar: 500 µm.
Supplementary Figure 4 Mediolateral distribution of cholinergic axons in the VTA.
(a) The axons used in the analyses in Fig. 1c and 2c were traced and digitized, as shown in this representative sections (n = 3 rats each). (b) The axonal density was normalized to detect areas of preferential innervation from either the PPN or the LDT. Whereas in the lateral and middle VTA we observed a similar pattern, in the medial VTA some differences were observed: PPN tends to innervate the most rostral regions of the VTA, where LDT innervation is low, but then drops in the most caudal regions, where LDT innervation is higher. This coincides with the low numbers of retrogradely labeled cholinergic neurons in the PPN when the injection was located in the caudal VTA. (c) Quantification of the total length cholinergic axons in the PPN and the LDT (t = 1.29, two-tailed t-test, P = 0.266). Bars represent SEM. * P < 0.05.
Supplementary Figure 5 Analysis of the response of a representative neuron to the laser stimulation.
(a) Three responses to the laser were obtained for this neuron. Spike events were extracted and merged into a single spike train (black trace). (b) Cumulative distribution function (CDF) of the three individual spike trains together with that of the merged spike train (black dots) were computed by increasing the CDF one unit each time a spike occurred (note that in order to maintain the same scale, the CDF of the merged spike train must be divided by the number of spike trains merged). Regression slope of the CDF was computed at each spike time by using a local (18 neighboring spikes) linear regression analysis (see grey dots on the black trace and dashed red lines for an example at three different time points, red dots). (c) Estimated instantaneous firing rate (regression slope) for the three individual trains and for the merged spike train where red dots correspond to the regression points marked in panel b. (d) Smoothed version and z-scored version of the instantaneous firing rate shown in panel c (for merged trace only). Horizontal lines correspond to the mean (full) and 5th and 95th percentile (dashed) values of the firing rate during pre-stimulus period (-10 to 0 s). Red trace demarks the response period of the neuron (i.e., a significant P < 0.05 increase in the firing rate).
Supplementary Figure 6 Controls for stimulation of cholinergic axons in the VTA.
(a) Following injection of AAV2-DIO-EF1a-eYFP into the PPN/LDT in ChAT::cre rats, individual TH+ neurons (n = 5 neurons) were recorded in vivo during blue light delivery and subsequently labeled with neurobiotin (b). The same protocol was followed as that used in figures 1 and 2. No change in firing was observed in neurons transduced with YFP only. Scale bar: 20 µm.
Supplementary Figure 7 Electrical stimulation of the PPN produces short-latency responses in DA neurons.
(a) A neuron that was recorded and labeled by the juxtacellular method and identified as dopaminergic by the expression of immunoreactivity for TH. (b) Raster plot and peri-stimulus time histogram (PSTH) of the neuron shown in a, following electrical stimulation of the PPN (bin size 1 ms). (b’) A representative spike following the stimulus (s; 0.5 ms duration, 0.5-0.8 mA, 0.5 Hz). Consistent with previous reports, short-latency action potentials were elicited in the DA neurons within a few milliseconds of the stimulus being delivered in the PPN; this contrasts with the slow modulatory effect of the optogenetic activation of PPN cholinergic afferents. (c) Short-latency responses were consistent across the DA neuronal population (n = 12 neurons in 5 rats). (d, e, e’) Non-DA neurons (i.e. TH-immunonegative, d) were also sampled (n = 4 neurons in 5 rats). PSTH (bin size 2 ms) showing a long-lasting inhibition (average time of inhibition: 30 ms +/- 8.6 ms, e). Responses in non-DA neurons were more heterogeneous.
Supplementary Figure 8 Nicotinic modulation of VTA neurons.
(a) Effect of carbachol puffing on VTA DA neurons (n = 9 neurons) that were labeled and identified as TH+. (b) Example of VTA neurons showing classical dopaminergic features with low spontaneous firing and/or presence of Ih current. (c) Carbachol puffs (200 µm) provoke excitatory postsynaptic potentials (EPSPs) that are blocked by mecamylamine (n = 9 neurons; 5 µM) but not by DhβE (1 µM) or methylcaconitine (500 nM). (d) Carbachol-induced EPSPs were not blocked by bicuculline (10 µM), CNQX (10 µM) or APV (10 µM) (n = 4 neurons). (e) The mecamylamine effect on EPSP size was significantly different to the control condition (t = 7.281, two-tailed t-test, P = 0.000342). (f) Effect of carbachol puffing on a VTA non-DA neuron, confirmed as TH- by immunofluorescence, and showing classical non-DA neuron features such as higher firing rate and lack of Ih current. (g) Carbachol puffing provokes robust EPSPs that are blocked by bath application of MLA (500 nM) but not by DHβE (1 µM) or mecamylamine (5 µM). Scale bar in fluorescent images: 20 µm. Acetylcholine has been reported to induce strong somatic excitation of dopamine neurons in the VTA (Eddine, R., et al. (2015) Sci Rep 5, 8184) and its effects consist of a presynaptic component, modulating glutamate release (Schilstrom, B., et al. (1998) Neuroscience 82, 781–789), and a postsynaptic component, involving nicotinic and muscarinic mechanisms (Gronier, B., et al. (2000) Psychopharmacology 147, 347-355; Mameli-Engvall, M., et al. (2006) Neuron 50, 911-921; Mansvelder, H.D., et al. (2002) Neuron 33, 905-919). Furthermore, muscarinic agonists increase the frequency of spontaneous action potentials in DA neurons (Lacey, M.G., et al. (1990) Pharmacol Exp Ther 253, 395-400; Scroggs, R.S., et al. (2001) J Neurophysiol 86, 2966-2972) and induce glutamate release concomitantly with a decrease in GABA release in the VTA (Grillner, P., et al. (2000) Neuroscience 96, 299-307; Mansvelder, H.D., et al. (2000) Neuron 27, 349-357). Our results are in agreement with other studies showing that mecamylamine was able to abolish the effects mediated by cholinergic agonists in DA neurons (Zhang, L., et al. (2005) J Physiol 586, 469-481) or following place preference associated with carbachol injections in the VTA (Ikemoto, S. & Wise R.A., et al. (2002) J Neurosci 22, 9895-9904). While our experiments seem to indicate an effect predominantly mediated by nicotinic receptors, muscarinic receptors are highly expressed in the VTA and are also likely to contribute to the modulation of DA neurons, as shown previously (Forster, G.L. and Blaha C.D. (2000) Eur J Neurosci 12, 3596–3604).
Supplementary Figure 9 Expression of vesicular acetylcholine transporter (VAChT) in YFP-labeled axons.
Axons expressing YFP in the VTA from both PPN- and LDT-transduced animals were tested for the presence of vesicular transporters by immunofluorescence. VAChT immunofluorescence was detected in the majority of axonal varicosities expressing YFP (arrowheads), as has also been reported in the striatum (Dautan et al (2014) J Neurosci 34, 4509-4518). In contrast, the vesicular glutamate transporter 2 (VGluT2) was never observed to co-localize with YFP in axonal varicosities, even though we detected large numbers of VGluT2-positive varicosities within the VTA. Thus, in agreement with in situ hybridization data (Wang and Morales (2009) Eur J Neurosci 29,340-358) the axons of cholinergic neurons from the brainstem lack VGluT2.
Supplementary Figure 10 Individual response of DA neurons to aversive (pinch) and laser stimulation.
Representative traces of two TH+ neurons recorded in vivo during a hind-paw pinch (red square) and laser stimulation (blue square) of PPN (a) or LDT (b) cholinergic axons in the VTA. Scale bar: 50 µm.
Supplementary Figure 11 Behavioral responses to the optogenetic activation of PPN and LDT axons are blocked by cholinergic receptor antagonists.
(a) Optogenetic activation of cholinergic axons in the VTA of PPN (n = 12 rats), LDT (n = 6 rats) or wild-type groups (n = 8 rats) failed to produce stimulation-locked effects on locomotor activity in the presence of cholinergic antagonists (i.p. 0.3 ml final volume, MLA: 6 mg/Kg; DHβE: 3 mg/Kg; atropine: 0.5 mg/Kg and mecamylamine: 1.0 mg/Kg). Locomotor activity in all groups was similar (2-way ANOVA; stimulation effect: F(2,19) = 0.589, P = 0.565; group effect: F(2,19) = 0.140, P = 0.871). (b) Cumulative distance travelled during laser stimulation (10 Hz, 50 ms, 8 s duration, every 2 min) of cholinergic axons in wild-type (green), PPN (gray) or LDT (red) groups following a systemic injection of a cocktail of cholinergic antagonists (1-way ANOVA: F(2, 21) = 1.722, P = 0.205). (c) Number of lever presses per minute during acquisition of the operant task for wild-type (green, n = 10), PPN (gray, n = 12) and LDT (red, n = 10) animals. No significant differences were observed (2-way ANOVA; day effect F(4,26) = 4.336, P = 0.08; group effect: F(2,29) = 0.174, P = 0.842; interaction F(8,54) = 1.038, P = 0.426).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 and Supplementary Tables 1 and 2 (PDF 1638 kb)
Rights and permissions
About this article
Cite this article
Dautan, D., Souza, A., Huerta-Ocampo, I. et al. Segregated cholinergic transmission modulates dopamine neurons integrated in distinct functional circuits. Nat Neurosci 19, 1025–1033 (2016). https://doi.org/10.1038/nn.4335
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4335
This article is cited by
-
Upper brainstem cholinergic neurons project to ascending and descending circuits
BMC Biology (2023)
-
The hyperexcitability of laterodorsal tegmentum cholinergic neurons accompanies adverse behavioral and cognitive outcomes of prenatal stress
Scientific Reports (2023)
-
A non-canonical GABAergic pathway to the VTA promotes unconditioned freezing
Molecular Psychiatry (2022)
-
Stereological estimations and neurochemical characterization of neurons expressing GABAA and GABAB receptors in the rat pedunculopontine and laterodorsal tegmental nuclei
Brain Structure and Function (2022)
-
Glutamate inputs from the laterodorsal tegmental nucleus to the ventral tegmental area are essential for the induction of cocaine sensitization in male mice
Psychopharmacology (2022)