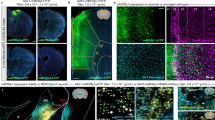
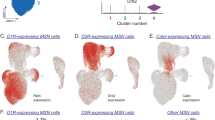
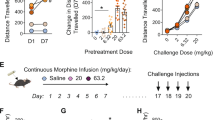
Abstract
μ-opioid receptors (MORs) are necessary for the analgesic and addictive effects of opioids such as morphine, but the MOR-expressing neuronal populations that mediate the distinct opiate effects remain elusive. Here we devised a new conditional bacterial artificial chromosome rescue strategy to show, in mice, that targeted MOR expression in a subpopulation of striatal direct-pathway neurons enriched in the striosome and nucleus accumbens, in an otherwise MOR-null background, restores opiate reward and opiate-induced striatal dopamine release and partially restores motivation to self administer an opiate. However, these mice lack opiate analgesia or withdrawal. We used Cre-mediated deletion of the rescued MOR transgene to establish that expression of the MOR transgene in the striatum, rather than in extrastriatal sites, is needed for the restoration of opiate reward. Our study demonstrates that a subpopulation of striatal direct-pathway neurons is sufficient to support opiate reward-driven behaviors and provides a new intersectional genetic approach to dissecting neurocircuit-specific gene function in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Le Merrer, J., Becker, J.A.J., Befort, K. & Kieffer, B.L. Reward processing by the opioid system in the brain. Physiol. Rev. 89, 1379–1412 (2009).
Wassum, K.M., Ostlund, S.B., Maidment, N.T. & Balleine, B.W. Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proc. Natl. Acad. Sci. USA 106, 12512–12517 (2009).
Nestler, E.J. Molecular mechanisms of opiate and cocaine addiction. Curr. Opin. Neurobiol. 7, 713–719 (1997).
Matthes, H.W.D. et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the μ-opioid–receptor gene. Nature 383, 819–823 (1996).
Mansour, A., Fox, C.A., Akil, H. & Watson, S.J. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 18, 22–29 (1995).
Crittenden, J.R. & Graybiel, A.M. Basal ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front. Neuroanat. 5, 59 (2011).
Gerfen, C.R. The neostriatal mosaic. I. Compartmental organization of projections from the striatum to the substantia nigra in the rat. J. Comp. Neurol. 236, 454–476 (1985).
Fujiyama, F. et al. Exclusive and common targets of neostriatofugal projections of rat striosome neurons: a single neuron-tracing study using a viral vector. Eur. J. Neurosci. 33, 668–677 (2011).
Watabe-Uchida, M., Zhu, L., Ogawa, S.K., Vamanrao, A. & Uchida, N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873 (2012).
Chuhma, N., Tanaka, K.F., Hen, R. & Rayport, S. Functional connectome of the striatal medium spiny neuron. J. Neurosci. 31, 1183–1192 (2011).
Gong, S. et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925 (2003).
Lobo, M.K., Karsten, S.L., Gray, M., Geschwind, D.H. & Yang, X.W. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat. Neurosci. 9, 443–452 (2006).
Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 (2001).
Sternini, C. et al. Agonist-selective endocytosis of μ opioid receptor by neurons in vivo. Proc. Natl. Acad. Sci. USA 93, 9241–9246 (1996).
Bielsky, I.F., Hu, S.-B., Ren, X., Terwilliger, E.F. & Young, L.J. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron 47, 503–513 (2005).
Maskos, U. et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436, 103–107 (2005).
Spealman, R.D. & Goldberg, S.R. Drug self-administration by laboratory animals: control by schedules of reinforcement. Annu. Rev. Pharmacol. Toxicol. 18, 313–339 (1978).
Sanchis-Segura, C. & Spanagel, R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict. Biol. 11, 2–38 (2006).
Patel, S.S. & Spencer, C.M. Remifentanil. Drugs 52, 417–427 (1996).
Koob, G.F. & Volkow, N.D. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238 (2010).
Williams, J.T., Christie, M.J. & Manzoni, O. Cellular and synaptic adaptations mediating opioid dependence. Physiol. Rev. 81, 299–343 (2001).
Porreca, F., Mosberg, H.I., Hurst, R., Hruby, V.J. & Burks, T.F. Roles of μ, δ and κ opioid receptors in spinal and supraspinal mediation of gastrointestinal transit effects and hot-plate analgesia in the mouse. J. Pharmacol. Exp. Ther. 230, 341–348 (1984).
Zachariou, V. et al. Essential role for RGS9 in opiate action. Proc. Natl. Acad. Sci. USA 100, 13656–13661 (2003).
Dang, M.T. et al. Disrupted motor learning and long-term synaptic plasticity in mice lacking NMDAR1 in the striatum. Proc. Natl. Acad. Sci. USA 103, 15254–15259 (2006).
Lüscher, C. & Malenka, R.C. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69, 650–663 (2011).
Johnson, S.W. & North, R.A. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci. 12, 483–488 (1992).
Chefer, V.I., Denoroy, L., Zapata, A. & Shippenberg, T.S. μ opioid receptor modulation of somatodendritic dopamine overflow: GABAergic and glutamatergic mechanisms. Eur. J. Neurosci. 30, 272–278 (2009).
Sulzer, D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 69, 628–649 (2011).
Wise, R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 5, 483–494 (2004).
Matsui, A. & Williams, J.T. Opioid-sensitive GABA inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons. J. Neurosci. 31, 17729–17735 (2011).
Olson, V.G. et al. Role of noradrenergic signaling by the nucleus tractus solitarius in mediating opiate reward. Science 311, 1017–1020 (2006).
Hnasko, T.S., Sotak, B.N. & Palmiter, R.D. Morphine reward in dopamine-deficient mice. Nature 438, 854–857 (2005).
Nader, K. & van der Kooy, D. Deprivation state switches the neurobiological substrates mediating opiate reward in the ventral tegmental area. J. Neurosci. 17, 383–390 (1997).
Laviolette, S.R., Gallegos, R.A., Henriksen, S.J. & van der Kooy, D. Opiate state controls bi-directional reward signaling via GABAA receptors in the ventral tegmental area. Nat. Neurosci. 7, 160–169 (2004).
Kravitz, A.V., Tye, L.D. & Kreitzer, A.C. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat. Neurosci. 15, 816–818 (2012).
Lobo, M.K. & Nestler, E.J. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front. Neuroanat. 5, 41 (2011).
Amemori, K., Gibb, L.G. & Graybiel, A.M. Shifting responsibly: the importance of striatal modularity to reinforcement learning in uncertain environments. Front. Hum. Neurosci. 5, 47 (2011).
Babovic, D. et al. Behavioural and anatomical characterization of mutant mice with targeted deletion of D1 dopamine receptor–expressing cells: response to acute morphine. J. Pharmacol. Sci. 121, 39–47 (2013).
Nagy, A. Cre recombinase: the universal reagent for genome tailoring. Genesis 26, 99–109 (2000).
Branda, C.S. & Dymecki, S.M. Talking about a revolution: the impact of site-specific recombinases on genetic analyses in mice. Dev. Cell 6, 7–28 (2004).
Yang, X.W., Model, P. & Heintz, N. Homologous recombination based modification in Escherichia coli and germline transmission in transgenic mice of a bacterial artificial chromosome. Nat. Biotechnol. 15, 859–865 (1997).
Gong, S., Yang, X.W., Li, C. & Heintz, N. Highly efficient modification of bacterial artificial chromosomes (BACs) using novel shuttle vectors containing the R6Kγ origin of replication. Genome Res. 12, 1992–1998 (2002).
Sakoori, K. & Murphy, N.P. Expression of morphine-conditioned place preference is more vulnerable than naloxone-conditioned place aversion to disruption by nociceptin in mice. Neurosci. Lett. 443, 108–112 (2008).
Murphy, N.P., Lam, H.A. & Maidment, N.T. A comparison of morphine-induced locomotor activity and mesolimbic dopamine release in C57BL6, 129Sv and DBA2 mice. J. Neurochem. 79, 626–635 (2001).
Bryant, C.D., Eitan, S., Sinchak, K., Fanselow, M.S. & Evans, C.J. NMDA receptor antagonism disrupts the development of morphine analgesic tolerance in male, but not female C57BL/6J mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R315–R326 (2006).
Bryant, C.D., Roberts, K.W., Byun, J.S., Fanselow, M.S. & Evans, C.J. Morphine analgesic tolerance in 129P3/J and 129S6/SvEv mice. Pharmacol. Biochem. Behav. 85, 769–779 (2006).
Sugino, K. et al. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat. Neurosci. 9, 99–107 (2006).
Tang, F. et al. RNA-Seq analysis to capture the transcriptome landscape of a single cell. Nat. Protoc. 5, 516–535 (2010).
Thomsen, M. & Caine, S.B. Chronic intravenous drug self-administration in rats and mice. Curr. Protoc. Neurosci. Chapter 9, Unit 9.20 (2005).
Murphy, N.P. & Maidment, N.T. Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J. Neurochem. 73, 179–186 (1999).
Acknowledgements
The research is supported by the University of California, Los Angeles (UCLA) Center for Opioid Receptors and Drugs of Abuse funded by the National Institute on Drug Abuse (NIDA) at the US National Institutes of Health (P50 DA005010). X.W.Y. is also supported in part by the David Weil Fund to the Semel Institute at UCLA and the Neuroscience of Brain Disorders Award from The McKnight Endowment Fund for Neuroscience. The Pdyn-MOR BAC transgenic mice were generated at the UCLA Transgenic Core Facility. Flow cytometry was performed by I. Williams and M. Zhou in the UCLA Jonsson Comprehensive Cancer Center (JCCC) and Center for AIDS Research Flow Cytometry Core Facility (funded by US National Institutes of Health awards CA-16042 and AI-28697 and by the JCCC, the UCLA AIDS Institute and the David Geffen School of Medicine at UCLA). W.G. and Y.E.S. are supported by the Transcriptome and Epigenetics Core (P50 DA005010) and by the Intellectual and Developmental Disabilities Research Center (IDDRC center grant NIH-P30HD004612). S.B.O. is supported by the NIDA (R01DA029035). Y.E.S. is also supported by the National Natural Science Foundation of China (NSFC, 90919057). N.M. and N.P.M. are partially supported by the Hatos Foundation.
Author information
Authors and Affiliations
Contributions
Y.C. and X.W.Y. designed the study, interpreted the results and wrote the manuscript. Y.C. performed experiments, analyzed the data and made Figures 1, 2, 3, 5 and 6a–g and Supplementary Figures 1, 2, 3, 4, 5, 6, 7, 8. S.B.O. and N.T.M. designed and did experiments for and made Figure 6h,i. A.S.J., J.D.J. and W.M.W. did experiments for and made Figure 4. W.G. and Y.E.S. contributed to Figure 1b. C.S.P. contributed to Figure 1 and Supplementary Figure 3. K.W.R., N.M., N.P.M., N.T.M., B.L.K. and C.J.E. contributed to the experimental design, actual experiments and data analyses for Figures 2, 3 and 5 and Supplementary Figures 6 and 7. C.C. and M.S.L. contributed to revision of the manuscript. X.W.Y. and C.J.E. contributed to Supplementary Figure 9.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Immunohistochemical staining of MOR in Drd1-GFP and Drd2-GFP mice.
(a-c) A representative double immunofluorescence staining shows the expression pattern of MOR (red, b, c) and GFP (green, a,c) in the GENSAT Drd1-GFP mouse brain. Scale bar = 50 μm. (d-f) A representative double immunofluorescence staining shows the expression pattern on of MOR (red, e and f) and GFP (green, d and f) the GENSAT Drd2-GFP mouse brain. Scale bar = 50 μm. (g-i) A representative double immunofluorescence staining shows the co-localization of MOR (red, b, c) and GFP (green, a,c) in the GENSAT Drd1-GFP mouse brain. Scale bar = 20 μm. (j-l) A representative double immunofluorescence staining shows the co-localization of MOR (red, e and f) and GFP (green, d and f) the GENSAT Drd2-GFP mouse brain. Scale bar = 20 μm.
Supplementary Figure 2 A example of FACS sorting of the GFP positive but propidium iodide negative neurons from WT (used as negative control) and Pdyn-GFP mice.
Same gating was used for the Drd2-GFP mice. Green dots within the selected area represents the GFP+/propidium iodide- cells been collected.
Supplementary Figure 3 Immunohistochemical staining of MOR in adult WT, MOR-KO and Rescue mice.
(a) WT; (b) MOR-KO; and (c) Rescue mice. The arrows show the expression of MOR in the cortex and brain stem. Ctx, cerebral cortex; Str, striatum; Tha, thalamus; Mid, midbrain, SN, substantia nigra; BS, brain stem. Scare bar = 1 mm.
Supplementary Figure 4 Western blot analysis of expression of striatal MOR in Rescue mice.
(a) Western blot performed on brain lysates isolated from Rescue, MOR-KO and WT control mice. (b) Quantification of relative MOR expression in the striatum. MOR levels were normalized to those observed in the WT mice (H(2)=32.143, p < 0.001, Non-parametric one-way ANOVA on ranking; n=4, WT; n=3, MOR-KO and n=6, Rescue). Values are mean ± SEM. Asterisks indicate p < 0.05.
Supplementary Figure 5 Pdyn-MOR mice and Rescue mice do not exhibit significant deficits in body weight or locomotor behaviors.
(a) The body weights of the WT, Pdyn-MOR, MOR-KO and Rescue mice are not significantly different (F(3, 44)=1.864, p = 0.1497, ANOVA, n = 12 for genotypes). (b and c) Locomotor activities in the open field test were obtained for adult WT, Pdyn-MOR, MOR-KO and Rescue mice (n=8 per genotype). No significant differences were observed in (b floorplane distance (cm) and (c) floorplane moves among the different genotypes. (F(3, 28) = 0.9993, p = 0.2778 for floorplane distance; F(3, 28) = 0.2574, p = 0.9061 for floorplane moves; One-Way ANOVA, n=8 for all groups)
Supplementary Figure 6 Morphine rewarding effect is rescued by Pdyn-BAC-driven expression of the MOR.
(a) An independent cohort of mice are used to show that the CPP deficit in the MOR-KO mice is restored to the WT level in the Rescue mice. (genotype x F(3, 41)=3.887, p = 0.0156, genotype x treatment interaction, two way ANOVA; n=7, WT+morphine group; n=6 in all other groups). (b) The Rgs9-Cre transgene alone has no significant deficits in CPP (F(4,71)=5,338, p = 0.0008; genotype x treatment interaction, two way ANOVA, n=7-9 per group).The WT, MOR-KO, and Rescue/Cre mice are the same group of littermate mice as those shown in Fig. 5b. Values are mean ± SEM. Triple asterisk indicates p < 0.001.
Supplementary Figure 7 Naloxone-precipitated morphine withdrawal syndrome is not significantly restored by Pdyn-BAC driven expression of MOR in the striatal direct-pathway MSN subpopulations.
(a) tremor; (b) wet dog shakes; (c) teeth chattering; (d) sniffing; (e) ptosis; (f) jumping and (g) diarrhea. Results are expressed as means ± SEM. Triple asterisks indicate p < 0.001 and single asterisk indicates p < 0.05.
Supplementary Figure 8 Immunohistochemistry staining of MOR in Rescue/Cre and Rescue mice.
(a). A low magnification image of Rescue/Cre mice showed the striatal MOR expression is abolished. (b-g) Higher magnificent images to compare MOR expression in Rescue mice (b,d,f) and Rescue/Cre mice (c,e,g) in the cortex (b,c), the brain stem (d,e) and substantia nigra (f,g). The result showed Rgs9-Cre can selectively remove in Rescue/Cre mice the expression of MOR in the striatal-direct pathway MSNs and their axonal terminals in SNc, but did not alter MOR expression in the cortex or brain stem compared to Rescue mice.
Supplementary Figure 9 The role of striatal direct-pathway MSNs in the striosome and NAc in regulating striatal dopamine release in our Rescue mice.
MOR is selectively expressed in a subpopulation of striatal direct-pathway MSNs (black dotted circle) located in the striosome and NAc, which form monosynaptic inputs to the DA neurons in VTA and SNc. Opioids (e.g. morphine) binding to MOR on this direct-pathway MSN subpopulation results in the restoration of striatal dopamine release and opiate-driven reward and reinforcement behaviors in the Rescue mice.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Table 1 (PDF 13781 kb)
Rights and permissions
About this article
Cite this article
Cui, Y., Ostlund, S., James, A. et al. Targeted expression of μ-opioid receptors in a subset of striatal direct-pathway neurons restores opiate reward. Nat Neurosci 17, 254–261 (2014). https://doi.org/10.1038/nn.3622
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3622
This article is cited by
-
Mu opioid receptors on hippocampal GABAergic interneurons are critical for the antidepressant effects of tianeptine
Neuropsychopharmacology (2022)
-
EGR3 regulates opioid-related nociception and motivation in male rats
Psychopharmacology (2022)
-
An opioid-gated thalamoaccumbal circuit for the suppression of reward seeking in mice
Nature Communications (2022)
-
Mesolimbic opioid-dopamine interaction is disrupted in obesity but recovered by weight loss following bariatric surgery
Translational Psychiatry (2021)
-
An endogenous opioid circuit determines state-dependent reward consumption
Nature (2021)