Abstract
It has been debated whether orientation selectivity in mouse primary visual cortex (V1) is derived from tuned lateral geniculate nucleus (LGN) inputs or computed from untuned LGN inputs. However, few studies have measured orientation tuning of LGN axons projecting to V1. We measured the response properties of mouse LGN axons terminating in V1 and found that LGN axons projecting to layer 4 were generally less tuned for orientation than axons projecting to more superficial layers of V1. We also found several differences in response properties between LGN axons and V1 neurons in layer 4. These results suggest that orientation selectivity of mouse V1 may not simply be inherited from LGN inputs, but could also depend on thalamocortical or V1 circuits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ohki, K., Chung, S., Ch'ng, Y.H., Kara, P. & Reid, R.C. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 433, 597–603 (2005).
Hubel, D.H. & Wiesel, T.N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J. Physiol. (Lond.) 160, 106–154 (1962).
Reid, R.C. & Alonso, J.M. Specificity of monosynaptic connections from thalamus to visual cortex. Nature 378, 281–284 (1995).
Marshel, J.H., Kaye, A.P., Nauhaus, I. & Callaway, E.M. Anterior-posterior direction opponency in the superficial mouse lateral geniculate nucleus. Neuron 76, 713–720 (2012).
Cruz-Martín, A. et al. A dedicated circuit links direction-selective retinal ganglion cells to the primary visual cortex. Nature 507, 358–361 (2014).
Piscopo, D.M., El-Danaf, R.N., Huberman, A.D. & Niell, C.M. Diverse visual features encoded in mouse lateral geniculate nucleus. J. Neurosci. 33, 4642–4656 (2013).
Scholl, B., Tan, A.Y., Corey, J. & Priebe, N.J. Emergence of orientation selectivity in the mammalian visual pathway. J. Neurosci. 33, 10616–10624 (2013).
Zhao, X., Chen, H., Liu, X. & Cang, J. Orientation-selective responses in the mouse lateral geniculate nucleus. J. Neurosci. 33, 12751–12763 (2013).
Li, Y.T., Ibrahim, L.A., Liu, B.H., Zhang, L.I. & Tao, H.W. Linear transformation of thalamocortical input by intracortical excitation. Nat. Neurosci. 16, 1324–1330 (2013).
Lien, A.D. & Scanziani, M. Tuned thalamic excitation is amplified by visual cortical circuits. Nat. Neurosci. 16, 1315–1323 (2013).
Clascá, F., Rubio-Garrido, P. & Jabaudon, D. Unveiling the diversity of thalamocortical neuron subtypes. Eur. J. Neurosci. 35, 1524–1532 (2012).
Antonini, A., Fagiolini, M. & Stryker, M.P. Anatomical correlates of functional plasticity in mouse visual cortex. J. Neurosci. 19, 4388–4406 (1999).
Ohmura, N., Kawasaki, K., Satoh, T. & Hata, Y. In vivo electroporation to physiologically identified deep brain regions in postnatal mammals. Brain Struct. Funct. 220, 1307–1316 (2015).
Glickfeld, L.L., Andermann, M.L., Bonin, V. & Reid, R.C. Cortico-cortical projections in mouse visual cortex are functionally target specific. Nat. Neurosci. 16, 219–226 (2013).
Matsui, T. & Ohki, K. Target dependence of orientation and direction selectivity of corticocortical projection neurons in the mouse V1. Front. Neural Circuits 7, 143 (2013).
Ji, N., Milkie, D.E. & Betzig, E. Adaptive optics via pupil segmentation for high-resolution imaging in biological tissues. Nat. Methods 7, 141–147 (2010).
White, E.L., Weinfeld, E. & Lev, D.L. Quantitative analysis of synaptic distribution along thalamocortical axons in adult mouse barrels. J. Comp. Neurol. 479, 56–69 (2004).
Chen, T.W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Jin, J., Wang, Y., Swadlow, H.A. & Alonso, J.M. Population receptive fields of ON and OFF thalamic inputs to an orientation column in visual cortex. Nat. Neurosci. 14, 232–238 (2011).
Ringach, D.L. On the origin of the functional architecture of the cortex. PLoS One 2, e251 (2007).
Smith, S.L. & Häusser, M. Parallel processing of visual space by neighboring neurons in mouse visual cortex. Nat. Neurosci. 13, 1144–1149 (2010).
Harris, J.A., Oh, S.W. & Zeng, H. Adeno-associated viral vectors for anterograde axonal tracing with fluorescent proteins in nontransgenic and cre driver mice. Curr. Protoc. Neurosci. Chapter 1 Unit 1.20. 1–18 (2012).
Paxinos, G. & Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates (Elsevier, 2004).
Kalatsky, V.A. & Stryker, M.P. New paradigm for optical imaging: temporally encoded maps of intrinsic signal. Neuron 38, 529–545 (2003).
Olsen, S.R., Bortone, D.S., Adesnik, H. & Scanziani, M. Gain control by layer six in cortical circuits of vision. Nature 483, 47–52 (2012).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Peirce, J.W. Generating stimuli for neuroscience using PsychoPy. Front. Neuroinform. 2, 10 (2008).
Swindale, N.V. Orientation tuning curves: empirical description and estimation of parameters. Biol. Cybern. 78, 45–56 (1998).
Wörgötter, F. & Eysel, U.T. Quantitative determination of orientational and directional components in the response of visual cortical cells to moving stimuli. Biol. Cybern. 57, 349–355 (1987).
Hawken, M.J. & Parker, A.J. Spatial properties of neurons in the monkey striate cortex. Proc. R. Soc. Lond. B Biol. Sci. 231, 251–288 (1987).
Acknowledgements
We thank T. Matsui for the technical advice at the beginning of the experiments, A. Honda for the assistance with the histology experiments, A. Honda and Y. Sono for animal care and genotyping, all of the members of Ohki laboratory for support and discussion, and the Research Support Center, Graduate School of Medical Sciences, Kyushu University for technical support. This work was supported by grants from CREST-JST (to K.O.), JSPS KAKENHI (grant number 25221001 to K.O.), JST, Strategic International Research Cooperative Program, SCIP (to K.O.), Grant-in-Aid for Scientific Research on Innovative Areas, “Glial assembly: a new regulatory machinery of brain function and disorders” (25117004 to K.O.). A part of this work was carried out under the Brain/MINDS by the MEXT of Japan.
Author information
Authors and Affiliations
Contributions
S.K. and K.O. designed the experiments. S.K. performed the experiments and analyzed the data. S.K. and K.O. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Expression of GCaMP6s in LGN and their axons in V1.
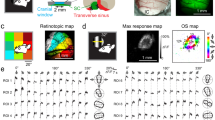
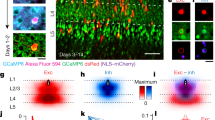
(a, b) Coronal section of LGN and V1 expressing GCaMP6s. a Coronal slice (bregma -2.3 mm) including thalamus confirmed that GCaMP6s expression was restricted within LGN. b The section of V1 was stained with fluorescent Nissl (red) and layers were assigned according to the size and density of cells (Anderson et al., Brain Res. 2009) (top). The same section from the top panel with GCaMP6s signal (green, bottom) is shown with assigned layers. (c) A part of V1 area (a bottom) was expanded. Intensity of GCaMP6s signal (LGN axons, green) was summed horizontally and plotted as normalized intensity on the right along the cortical depth. LGN axons distributing in layer 4 were particullary numerous and much less amount in layers 1 and 2/3. Small numbers of axons can be also seen in layers 5 and 6. We repeated the experiments three times. Previous studies showed the LGN projection pattern to V1 in higher mammals. In cat, X and Y cells mainly target layers 4 and 6, while W cells mainly target layer 1 and some amount to layers 3, 4a, 5a (Boyd & Matsubara, J. Comp. Neurol. 1996). In macaque monkey, similar to cat, P and M cells target mainly layer 4 and less amount layer 6, while K cells target mainly layers 1 and 2/3 (Lund, Annu. Rev. Neurosci. 1988). Therefore, projection patterns from LGN to V1 in mouse is similar to cat and macaque in principle. (d) Three-dimensionally reconstructed image of V1 from Scnn1a-cre/Ai14 mouse in vivo. Most of the cell bodies (red) can be seen at the depth of ~400μm from the pia mater. (e) Merged image of LGN axons (green) and layer 4 neurons (red) obtained from Scnn1a-cre/Ai14 mouse. LGN axons (green) can be seen among layer 4 neurons (red). Some imagings from layer 4 were obtained by using Scnn1a-cre/Ai14 mice (11 mice). (f, g, h) An example of cranial window for the in vivo imaging (f). Intrinsic optical imaging was performed and retinotopic map was created to determine the V1/V2 border (11 mice, g). tdTomato (red) was co-expressed with GCaMP6s (green) in LGN axons. Because GCaMP6s signal is low at the base level, we used the co-expressed tdTomato signal to find the area to image. Two-photon calcium imagings of LGN axons were typically obtained several ROIs (eg. white rectangles) within V1 (h). The dotted line indicates V1/V2 border determined from the retinotopic map (f-h).
Supplementary Figure 2 Orientation selectivity of LGN axons in layer 1 and 4.
Orientation maps of LGN axons recorded in layer 1 (a, n = 123 from 37 mice) and layer 4 (b, n = 98 from 30 mice). Colored pixels indicate the orientation selective [hue: preferred orientation, lightness: response magnitude, and saturation: gOSI]. White pixels indicate responsive but orientation non-selective. Orientation selectivity of LGN axons was typically much lower in layer 4 than layer 1.
Supplementary Figure 3 Proportion of orientation selective LGN axons and V1 neurons.
Histograms of proportion of orientation selective LGN boutons in layer 1 (a, n = 123 images from 37 mice), 2/3 (b, n = 97 images from 36 mice) and 4 (c, n = 98 images from 30 mice) and V1 neurons in layer 2/3 (d, n = 26 volume images from 14 mice) and 4 (e, n = 14 volume images from 10 mice) were shown (see Online Methods for criteria). Proportion of orientation selective LGN axons in layer 4 (c) was significantly lower than those in layers 1 and 2/3 LGN axons (a, b) and V1 neurons (d, e) (p < 0.001, Mann-Whitney U-test with Bonferroni correction).
Supplementary Figure 4 Point spread function in layer 1 and 4 after spherical aberration correction.
The image sizes of LGN axonal shaft were measured in layer 1 and 4 (10 axonal shafts from each layer). These values were used to estimate the PSF in layers 1 and 4. Full width at the half maximum (FWHM) of axonal shaft for lateral and axial-directions were 0.48 ± 0.03 µm and 2.83 ± 0.12 µm for layer 1 and 0.58 ± 0.05 µm and 3.71 ± 0.15 µm for layer 4 respectively. The image size of lateral direction was not statistically different between layers 1 and 4 (a: p = 0.06 paired t-test). The image size in axial-direction was statistically different between layers 1 and 4 (d: p < 0.01 paired t-test). Despite of statistical difference of image sizes, expansion of image size of shafts in layer 4 was not so big (1.3 times lager than that in layer 1), indicating PSF is mostly maintained in layer 4. Fluorescence profiles of lateral and axial directions and their Gaussian fittings are shown for layer 1 (b, c) and layer 4 (e, f) respectively.
Supplementary Figure 5 Relation between gOSI (or OSI) and contamination ratio.
gOSI (a) and OSI (b) were plotted as a function of contamination ratio in layer 1 (n = 2,924 boutons from 37 mice), 2/3 (n = 2,818 boutons from 36 mice) and 4 (n = 1,722 boutons from 30 mice) (see Methods section for the calculation formula of gOSI and OSI). In our studies, raw calcium signal data to visual stimulations were corrected to minimize the out-of-focus signal contamination. The correction was done by subtracting the out-of-focus signal multiplied by the contamination ratio from the raw data (see Online Methods). We confirmed that our conclusion was affected neither by the contamination ratio nor by which formula to use to calculate orientation selectivity (gOSI or OSI).
Supplementary Figure 6 Relation between density of LGN axons and gOSI in layer 1 and 4.
(a) To examine the possibility that contamination with untuned neuropil signal from dense layer 4 axons lowered the orientation selectivity of LGN axons in layer 4, we compared the gOSI of boutons in images with densely versus sparsely labeled layer 4 and layer 1. We plotted the gOSI as a function of density of responsive boutons for layer 1 and layer 4 LGN axons. Regression analysis revealed that the correlations between mean gOSI and density of responsive boutons were statistically significant for both layers 1 (n = 123 images from 37 mice, p < 0.01) and 4 (n = 98 images from 30 mice, p < 0.001). The slopes of regression lines for layer 1 (gOSI = -0.029 x density of responsive boutons + 0.386) and layer 4 (gOSI = -0.025 x density of responsive boutons + 0.285) were not statistically different (p = 0.66). Therefore, we assumed these slopes were parallel and we performed analysis of covariance. We found the distributions of gOSIs for these layers were statistically different (p < 0.001) after removing the effect of density of responsive boutons. (b) Representative images of LGN boutons were shown from low and high responsive bouton density images of layer 1 and layer 4. These results indicated that our conclusion that the orientation selectivity was lower in layer 4 LGN axons than in layer 1 was not due to the higher axonal density in layer 4 than in layer 1.
Supplementary Figure 7 Distributions of OSI of LGN axons and V1 neurons.
Distribution of OSI from LGN axons in layer 1 (a, n = 2,924 boutons from 37 mice), 2/3 (b, n = 2,818 boutons from 36 mice) and 4 (c, n = 1,722 boutons from 30 mice) and V1 neurons in layer 2/3 (d, n = 9,629 neurons from14 mice) and 4 (e, n = 5,102 neurons from10 mice) are plotted. Distribution from layer 4 LGN axons was significantly different from layer 1 LGN axons and V1 layers 2/3 and 4 neurons (p < 0.001, Mann-Whitney U-test with Bonferroni correction). We obtained the similar results from OSI as from gOSI. Orientation selectivity was overall lower in layer 4 LGN axons than LGN axons in superficial layers and V1 neurons.
Supplementary Figure 8 Relation between the orientation bias and the strength of orientation selectivity.
We observed that LGN axons had strong orientation bias (Figure 3f-h). To know if this bias for orientation preferences has any relationship with orientation selectivity, boutons were divided into two groups according to the strength of orientation selectivity (gOSI); one group has gOSI between 0.33 and 0.67 (a-e, moderate), another has gOSI more than 0.67 (f-j, strong). Distribution of preferred orientation in each group was plotted for LGN axons (a-c, f-h) and V1 neurons (d,e, i,j). In layers 1 and 2/3, orientation bias was not related with strength of orientation selectivity. However, in layer 4, cardinal bias toward 90 degree became more evident in the group of strongly orientation selective boutons (gOSI > 0.67). Therefore, there was a laminar difference in the relation between orientation selectivity and the orientation bias. These results may suggest that the source of strongly orientation selective inputs to layer 4 and to the superficial layers might be different. n indicates number of boutons.
Supplementary Figure 9 Proportion of direction selective LGN axons and V1 neurons.
Histograms of proportion of direction selective LGN boutons in layer 1 (a, n = 123 images from 37 mice), 2/3 (b, n = 97 images from 36 mice) and 4 (c, n = 98 images from 30 mice) and V1 neurons in layer 2/3 (d, n = 13 images from 5 mice) and 4 (e, n = 12 images from 6 mice) were shown (see Online Methods for criteria). Proportion of direction selective LGN boutons in layer 4 (c) was significantly lower than those in layer 1 (a, p<0.001, Mann-Whitney U-test with Bonferroni correction) and V1 neurons in layer 4 (e, p<0.01, Mann-Whitney U-test with Bonferroni correction).
Supplementary Figure 10 Distribution of preferred directions of LGN axons.
Distribution of preferred direction of LGN axons in layer 1 (a, n = 1,954 boutons from 37 mice), 2/3 (b, n = 1,630 boutons from 36 mice) and 4 (c, n = 931 boutons from 30 mice). They show cardinal bias which is less prominent (see Supplementary Fig. 11 for the possible reason) than preferred orientation (see Fig. 3). Red triangles indicate cardinal directions.
Supplementary Figure 11 Dissociation between preferred orientations and directions of LGN axons.
Dissociation between preferred orientation and direction. (a-c) The distribution of bin difference between preferred orientation and direction from LGN axons in layer 1 (a, n = 1,235 boutons from 37 mice), 2/3 (b, n = 1,019 boutons from 36 mice), and 4 (c, n = 495 boutons from 30 mice). Although most of the boutons (~75 %) showed that preferred orientation and direction fell into the same bin (a - c), the rest of boutons were in the next bin. In such cases, the polar plots of the tuning curves showed that two direction peaks were not in the perfect opposition, which caused the dissociation of preferred orientation and direction by more than 15 degrees (d - f). The dissociation contributed to weaker direction bias (Supplementary Fig. 10) than the orientation bias (Fig. 3f-j).
Supplementary Figure 12 Spatial frequency tunings of LGN axons and V1 neurons.
(a-e) Distribution of preferred spatial frequency was shown as histograms for LGN axons in layer 1 (a, n = 3,817 boutons from 20 mice), 2/3 (b, n = 2,669 boutons from 18 mice) and 4 (c, n = 5,576 boutons from 20 mice) and V1 neurons in layer 2/3 (d, n = 7,635 neurons from 10 mice) and 4 (e, n = 6,210 neurons from 9 mice). Mean preferred frequency of layer 4 LGN axons (c) was statistically different from other layers’ axons (a, b) and V1 neurons (d, e) (p < 0.001 with layer 1 axons and V1 neurons, p < 0.05 with layer 2/3 axons, Mann-Whitney U-test with Bonferroni correction). (f) Proportion of SPF selective LGN axons and V1 neurons were not statistically different (p > 0.05, Mann-Whitney U-test with Bonferroni correction). Mean preferred SPF was significantly lower in layer 4 LGN axons than layer 4 V1 neurons, suggesting further computation in the cortical circuit.
Supplementary Figure 13 Summary.
Summary. Percentages of strongly-selective (gOSI > 0.67), moderately-selective (0.33 < gOSI < 0.67), and weakly-selective or non-selective (gOSI < 0.33) boutons among responsive LGN boutons in V1 layers 1, 2/3, and 4, and neurons among responsive neurons in V1 layers 2/3 and 4 are indicated.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13 and Supplementary Tables 1–3 (PDF 1199 kb)
Rights and permissions
About this article
Cite this article
Kondo, S., Ohki, K. Laminar differences in the orientation selectivity of geniculate afferents in mouse primary visual cortex. Nat Neurosci 19, 316–319 (2016). https://doi.org/10.1038/nn.4215
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4215
This article is cited by
-
Haploinsufficiency of Shank3 increases the orientation selectivity of V1 neurons
Scientific Reports (2022)
-
Portrait of visual cortical circuits for generating neural oscillation dynamics
Cognitive Neurodynamics (2021)
-
Wide and Deep Imaging of Neuronal Activities by a Wearable NeuroImager Reveals Premotor Activity in the Whole Motor Cortex
Scientific Reports (2019)
-
In vivo wide-field calcium imaging of mouse thalamocortical synapses with an 8 K ultra-high-definition camera
Scientific Reports (2018)
-
Functional modulation of primary visual cortex by the superior colliculus in the mouse
Nature Communications (2018)