Abstract
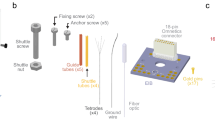
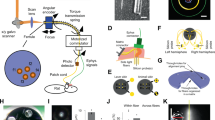
Recent advances in optogenetics have improved the precision with which defined circuit elements can be controlled optically in freely moving mammals; in particular, recombinase-dependent opsin viruses, used with a growing pool of transgenic mice expressing recombinases, allow manipulation of specific cell types. However, although optogenetic control has allowed neural circuits to be manipulated in increasingly powerful ways, combining optogenetic stimulation with simultaneous multichannel electrophysiological readout of isolated units in freely moving mice remains a challenge. We designed and validated the optetrode, a device that allows for colocalized multi-tetrode electrophysiological recording and optical stimulation in freely moving mice. Optetrode manufacture employs a unique optical fiber-centric coaxial design approach that yields a lightweight (2 g), compact and robust device that is suitable for behaving mice. This low-cost device is easy to construct (2.5 h to build without specialized equipment). We found that the drive design produced stable high-quality recordings and continued to do so for at least 6 weeks following implantation. We validated the optetrode by quantifying, for the first time, the response of cells in the medial prefrontal cortex to local optical excitation and inhibition, probing multiple different genetically defined classes of cells in the mouse during open field exploration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
11 December 2011
In the version of this article initially published online, the middle initial of author Lisa A. Gunaydin was missing. The error has been corrected for the print, PDF and HTML versions of this article.
References
Nestler, E.J. & Hyman, S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 13, 1161–1169 (2010).
Costa, R.M. et al. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature 415, 526–530 (2002).
Leypold, B.G. et al. Altered sexual and social behaviors in trp2 mutant mice. Proc. Natl. Acad. Sci. USA 99, 6376–6381 (2002).
Boyden, E.S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Zhang, F. et al. Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–639 (2007).
Petreanu, L., Huber, D., Sobczyk, A. & Svoboda, K. Channelrhodopsin-2–assisted circuit mapping of long-range callosal projections. Nat. Neurosci. 10, 663–668 (2007).
Adamantidis, A.R., Zhang, F., Aravanis, A.M., Deisseroth, K. & de Lecea, L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 450, 420–424 (2007).
Arenkiel, B.R. et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 54, 205–218 (2007).
Huang, Z.J., Yu, W., Lovett, C. & Tonegawa, S. Cre/loxP recombination-activated neuronal markers in mouse neocortex and hippocampus. Genesis 32, 209–217 (2002).
Lindeberg, J. et al. Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis 40, 67–73 (2004).
Gong, S. et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J. Neurosci. 27, 9817–9823 (2007).
Young, P. et al. Single-neuron labeling with inducible Cre-mediated knockout in transgenic mice. Nat. Neurosci. 11, 721–728 (2008).
Tsai, H.C. et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324, 1080–1084 (2009).
Sohal, V.S., Zhang, F., Yizhar, O. & Deisseroth, K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702 (2009).
Gradinaru, V. et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141, 154–165 (2010).
Gradinaru, V. et al. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J. Neurosci. 27, 14231–14238 (2007).
Brown, E.N., Purdon, P.L. & Van Dort, C.J. General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu. Rev. Neurosci. 34, 601–628 (2011).
McNaughton, B.L., O'Keefe, J. & Barnes, C.A. The stereotrode: a new technique for simultaneous isolation of several single units in the central nervous system from multiple unit records. J. Neurosci. Methods 8, 391–397 (1983).
Wilson, M.A. & McNaughton, B.L. Dynamics of the hippocampal ensemble code for space. Science 261, 1055–1058 (1993).
Gray, C.M., Maldonado, P.E., Wilson, M. & McNaughton, B.L.J. Tetrodes markedly improve the reliability and yield of multiple single-unit isolation from multi-unit recordings in cat striate cortex. J. Neurosci. Methods 63, 43–54 (1995).
Jog, M.S. et al. Tetrode technology: advances in implantable hardware, neuroimaging, and data analysis techniques. J. Neurosci. Methods 117, 141–152 (2002).
Nakazawa, K. et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 297, 211–218 (2002).
Wiltgen, B.J., Brown, R.A., Talton, L.E. & Silva, A.J. New circuits for old memories: the role of the neocortex in consolidation. Neuron 44, 101–108 (2004).
Chawla, M.K. & Barnes, C.A. Hippocampal granule cells in normal aging: insights from electrophysiological and functional imaging experiments. Prog. Brain Res. 163, 661–678 (2007).
Gerrard, J.L., Burke, S.N., McNaughton, B.L. & Barnes, C.A. Sequence reactivation in the hippocampus is impaired in aged rats. J. Neurosci. 28, 7883–7890 (2008).
Scanziani, M. & Häusser, M. Electrophysiology in the age of light. Nature 461, 930–939 (2009).
McHugh, T.J., Blum, K.I., Tsien, J.Z., Tonegawa, S. & Wilson, M.A. Impaired hippocampal representation of space in CA1-specific NMDAR1 knockout mice. Cell 87, 1339–1349 (1996).
Jeantet, Y. & Cho, Y.H. Design of a twin tetrode microdrive and headstage for hippocampal single unit recordings in behaving mice. J. Neurosci. Methods 129, 129–134 (2003).
Royer, S. et al. Multi-array silicon probes with integrated optical fibers: light-assisted perturbation and recording of local neural circuits in the behaving animal. Eur. J. Neurosci. 31, 2279–2291 (2010).
Schmitzer-Tobert, N., Jackson, J., Henze, D., Harris, K. & Redish, A.D. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience 131, 1–11 (2005).
Aravanis, A.M. et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng. 4, S143–S156 (2007).
Diester, I. et al. An optogenetic toolbox designed for primates. Nat. Neurosci. 14, 387–397 (2011).
Benabid, A.L., Chabardes, S., Mitrofanis, J. & Pollak, P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson's disease. Lancet Neurol. 8, 67–81 (2009).
Ponce, F.A. & Lozano, A.M. Deep brain stimulation state of the art and novel stimulation targets. Prog. Brain Res. 184, 311–324 (2010).
Mayberg, H.S. Targeted electrode-based modulation of neural circuits for depression. J. Clin. Invest. 119, 717–725 (2009).
Histed, M.H., Bonin, V. & Reid, R.C. Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron 63, 508–522 (2009).
Thiel, G., Greengard, P. & Südhof, T.C. Characterization of tissue-specific transcription by the human synapsin I gene promoter. Proc. Natl. Acad. Sci. USA 88, 3431–3435 (1991).
Hippenmeyer, S. et al. A Developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 3, e159 (2005).
Britton, D.R. & Britton, K.T. A sensitive open field measure of anxiolytic drug activity. Pharmacol. Biochem. Behav. 15, 577–582 (1981).
Gradinaru, V., Mogri, M., Thompson, K.R., Henderson, J.M. & Deisseroth, K. Optical deconstruction of parkinsonian neural circuitry. Science 324, 354–359 (2009).
Bacon, S.J., Headlam, A.J., Gabbott, P.L. & Smith, A.D. Amygdala input to medial prefrontal cortex (mPFC) in the rat: a light and electron microscope study. Brain Res. 720, 211–219 (1996).
Yizhar, O. et al. Neocortical excitation-inhibition balance in information processing and social dysfunction. Nature 477, 171–178 (2011).
Halassa, M.M. et al. Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nat. Neurosci. 14, 1118–1120 (2011).
Acknowledgements
We thank S. Arber for the PV::Cre transgenic mouse line. P.A. thanks D.G. Walker for advice on mechanical design. P.A. was supported by a Dean's fellowship from Stanford University School of Medicine, I.W. was supported by the Helen Hay Whitney Foundation, I.G. was supported by a Machiah fellowship and the Weizmann Institute Women in Science award, L.G. was supported by a National Science Foundation Integrative Graduate Education and Research Traineeship Award, and L.A.G. was supported by a BioX fellowship from Stanford University. L.M.F. and K.D. received support from a GO grant from the National Institute of Neurological Disorders and Stroke. Full funding information for K.D. is listed at http://www.stanford.edu/group/dlab/optogenetics/funding/ and includes the Gatsby Charitable Foundation, the Defense Advanced Research Projects Agency Reorganization and Plasticity to Accelerate Injury Recovery Program, the California Institute for Regenerative Medicine, the McKnight Foundation, the National Institute of Mental Health, and the National Institute on Drug Abuse.
Author information
Authors and Affiliations
Contributions
P.A., A.S.A. and K.D. designed the experiments and analyzed the data. P.A. devised and designed the device. P.A. and A.S.A. collected and analyzed electrophysiological and behavioral data. P.A., A.S.A. and I.G. performed immunohistochemical processing and confocal imaging. M.W. and I.W. aided in the development of the device and surgical procedure. M.W. and L.M.F. aided with neuronal sorting procedures. L.G. aided in statistical analysis. L.A.G. contributed data regarding defined-projection manipulation. L.M.F. aided the analysis of the electrophysiological data. K.D. supervised all aspects of the work. P.A., A.S.A. and K.D. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Note (PDF 2190 kb)
Rights and permissions
About this article
Cite this article
Anikeeva, P., Andalman, A., Witten, I. et al. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat Neurosci 15, 163–170 (2012). https://doi.org/10.1038/nn.2992
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2992
This article is cited by
-
An optrode array for spatiotemporally-precise large-scale optogenetic stimulation of deep cortical layers in non-human primates
Communications Biology (2024)
-
Neuronal dynamics direct cerebrospinal fluid perfusion and brain clearance
Nature (2024)
-
Fully bioresorbable hybrid opto-electronic neural implant system for simultaneous electrophysiological recording and optogenetic stimulation
Nature Communications (2024)
-
Design and validation of a low-cost photomodulator for in vivo photoactivation of a mGluR5 inhibitor
Biomedical Engineering Letters (2024)
-
Polydimethylsiloxane as a more biocompatible alternative to glass in optogenetics
Scientific Reports (2023)