Abstract
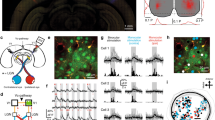
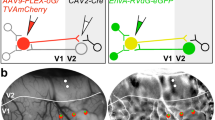
Neurons in primary sensory cortex have diverse response properties, whereas higher cortical areas are specialized. Specific connectivity may be important for areal specialization, particularly in the mouse, where neighboring neurons are functionally diverse. To examine whether higher visual areas receive functionally specific input from primary visual cortex (V1), we used two-photon calcium imaging to measure responses of axons from V1 arborizing in three areas with distinct spatial and temporal frequency preferences. We found that visual preferences of presynaptic boutons in each area were distinct and matched the average preferences of recipient neurons. This specificity could not be explained by organization within V1 and instead was due to both a greater density and greater response amplitude of functionally matched boutons. Projections from a single layer (layer 5) and from secondary visual cortex were also matched to their target areas. Thus, transmission of specific information to downstream targets may be a general feature of cortico-cortical communication.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Dräger, U.C. Receptive fields of single cells and topography in mouse visual cortex. J. Comp. Neurol. 160, 269–290 (1975).
Niell, C.M. & Stryker, M.P. Highly selective receptive fields in mouse visual cortex. J. Neurosci. 28, 7520–7536 (2008).
Gao, E., DeAngelis, G.C. & Burkhalter, A. Parallel input channels to mouse primary visual cortex. J. Neurosci. 30, 5912–5926 (2010).
Andermann, M.L., Kerlin, A.M., Roumis, D.K., Glickfeld, L.L. & Reid, R.C. Functional specialization of mouse higher visual cortical areas. Neuron 72, 1025–1039 (2011).
Marshel, J.H., Garrett, M.E., Nauhaus, I. & Callaway, E.M. Functional specialization of seven mouse visual cortical areas. Neuron 72, 1040–1054 (2011).
Roth, M.M., Helmchen, F. & Kampa, B.M. Distinct functional properties of primary and posteromedial visual area of mouse neocortex. J. Neurosci. 32, 9716–9726 (2012).
Ohki, K., Chung, S., Ch'ng, Y.H., Kara, P. & Reid, R.C. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 433, 597–603 (2005).
Bonin, V., Histed, M.H., Yurgenson, S. & Reid, R.C. Local diversity and fine-scale organization of receptive fields in mouse visual cortex. J. Neurosci. 31, 18506–18521 (2011).
Ohki, K. & Reid, R.C. Specificity and randomness in the visual cortex. Curr. Opin. Neurobiol. 17, 401–407 (2007).
Ko, H. et al. Functional specificity of local synaptic connections in neocortical networks. Nature 473, 87–91 (2011).
Livingstone, M.S. & Hubel, D.H. Specificity of cortico-cortical connections in monkey visual system. Nature 304, 531–534 (1983).
DeYoe, E.A. & Van Essen, D.C. Segregation of efferent connections and receptive field properties in visual area V2 of the macaque. Nature 317, 58–61 (1985).
Livingstone, M.S. & Hubel, D.H. Connections cytochrome between layer 4B of area 17 and the thick oxidase stripes of area 18 in the squirrel monkey. J. Neurosci. 7, 3371–3377 (1987).
Shipp, S. & Zeki, S. The organization of connections between areas V5 and V2 in macaque monkey visual cortex. Eur. J. Neurosci. 1, 333–354 (1989).
Sincich, L.C. & Horton, J.C. Independent projection streams from macaque striate cortex to the second visual area and middle temporal area. J. Neurosci. 23, 5684–5692 (2003).
Nassi, J.J. & Callaway, E.M. Specialized circuits from primary visual cortex to V2 and area MT. Neuron 55, 799–808 (2007).
Nassi, J.J. & Callaway, E.M. Parallel processing strategies of the primate visual system. Nat. Rev. Neurosci. 10, 360–372 (2009).
Johnson, R.R. & Burkhalter, A. A polysynaptic feedback circuit in rat visual cortex. J. Neurosci. 17, 7129–7140 (1997).
Movshon, J.A. & Newsome, W.T. Visual response properties of striate cortical neurons projecting to area MT in macaque monkeys. J. Neurosci. 16, 7733–7741 (1996).
Ferraina, S., Paré, M. & Wurtz, R.H. Comparison of cortico-cortical and cortico-collicular signals for the generation of saccadic eye movements. J. Neurophysiol. 87, 845–858 (2002).
Sato, T.R. & Svoboda, K. The functional properties of barrel cortex neurons projecting to the primary motor cortex. J. Neurosci. 30, 4256–4260 (2010).
Jarosiewicz, B., Schummers, J., Malik, W.Q., Brown, E.N. & Sur, M. Functional biases in visual cortex neurons with identified projections to higher cortical targets. Curr. Biol. 22, 269–277 (2012).
Wang, Q. & Burkhalter, A. Area map of mouse visual cortex. J. Comp. Neurol. 502, 339–357 (2007).
Wang, Q., Gao, E. & Burkhalter, A. Gateways of ventral and dorsal streams in mouse visual cortex. J. Neurosci. 31, 1905–1918 (2011).
Van den Bergh, G., Zhang, B., Arckens, L. & Chino, Y.M. Receptive-field properties of V1 and V2 neurons in mice and macaque monkeys. J. Comp. Neurol. 518, 2051–2070 (2010).
Coogan, T.A. & Burkhalter, A. Hierarchical organization of areas in rat visual cortex. J. Neurosci. 13, 3749–3772 (1993).
Brenowitz, S.D. & Regehr, W.G. Reliability and heterogeneity of calcium signaling at single presynaptic boutons of cerebellar granule cells. J. Neurosci. 27, 7888–7898 (2007).
Dreosti, E., Odermatt, B., Dorostkar, M.M. & Lagnado, L. A genetically encoded reporter of synaptic activity in vivo. Nat. Methods 6, 883–889 (2009).
Petreanu, L. et al. Activity in motor-sensory projections reveals distributed coding in somatosensation. Nature 489, 299–303 (2012).
Nikolaou, N. et al. Parametric functional maps of visual inputs to the tectum. Neuron 76, 317–324 (2012).
Churchland, A.K. & Lisberger, S.G. Discharge properties of MST neurons that project to the frontal pursuit area in macaque monkeys. J. Neurophysiol. 94, 1084–1090 (2005).
Swadlow, H.A. & Weyand, T.G. Efferent systems of the rabbit visual cortex: laminar distribution of the cells of origin, axonal conduction velocities, and identification of axonal branches. J. Comp. Neurol. 203, 799–822 (1981).
Swadlow, H.A. Neocortical efferent neurons with very slowly conducting axons: strategies for reliable antidromic identification. J. Neurosci. Methods 79, 131–141 (1998).
Berezovskii, V.K., Nassi, J.J. & Born, R.T. Segregation of feedforward and feedback projections in mouse visual cortex. J. Comp. Neurol. 519, 3672–3683 (2011).
Simmons, P.A., Lemmon, V. & Pearlman, A.L. Afferent and efferent connections of the striate and extrastriate visual cortex of the normal and reeler mouse. J. Comp. Neurol. 211, 295–308 (1982).
Jia, H., Rochefort, N.L., Chen, X. & Konnerth, A. Dendritic organization of sensory input to cortical neurons in vivo. Nature 464, 1307–1312 (2010).
Dong, H., Wang, Q., Valkova, K., Gonchar, Y. & Burkhalter, A. Experience-dependent development of feedforward and feedback circuits between lower and higher areas of mouse visual cortex. Vision Res. 44, 3389–3400 (2004).
Molyneaux, B.J. et al. Novel subtype-specific genes identify distinct subpopulations of callosal projection neurons. J. Neurosci. 29, 12343–12354 (2009).
Hata, Y., Tsumoto, T. & Stryker, M.P. Selective pruning of more active afferents when cat visual cortex is pharmacologically inhibited. Neuron 22, 375–381 (1999).
Brown, S.P. & Hestrin, S. Intracortical circuits of pyramidal neurons reflect their long-range axonal targets. Nature 457, 1133–1136 (2009).
Morishima, M. & Kawaguchi, Y. Recurrent connection patterns of corticostriatal pyramidal cells in frontal cortex. J. Neurosci. 26, 4394–4405 (2006).
Yoshimura, Y., Dantzker, J. & Callaway, E.M. Excitatory cortical neurons form fine-scale functional networks. Nature 433, 868–873 (2005).
Bosking, W.H., Zhang, Y., Schofield, B. & Fitzpatrick, D. Orientation selectivity and the arrangement of horizontal connections in tree shrew striate cortex. J. Neurosci. 17, 2112–2127 (1997).
Allen, C.B., Celikel, T. & Feldman, D.E. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat. Neurosci. 6, 291–299 (2003).
Zhao, C., Dreosti, E. & Lagnado, L. Homeostatic synaptic plasticity through changes in presynaptic calcium influx. J. Neurosci. 31, 7492–7496 (2011).
Hofer, S.B. et al. Differential connectivity and response dynamics of excitatory and inhibitory neurons in visual cortex. Nat. Neurosci. 14, 1045–1052 (2011).
Bock, D.D. et al. Network anatomy and in vivo physiology of visual cortical neurons. Nature 471, 177–182 (2011).
Niell, C.M. & Stryker, M.P. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479 (2010).
Wang, Q., Sporns, O. & Burkhalter, A. Network analysis of corticocortical connections reveals ventral and dorsal processing streams in mouse visual cortex. J. Neurosci. 32, 4386–4399 (2012).
Felleman, D.J. & Van Essen, D.C. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1, 1–47 (1991).
Gong, S. et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J. Neurosci. 27, 9817–9823 (2007).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Tian, L. et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 6, 875–881 (2009).
Husson, T.R., Mallik, A.K., Zhang, J.X. & Issa, N.P. Functional imaging of primary visual cortex using flavoprotein autofluorescence. J. Neurosci. 27, 8665–8675 (2007).
Braitenberg, V. & Schüz, A. Anatomy of the Cortex: Statistics and Geometry (Springer, 1991).
Kerlin, A.M., Andermann, M.L., Berezovskii, V.K. & Reid, R.C. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron 67, 858–871 (2010).
Acknowledgements
We thank A. Caiado, G. Goldey, M. Kirk, C. Mazur and D. Roumis for surgical assistance; J. Curry, A. Moffa and W. Wray for behavioral habituation of mice; S. Yurgenson for technical contributions to visual stimulation and eye-tracking software; and A. Vagodny for technical assistance. We also thank V. Berezovskii, R. Born, S. Chatterjee, M. Histed, C. Hull, A. Kerlin, W. Lee, J. Maunsell and W. Regehr for advice and discussion at all stages of the project. This work was supported by the US National Institutes of Health (R01 EY018742 and R01 EY010115) and by fellowships from the Helen Hay Whitney Foundation (L.L.G. and M.L.A.) and the Ludcke Foundation and Pierce Charitable Trust (M.L.A.).
Author information
Authors and Affiliations
Contributions
L.L.G. performed the experiments. L.L.G., M.L.A. and V.B. analyzed the data. V.B. built the experimental setup. L.L.G., M.L.A., V.B. and R.C.R. designed the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 1839 kb)
Supplementary Movie 1
Two-photon imaging of calcium transients in axonal projections. Changes in fluorescence (dF/F at 10× speed) in response to the presentation of visual stimuli (inset, 0.5× speed) for 10 individual trials. This is from the same experiment as shown in Figure 1c,d; field of view is in area PM approximately 90 μm below the surface. Range of dF/F: −0.5 to 2.5. (AVI 14409 kb)
Supplementary Movie 2
Average time course in response to visual stimuli. Time course of changes in fluorescence (dF/F at 5× speed) in response to the presentation of 9 different visual stimuli (inset, 0.5× speed) spaced at 2 octaves (1, 4 and 15 Hz at 0.02, 0.08 and 0.32 c.p.d.). The response to each stimulus is the average of 24 presentations. Same experiment as shown in Figure 1c,d. Range of dF/F: −0.15 to 0.8. (AVI 12969 kb)
Rights and permissions
About this article
Cite this article
Glickfeld, L., Andermann, M., Bonin, V. et al. Cortico-cortical projections in mouse visual cortex are functionally target specific. Nat Neurosci 16, 219–226 (2013). https://doi.org/10.1038/nn.3300
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3300
This article is cited by
-
Polymer Physics-Based Classification of Neurons
Neuroinformatics (2023)
-
Diversity of spatiotemporal coding reveals specialized visual processing streams in the mouse cortex
Nature Communications (2022)
-
Visual evoked feedforward–feedback traveling waves organize neural activity across the cortical hierarchy in mice
Nature Communications (2022)
-
Existing function in primary visual cortex is not perturbed by new skill acquisition of a non-matched sensory task
Nature Communications (2022)
-
Anatomical and functional connectomes underlying hierarchical visual processing in mouse visual system
Brain Structure and Function (2022)