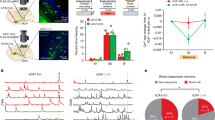
Abstract
Memory is supported by a specific ensemble of neurons distributed in the brain that form a unique memory trace. We previously showed that neurons in the lateral amygdala expressing elevated levels of cAMP response-element binding protein are preferentially recruited into fear memory traces and are necessary for the expression of those memories. However, it is unknown whether artificially activating just these selected neurons in the absence of behavioral cues is sufficient to recall that fear memory. Using an ectopic rat vanilloid receptor TRPV1 and capsaicin system, we found that activating this specific ensemble of neurons was sufficient to recall established fear memory. Furthermore, this neuronal activation induced a reconsolidation-like reorganization process, or strengthening of the fear memory. Thus, our findings establish a direct link between the activation of specific ensemble of neurons in the lateral amygdala and the recall of fear memory and its subsequent modifications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
12 December 2013
In the version of this article initially published online, the P value in Figure 4a was given as **P < 0.01 instead of *P < 0.05 and the y axis of Figure 1e was labeled “Colocalized cells (%)” instead of “Normalized TRPV1.” The errors have been corrected for the print, PDF and HTML versions of this article.
References
Roediger, H.L., Dudai, Y. & Fitzpatrick, S.M. Science of Memory: Concepts (Oxford University Press, Oxford, 2007).
Guzowski, J.F., McNaughton, B.L., Barnes, C.A. & Worley, P.F. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat. Neurosci. 2, 1120–1124 (1999).
LeDoux, J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184 (2000).
Repa, J.C. et al. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat. Neurosci. 4, 724–731 (2001).
Rumpel, S., LeDoux, J., Zador, A. & Malinow, R. Postsynaptic receptor trafficking underlying a form of associative learning. Science 308, 83–88 (2005).
Reijmers, L.G., Perkins, B.L., Matsuo, N. & Mayford, M. Localization of a stable neural correlate of associative memory. Science 317, 1230–1233 (2007).
Han, J.H. et al. Neuronal competition and selection during memory formation. Science 316, 457–460 (2007).
Han, J.H. et al. Selective erasure of a fear memory. Science 323, 1492–1496 (2009).
Zhou, Y. et al. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat. Neurosci. 12, 1438–1443 (2009).
Dudai, Y. The restless engram: consolidations never end. Annu. Rev. Neurosci. 35, 227–247 (2012).
Misanin, J.R., Miller, R.R. & Lewis, D.J. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science 160, 554–555 (1968).
Nader, K., Schafe, G.E. & Le Doux, J.E. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406, 722–726 (2000).
Bunch, M.E.M. W.K. The retention in rats of incompletely learned maze solution for short intervals of time. J. Comp. Psychol. 16, 385–409 (1933).
Wilson, M.A. & McNaughton, B.L. Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679 (1994).
Buzsáki, G. The hippocampo-neocortical dialogue. Cereb. Cortex 6, 81–92 (1996).
Sara, S.J. Strengthening the shaky trace through retrieval. Nat. Rev. Neurosci. 1, 212–213 (2000).
Pickens, C.L., Golden, S.A., Adams-Deutsch, T., Nair, S.G. & Shaham, Y. Long-lasting incubation of conditioned fear in rats. Biol. Psychiatry 65, 881–886 (2009).
Inda, M.C., Muravieva, E.V. & Alberini, C.M. Memory retrieval and the passage of time: from reconsolidation and strengthening to extinction. J. Neurosci. 31, 1635–1643 (2011).
Zemelman, B.V., Nesnas, N., Lee, G.A. & Miesenbock, G. Photochemical gating of heterologous ion channels: remote control over genetically designated populations of neurons. Proc. Natl. Acad. Sci. USA 100, 1352–1357 (2003).
Shapiro, M.G., Frazier, S.J. & Lester, H.A. Unparalleled control of neural activity using orthogonal pharmacogenetics. ACS Chem. Neurosci. 3, 619–629 (2012).
Arenkiel, B.R., Klein, M.E., Davison, I.G., Katz, L.C. & Ehlers, M.D. Genetic control of neuronal activity in mice conditionally expressing TRPV1. Nat. Methods 5, 299–302 (2008).
Güler, A.D. et al. Transient activation of specific neurons in mice by selective expression of the capsaicin receptor. Nat. Commun. 3, 746 (2012).
Cavanaugh, D.J. et al. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J. Neurosci. 31, 5067–5077 (2011).
Frankland, P.W. et al. Consolidation of CS and US representations in associative fear conditioning. Hippocampus 14, 557–569 (2004).
Josselyn, S.A. et al. Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J. Neurosci. 21, 2404–2412 (2001).
Monfils, M.H., Cowansage, K.K., Klann, E. & LeDoux, J.E. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science 324, 951–955 (2009).
Esteban, J.A. et al. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat. Neurosci. 6, 136–143 (2003).
Hu, H. et al. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell 131, 160–173 (2007).
Lee, H.K. et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112, 631–643 (2003).
Oh, M.C., Derkach, V.A., Guire, E.S. & Soderling, T.R. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J. Biol. Chem. 281, 752–758 (2006).
Huff, M.L., Miller, R.L., Deisseroth, K., Moorman, D.E. & LaLumiere, R.T. Posttraining optogenetic manipulations of basolateral amygdala activity modulate consolidation of inhibitory avoidance memory in rats. Proc. Natl. Acad. Sci. USA 110, 3597–3602 (2013).
Cole, C.J. et al. MEF2 negatively regulates learning-induced structural plasticity and memory formation. Nat. Neurosci. 15, 1255–1264 (2012).
Liu, X. et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012).
Johansen, J.P. et al. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc. Natl. Acad. Sci. USA 107, 12692–12697 (2010).
Quirk, G.J., Repa, C. & LeDoux, J.E. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron 15, 1029–1039 (1995).
Quirk, G.J., Armony, J.L. & LeDoux, J.E. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron 19, 613–624 (1997).
Goosens, K.A., Hobin, J.A. & Maren, S. Auditory-evoked spike firing in the lateral amygdala and Pavlovian fear conditioning: mnemonic code or fear bias? Neuron 40, 1013–1022 (2003).
Carlezon, W.A. Jr., Nestler, E.J. & Neve, R.L. Herpes simplex virus–mediated gene transfer as a tool for neuropsychiatric research. Crit. Rev. Neurobiol. 14, 47–67 (2000).
Barrot, M. et al. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc. Natl. Acad. Sci. USA 99, 11435–11440 (2002).
Han, J.H. et al. Increasing CREB in the auditory thalamus enhances memory and generalization of auditory conditioned fear. Learn. Mem. 15, 443–453 (2008).
Olson, V.G. et al. Regulation of drug reward by cAMP response element-binding protein: evidence for two functionally distinct subregions of the ventral tegmental area. J. Neurosci. 25, 5553–5562 (2005).
Chao, J.R. et al. Characterization of the mouse adenylyl cyclase type VIII gene promoter: regulation by cAMP and CREB. Eur. J. Neurosci. 16, 1284–1294 (2002).
Neve, R.L., Neve, K.A., Nestler, E.J. & Carlezon, W.A. Jr. Use of herpes virus amplicon vectors to study brain disorders. Biotechniques 39, 381–391 (2005).
Paxinos, G. & Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates 3rd edn. (Academic Press, San Diego, 2007).
Acknowledgements
We thank all our laboratory members for critical discussions and helpful comments on this study and U. Oh (Seoul National University) for rat TRPV1 cDNA plasmid. This work was supported by a National Research Foundation of Korea grant funded by the Korean government (2011-0005755 and 2011-0013173) and the KAIST High Risk High Return Project. J.-H.H. was supported by a TJ Park Science Fellowship, J.K. was supported by the BK21 project of Korean Research Foundation, J.-T.K. was supported by the project of Global Ph.D. Fellowship that National Research Foundation of Korea conducts from 2011, and H.-S.K. was supported by the World Class Institute program of the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (WCI 2009-003).
Author information
Authors and Affiliations
Contributions
J.-H.H. designed and directed the study. J.K. and J.-H.H. designed the HSV vector and the imaging, western blot and memory reconsolidation experiments. S.A.J. and J.-H.H. designed the memory strengthening experiment. J.-T.K. and J.-H.H. designed the in vivo recording and memory recall experiments. J.K. performed HSV vector construction, virus packaging, imaging, western blot and the memory reconsolidation experiment. J.K. and H.-S.K. performed the memory strengthening experiment. J.-T.K. performed in vivo recording and the memory recall experiment. J.K. and J.-H.H. analyzed the imaging, western blot and memory reconsolidation experiments. J.-T.K. and J.-H.H. analyzed the in vivo recording and memory recall experiments. J.K., H.-S.K. and J.-H.H. analyzed the memory strengthening experiments. J.K., J.-T.K., H.-S.K., S.A.J. and J.-H.H. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
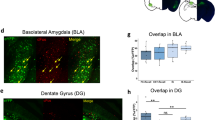
Supplementary Figure 1 HSV viral vector constructs infected similar percentage of LA neurons.
(a) Representative confocal images showing restricted expression of the transgene in the LA (outlined with a dotted line) by HSV-mediated gene transfer. GFP was used as an expression marker. Scale bar = 200 μm. (b) Histogram of cell counting result. The percentage number of cell expressing GFP in LA for each group is shown. HSV constructs used in this study infected similar percentages (approximately 15% to 20%) of cells in the LA with no significant differences among groups (n = 9, GFP; n = 11, CREB; n = 9, GFP-TR; n = 19, CREB-TR).
Supplementary Figure 2 Freezing was not induced by drug in mice trained with unpaired conditioning protocol.
Behavior procedure and histogram showing the percentage of freezing induced by drug. All mice were injected with CREB-TR vector. During training, shock (0.5 mA) was delivered first and 1 minute later tone was presented for 30 seconds. One group of mice was infused with capsaicin (n = 8) for drug-induced freezing test and the other group of mice infused with vehicle (n = 9) as a control.
Supplementary Figure 3 Activating a subset of random neurons in LA by drug did not induce freezing in mice with strong fear memory or elevated CREB.
(a) Mice in GFP-TR group (Fig. 3) were divided into two subgroups, Strong (>50% freezing, n = 3) and Weak (<50% freezing, n = 5), according to their freezing level in the auditory CS retention test. Although CS-induced freezing was much greater in the Strong group (82.4% ± 4.8%) than in the Weak group (27.5% ± 6.8%), mice in the Strong group did not exhibit significant drug-induced freezing and there was no significant difference in capsaicin-induced freezing between Strong (5.3% ± 2.6%) and Weak (5.0% ± 1.7%) groups. The freezing during 10–15 minutes period (Fig. 3b) was used for analysis. (b) Behavior procedure and histogram showing the percentage of freezing induced by drug (5-minute bins). In this experiment, GFP-TR vector was first injected and 2 days later CREB alone vector injected again within the LA but at slightly different site. During testing, one group of mice was administered capsaicin (n = 5) and the other group of mice vehicle (n = 4) as a control.
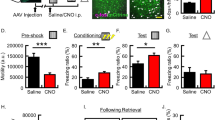
Supplementary Figure 4 Reconsolidation process was intact in viral vector injected mice.
(a) Behavior experimental procedure. Mice were tested before (Test 1) and 24 hours after (Test 2) anisomycin or vehicle infusion. During test session, an auditory CS was presented and CS-induced freezing was monitored. (b) Histograms showing behavioral results. Mice were injected with either CREB-TR (n = 12 for VEH and n = 7 for ANI) or GFP-TR (n = 4 for VEH and n = 8 for ANI) viral vectors and trained for auditory fear conditioning. **P > 0.01, ***P > 0.001.
Supplementary Figure 5 Anisomycin treatment after control vehicle injection instead of capsaicin had no amnesic effect on auditory fear memory.
(a) Behavior experimental procedure. Twenty-four hours after training, vehicle was administered, followed by anisomycin or vehicle treatment. (b) Histogram showing behavioral results. Mice were injected with either CREB-TR (n = 12 for VEH and n = 9 for ANI) or GFP-TR (n = 4 for VEH and n = 4 for ANI) viral vectors and trained for auditory fear conditioning.
Supplementary Figure 6 No differences in the expression level of tGluA1 and pGluA1 across different groups (CREB-TR, GFP-TR and Post CREB-TR) with no drug or control vehicle injection.
(a) Experimental procedures for western blot analysis. Top: CREB-TR and GFP-TR groups. Bottom: Post CREB-TR group. Quantification of tGluA1 and pGluA1 protein expression in LA of mice with no injection (b) or vehicle injection (d) by western blots. Representative blot images (b, d) and histogram (c, e) showing normalized level of tGluA1 and pGluA1 protein expression (right) are shown. For the comparison, the expression level of tGluA1 and pGluA1 protein was normalized to control β-actin (n = 5 for all conditions). Full immunoblots are shown in Supplementary Figure 8.
Supplementary Figure 7 Fear memory was strengthened naturally by multiple reactivations of memory trace by CS under the conditions parallel to drug-induced reactivations.
(a) Behavior experimental procedure. (b) Histogram showing behavior results. The percentage of freezing before (pre CS) and after (CS) tone presentation during testing is shown. Mice were injected with CREB-TR viral vector and trained for auditory fear conditioning with relatively weak shock (0.4 mA). One group of mice (n = 7, 3 CS) were exposed to 10-minute CS tone at the homecage once per day over 3 days for memory reactivations. In control group (n = 10, No CS), all procedures were same as except no CS was presented during reactivation period. Baseline freezing (pre CS) was determined by measuring freezing for 2 minutes right before tone CS presentation. *P > 0.05.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 721 kb)
Rights and permissions
About this article
Cite this article
Kim, J., Kwon, JT., Kim, HS. et al. Memory recall and modifications by activating neurons with elevated CREB. Nat Neurosci 17, 65–72 (2014). https://doi.org/10.1038/nn.3592
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3592
This article is cited by
-
Engram neurons: Encoding, consolidation, retrieval, and forgetting of memory
Molecular Psychiatry (2023)
-
Formation and fate of an engram in the lateral amygdala supporting a rewarding memory in mice
Neuropsychopharmacology (2023)
-
Optogenetic inhibition of the dorsal hippocampus CA3 region during early-stage cocaine-memory reconsolidation disrupts subsequent context-induced cocaine seeking in rats
Neuropsychopharmacology (2022)
-
Chronic administration of Tat-GluR23Y ameliorates cognitive dysfunction targeting CREB signaling in rats with amyloid beta neurotoxicity
Metabolic Brain Disease (2021)
-
Cortico-amygdala interaction determines the insular cortical neurons involved in taste memory retrieval
Molecular Brain (2020)