Abstract
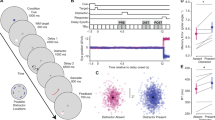
Selective attention filters information to limit what is encoded and maintained in working memory. Although the prefrontal cortex (PFC) is central to both selective attention and working memory, the underlying neural processes that link these cognitive abilities remain elusive. Using functional magnetic resonance imaging to guide repetitive transcranial magnetic stimulation with electroencephalographic recordings in humans, we perturbed PFC function at the inferior frontal junction in participants before they performed a selective-attention, delayed-recognition task. This resulted in diminished top-down modulation of activity in posterior cortex during early encoding stages, which predicted a subsequent decrement in working memory accuracy. Participants with stronger fronto-posterior functional connectivity displayed greater disruptive effects. Our data further suggests that broad alpha-band (7–14 Hz) phase coherence subserved this long-distance top-down modulation. These results suggest that top-down modulation mediated by the prefrontal cortex is a causal link between early attentional processes and subsequent memory performance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Posner, M.I. Orienting of attention. Q. J. Exp. Psychol. 32, 3–25 (1980).
Baddeley, A. Working Memory (Oxford University Press, Oxford, 1986).
Duncan, J. & Humphreys, G.W. Visual search and stimulus similarity. Psychol. Rev. 96, 433–458 (1989).
Näätänen, R. Processing negativity: an evoked potential reflection of selective attention. Psychol. Bull. 92, 605–640 (1982).
LaBar, K.S., Gitelman, D.R., Parrish, T.B. & Mesulam, M. Neuroanatomic overlap of working memory and spatial attention networks: a functional MRI comparison within subjects. Neuroimage 10, 695–704 (1999).
Awh, E. & Jonides, J. Overlapping mechanisms of attention and spatial working memory. Trends Cogn. Sci. 5, 119–126 (2001).
Kastner, S. & Ungerleider, L.G. Mechanisms of visual attention in the human cortex. Annu. Rev. Neurosci. 23, 315–341 (2000).
Gazzaley, A., Cooney, J.W., McEvoy, K., Knight, R.T. & D'Esposito, M. Top-down enhancement and suppression of the magnitude and speed of neural activity. J. Cogn. Neurosci. 17, 507–517 (2005).
Rainer, G., Asaad, W.F. & Miller, E.K. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature 393, 577–579 (1998).
Moran, J. & Desimone, R. Selective attention gates visual processing in the extrastriate cortex. Science 229, 782–784 (1985).
Fuster, J.M., Bauer, R.H. & Jervey, J.P. Functional interactions between inferotemporal and prefrontal cortex in a cognitive task. Brain Res. 330, 299–307 (1985).
Zanto, T.P. & Gazzaley, A. Neural suppression of irrelevant information underlies optimal working memory performance. J. Neurosci. 29, 3059–3066 (2009).
Rutman, A.M., Clapp, W.C., Chadick, J.Z. & Gazzaley, A. Early top-down control of visual processing predicts working memory performance. J. Cogn. Neurosci. 22, 1224–1234 (2010).
Ungerleider, L.G., Gaffan, D. & Pelak, V.S. Projections from inferior temporal cortex to prefrontal cortex via the uncinate fascicle in rhesus monkeys. Exp. Brain Res. 76, 473–484 (1989).
Webster, M.J., Bachevalier, J. & Ungerleider, L.G. Connections of inferior temporal areas TEO and TE with parietal and frontal cortex in macaque monkeys. Cereb. Cortex 4, 470–483 (1994).
Rossi, A.F., Pessoa, L., Desimone, R. & Ungerleider, L.G. The prefrontal cortex and the executive control of attention. Exp. Brain Res. 192, 489–497 (2009).
Corbetta, M. & Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215 (2002).
Gazzaley, A. et al. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb. Cortex 17, i125–i135 (2007).
Barceló, F., Suwazono, S. & Knight, R.T. Prefrontal modulation of visual processing in humans. Nat. Neurosci. 3, 399–403 (2000).
Taylor, P.C.J., Nobre, A.C. & Rushworth, M.F.S. FEF TMS affects visual cortical activity. Cereb. Cortex 17, 391–399 (2007).
Capotosto, P., Babiloni, C., Romani, G.L. & Corbetta, M. Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. J. Neurosci. 29, 5863–5872 (2009).
Ruff, C.C. et al. Distinct causal influences of parietal versus frontal areas on human visual cortex: evidence from concurrent TMS-fMRI. Cereb. Cortex 18, 817–827 (2008).
Miller, B.T., Vytlacil, J., Fegen, D., Pradhan, S. & D'Esposito, M. The prefrontal cortex modulates category selectivity in human extrastriate cortex. J. Cogn. Neurosci. 23, 1–10 (2011).
Zanto, T.P., Rubens, M.T., Bollinger, J. & Gazzaley, A. Top-down modulation of visual feature processing: the role of the inferior frontal junction. Neuroimage 53, 736–745 (2010).
Zanto, T.P., Toy, B. & Gazzaley, A. Delays in neural processing during working memory encoding in normal aging. Neuropsychologia 48, 13–25 (2010).
Zhang, W. & Luck, S.J. Feature-based attention modulates feedforward visual processing. Nat. Neurosci. 12, 24–25 (2009).
Klimesch, W., Freunberger, R. & Sauseng, P. Oscillatory mechanisms of process binding in memory. Neurosci. Biobehav. Rev. 34, 1002–1014 (2010).
Zeki, S. et al. A direct demonstration of functional specialization in human visual cortex. J. Neurosci. 11, 641–649 (1991).
Chawla, D., Rees, G. & Friston, K.J. The physiological basis of attentional modulation in extrastriate visual areas. Nat. Neurosci. 2, 671–676 (1999).
Rissman, J., Gazzaley, A. & D'Esposito, M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage 23, 752–763 (2004).
Gazzaley, A., Rissman, J. & Desposito, M. Functional connectivity during working memory maintenance. Cogn. Affect. Behav. Neurosci. 4, 580–599 (2004).
Pascual-Leone, A. et al. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J. Clin. Neurophysiol. 15, 333–343 (1998).
Thut, G. & Pascual-Leone, A. A review of combined TMS-EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience. Brain Topogr. 22, 219–232 (2010).
Anllo-Vento, L. & Hillyard, S.A. Selective attention to the color and direction of moving stimuli: electrophysiological correlates of hierarchical feature selection. Percept. Psychophys. 58, 191–206 (1996).
Morishima, Y. et al. Task-specific signal transmission from prefrontal cortex in visual selective attention. Nat. Neurosci. 12, 85–91 (2009).
Fuggetta, G., Pavone, E.F., Walsh, V., Kiss, M. & Eimer, M. Cortico-cortical interactions in spatial attention: a combined ERP/TMS study. J. Neurophysiol. 95, 3277–3280 (2006).
Gazzaley, A. et al. Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proc. Natl. Acad. Sci. USA 105, 13122–13126 (2008).
Silver, M.A. & Kastner, S. Topographic maps in human frontal and parietal cortex. Trends Cogn. Sci. 13, 488–495 (2009).
Derrfuss, J., Brass, M. & von Cramon, D.Y. Cognitive control in the posterior frontolateral cortex: evidence from common activations in task coordination, interference control and working memory. Neuroimage 23, 604–612 (2004).
Brass, M. & von Cramon, D.Y. Decomposing components of task preparation with functional magnetic resonance imaging. J. Cogn. Neurosci. 16, 609–620 (2004).
Ungerleider, L.G., Courtney, S.M. & Haxby, J.V. A neural system for human visual working memory. Proc. Natl. Acad. Sci. USA 95, 883–890 (1998).
Sauseng, P. & Klimesch, W. What does phase information of oscillatory brain activity tell us about cognitive processes? Neurosci. Biobehav. Rev. 32, 1001–1013 (2008).
Thut, G., Nietzel, A., Brandt, S.A. & Pascual-Leone, A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J. Neurosci. 26, 9494–9502 (2006).
Sauseng, P., Klimesch, W., Schabus, M. & Doppelmayr, M. Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. Int. J. Psychophysiol. 57, 97–103 (2005).
Bollinger, J., Rubens, M.T., Zanto, T.P. & Gazzaley, A. Expectation-driven changes in cortical functional connectivity influence working-memory and long-term memory performance. J. Neurosci. 30, 14399–14410 (2010).
Desimone, R., Miller, E.K. & Chelazzi, L. Interaction of neural systems for attention and memory. in Large-Scale Theories of Neuronal Function (eds. C. Koch & J. Davis) 75–91 (MIT Press, Cambridge, Massachusetts, 1994).
Awh, E., Vogel, E.K. & Oh, S.H. Interactions between attention and working memory. Neuroscience 139, 201–208 (2006).
Schoenfeld, M.A. et al. Spatio-temporal analysis of feature-based attention. Cereb. Cortex 17, 2468–2477 (2007).
Grave de Peralta Menendez, R., Murray, M.M., Michel, C.M., Martuzzi, R. & Andino, S.L.G. Electrical neuroimaging based on biophysical constraints. Neuroimage 21, 527–539 (2004).
Lachaux, J.P., Rodriguez, E., Martinerie, J. & Varela, F.J. Measuring phase synchrony in brain signals. Hum. Brain Mapp. 8, 194–208 (1999).
Acknowledgements
We would like to thank V. Barres and C. Gruson-Daniel for their assistance. This work was supported by US National Institutes of Health grants 1F32AG030249-01A2 (T.P.Z.) and 5R01AG030395 (A.G.).
Author information
Authors and Affiliations
Contributions
T.P.Z., A.T. and A.G. conceptualized and designed the task. T.P.Z. and M.T.R. performed the experiment. T.P.Z. analyzed the data. T.P.Z. and A.G. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3, Supplementary Tables 1 and 2, Supplementary Results and Supplementary Discussion (PDF 801 kb)
Rights and permissions
About this article
Cite this article
Zanto, T., Rubens, M., Thangavel, A. et al. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat Neurosci 14, 656–661 (2011). https://doi.org/10.1038/nn.2773
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2773
This article is cited by
-
Exploring the impact of intensified multiple session tDCS over the left DLPFC on brain function in MCI: a randomized control trial
Scientific Reports (2024)
-
Dynamic layer-span connecting spiking neural networks with backpropagation training
Complex & Intelligent Systems (2024)
-
Temptation shapes dishonesty and can alter working memory
Current Psychology (2023)
-
Differences of resource allocation to active and passive states in visual working memory
Psychological Research (2023)
-
Modulation of resting-state networks following repetitive transcranial alternating current stimulation of the dorsolateral prefrontal cortex
Brain Structure and Function (2023)