Abstract
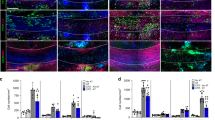
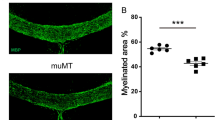
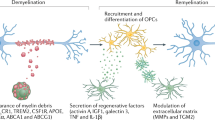
The lack of therapies for progressive multiple sclerosis highlights the need to understand the regenerative process of remyelination that can follow CNS demyelination. This involves an innate immune response consisting of microglia and macrophages, which can be polarized to distinct functional phenotypes: pro-inflammatory (M1) and anti-inflammatory or immunoregulatory (M2). We found that a switch from an M1- to an M2-dominant response occurred in microglia and peripherally derived macrophages as remyelination started. Oligodendrocyte differentiation was enhanced in vitro with M2 cell conditioned media and impaired in vivo following intra-lesional M2 cell depletion. M2 cell densities were increased in lesions of aged mice in which remyelination was enhanced by parabiotic coupling to a younger mouse and in multiple sclerosis lesions that normally show remyelination. Blocking M2 cell–derived activin-A inhibited oligodendrocyte differentiation during remyelination in cerebellar slice cultures. Thus, our results indicate that M2 cell polarization is essential for efficient remyelination and identify activin-A as a therapeutic target for CNS regeneration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Banati, R.B., Gehrmann, J., Schubert, P. & Kreutzberg, G.W. Cytotoxicity of microglia. Glia 7, 111–118 (1993).
Cash, E., Zhang, Y. & Rott, O. Microglia present myelin antigens to T cells after phagocytosis of oligodendrocytes. Cell. Immunol. 147, 129–138 (1993).
Kotter, M.R., Li, W.W., Zhao, C. & Franklin, R.J.M. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J. Neurosci. 26, 328–332 (2006).
Ruckh, J.M. et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 10, 96–103 (2012).
Kotter, M.R., Zhao, C., van, R.N. & Franklin, R.J.M. Macrophage-depletion induced impairment of experimental CNS remyelination is associated with a reduced oligodendrocyte progenitor cell response and altered growth factor expression. Neurobiol. Dis. 18, 166–175 (2005).
Ruffell, D. et al. A CREB-C/EBPbeta cascade induces M2 macrophage–specific gene expression and promotes muscle injury repair. Proc. Natl. Acad. Sci. USA 106, 17475–17480 (2009).
Dayan, V. et al. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res. Cardiol. 106, 1299–1310 (2011).
Deonarine, K. et al. Gene expression profiling of cutaneous wound healing. J. Transl. Med. 5, 11 (2007).
Edwards, J.P., Zhang, X., Frauwirth, K.A. & Mosser, D.M. Biochemical and functional characterization of three activated macrophage populations. J. Leukoc. Biol. 80, 1298–1307 (2006).
Gensert, J.M. & Goldman, J.E. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron 19, 197–203 (1997).
Nait-Oumesmar, B. et al. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur. J. Neurosci. 11, 4357–4366 (1999).
Arnett, H.A. et al. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science 306, 2111–2115 (2004).
Etxeberria, A., Mangin, J.M., Aguirre, A. & Gallo, V. Adult-born SVZ progenitors receive transient synapses during remyelination in corpus callosum. Nat. Neurosci. 13, 287–289 (2010).
Woodruff, R.H. & Franklin, R.J.M. The expression of myelin protein mRNAs during remyelination of lysolecithin-induced demyelination. Neuropathol. Appl. Neurobiol. 25, 226–235 (1999).
Ajami, B., Bennett, J.L., Krieger, C., McNagny, K.M. & Rossi, F.M. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 14, 1142–1149 (2011).
Voss, E.V. et al. Characterisation of microglia during de- and remyelination: can they create a repair promoting environment? Neurobiol. Dis. 45, 519–528 (2012).
Saederup, N. et al. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS ONE 5, e13693 (2010).
Huang, J.K. et al. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat. Neurosci. 14, 45–53 (2011).
Pendino, K.J., Meidhof, T.M., Heck, D.E., Laskin, J.D. & Laskin, D.L. Inhibition of macrophages with gadolinium chloride abrogates ozone-induced pulmonary injury and inflammatory mediator production. Am. J. Respir. Cell Mol. Biol. 13, 125–132 (1995).
Abdel-Zaher, A.O., Abdel-Rahman, M.M., Hafez, M.M. & Omran, F.M. Role of nitric oxide and reduced glutathione in the protective effects of aminoguanidine, gadolinium chloride and oleanolic acid against acetaminophen-induced hepatic and renal damage. Toxicology 234, 124–134 (2007).
Mizgerd, J.P., Molina, R.M., Stearns, R.C., Brain, J.D. & Warner, A.E. Gadolinium induces macrophage apoptosis. J. Leukoc. Biol. 59, 189–195 (1996).
Hardonk, M.J., Dijkhuis, F.W., Hulstaert, C.E. & Koudstaal, J. Heterogeneity of rat liver and spleen macrophages in gadolinium chloride-induced elimination and repopulation. J. Leukoc. Biol. 52, 296–302 (1992).
Marín-Teva, J.L. et al. Microglia promote the death of developing Purkinje cells. Neuron 41, 535–547 (2004).
Serrats, J. et al. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron 65, 94–106 (2010).
Das, J. et al. A critical role for NF-kappa B in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nat. Immunol. 2, 45–50 (2001).
Jang, E. et al. Secreted protein lipocalin-2 promotes microglial M1 polarization. FASEB J. 27, 1176–1190 (2012).
Fancy, S.P. et al. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat. Neurosci. 14, 1009–1016 (2011).
Yuen, T.J. et al. Identification of endothelin 2 as an inflammatory factor that promotes central nervous system remyelination. Brain 136, 1035–1047 (2013).
He, J.T. et al. Neuroprotective effects of exogenous activin A on oxygen-glucose deprivation in PC12 cells. Molecules 17, 315–327 (2012).
Sakai, T. & Xu, Y. Stem cells decreased neuronal cell death after hypoxic stress in primary fetal rat neurons in vitro. Cell Transplant. 21, 355–364 (2012).
de Kretser, D.M., O'Hehir, R.E., Hardy, C.L. & Hedger, M.P. The roles of activin A and its binding protein, follistatin, in inflammation and tissue repair. Mol. Cell Endocrinol. 359, 101–106 (2012).
Ahn, M. et al. Immunohistochemical study of arginase-1 in the spinal cords of Lewis rats with experimental autoimmune encephalomyelitis. Brain Res. 1453, 77–86 (2012).
Attisano, L., Wrana, J.L., Montalvo, E. & Massague, J. Activation of signaling by the activin receptor complex. Mol. Cell Biol. 16, 1066–1073 (1996).
Matzuk, M.M., Kumar, T.R. & Bradley, A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature 374, 356–360 (1995).
Matzuk, M.M. Functional analysis of mammalian members of the transforming growth factor-beta superfamily. Trends Endocrinol. Metab. 6, 120–127 (1995).
Mikita, J. et al. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Mult. Scler. 17, 2–15 (2011).
Olah, M. et al. Identification of a microglia phenotype supportive of remyelination. Glia 60, 306–321 (2012).
Liang, X., Draghi, N.A. & Resh, M.D. Signaling from integrins to Fyn to Rho family GTPases regulates morphologic differentiation of oligodendrocytes. J. Neurosci. 24, 7140–7149 (2004).
Thurnherr, T. et al. Cdc42 and Rac1 signaling are both required for and act synergistically in the correct formation of myelin sheaths in the CNS. J. Neurosci. 26, 10110–10119 (2006).
Flores, A.I. et al. Constitutively active Akt induces enhanced myelination in the CNS. J. Neurosci. 28, 7174–7183 (2008).
Guardiola-Diaz, H.M., Ishii, A. & Bansal, R. Erk1/2 MAPK and mTOR signaling sequentially regulates progression through distinct stages of oligodendrocyte differentiation. Glia 60, 476–486 (2012).
Diemel, L.T., Jackson, S.J. & Cuzner, M.L. Role for TGF-beta1, FGF-2 and PDGF-AA in a myelination of CNS aggregate cultures enriched with macrophages. J. Neurosci. Res. 74, 858–867 (2003).
McKinnon, R.D., Piras, G., Ida, J.A. Jr. & Dubois-Dalcq, M. A role for TGF-beta in oligodendrocyte differentiation. J. Cell Biol. 121, 1397–1407 (1993).
Kigerl, K.A. et al. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 29, 13435–13444 (2009).
Liao, B., Zhao, W., Beers, D.R., Henkel, J.S. & Appel, S.H. Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS. Exp. Neurol. 237, 147–152 (2012).
Kuhlmann, T. et al. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 131, 1749–1758 (2008).
Schaier, M. et al. Role of FTY720 on M1 and M2 macrophages, lymphocytes, and chemokines in 5/6 nephrectomized rats. Am. J. Physiol. Renal Physiol. 297, F769–F780 (2009).
Weber, M.S. et al. Type II monocytes modulate T cell–mediated central nervous system autoimmune disease. Nat. Med. 13, 935–943 (2007).
Acknowledgements
We thank the UK Multiple Sclerosis Tissue Bank for providing human brain tissue, F. Roncaroli (Imperial College London) for neuropathological diagnosis of lesions and R. Nicholas (Imperial College London) for providing clinical history of patients. We also thank W. Mungall, J. Huang, M. Harrisingh, A. Jarjour, M. Bechler, M. Swire, A.-C. Nunes-Fonseca, D. Morrison, and C. Watkins for technical assistance. This work was funded by the UK Multiple Sclerosis Society (R.J.M.F. and C.ff.-C.) and the Wellcome Trust (A.W. and C.ff.-C.), and V.E.M. holds a post-doctoral fellowship from the Multiple Sclerosis Society of Canada.
Author information
Authors and Affiliations
Contributions
V.E.M. conceived the project, designed and carried out the experiments, performed data acquisition, quantification and analysis, and wrote the manuscript. A.B. and A.W. assisted in in vivo studies and A.W. assisted in data interpretation. J.-W.Z. contributed to analysis and quantification of parabiosis lesion tissue. A.B., A.W., T.J.Y. and P.v.W. performed lesioning experiments to provide lesion tissue and assisted in tissue selection. J.M.R., J.L.S., A.J.W. and R.J.M.F. provided parabiosis lesion tissue. R.J.M.F. assisted in study design, data interpretation and manuscript writing. C.ff.-C. supervised the project, assisted in study design, data interpretation, figure preparation and writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
V.E.M., R.J.M.F. and C.f-C have submitted a patent application pertaining to the use of activin/activin signalling for oligodendrocyte differentiation and/or remyelination.
Supplementary information
Supplementary Figures and Text
Supplementary Figures 1–9 and Supplementary Table 1 (PDF 7360 kb)
Rights and permissions
About this article
Cite this article
Miron, V., Boyd, A., Zhao, JW. et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci 16, 1211–1218 (2013). https://doi.org/10.1038/nn.3469
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3469
This article is cited by
-
Mitochondrial and metabolic dysfunction of peripheral immune cells in multiple sclerosis
Journal of Neuroinflammation (2024)
-
Myeloid cell-associated aromatic amino acid metabolism facilitates CNS myelin regeneration
npj Regenerative Medicine (2024)
-
Hexahydrocurcumin attenuated demyelination and improved cognitive impairment in chronic cerebral hypoperfusion rats
Inflammopharmacology (2024)
-
Aerobic Glycolysis Induced by mTOR/HIF-1α Promotes Early Brain Injury After Subarachnoid Hemorrhage via Activating M1 Microglia
Translational Stroke Research (2024)
-
Regulation of Microglial Activation by Wnt/β-Catenin Signaling After Global Cerebral Ischemia in Mice
Molecular Neurobiology (2024)