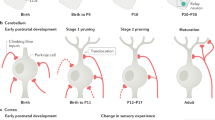
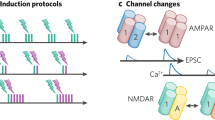
Abstract
Many proteins have been implicated in synaptic and experience-dependent plasticity. However, few demonstrate the exquisite regulation of expression and breadth of functional importance as the immediate early gene product Arc. Here we review and attempt to synthesize the disparate views of Arc in neuronal function. The main conclusion garnered from this body of work is that Arc is a critical effector molecule downstream of many molecular signaling pathways and that dysregulation of Arc expression can have dire consequences for normal brain function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Davis, H.P. & Squire, L.R. Protein synthesis and memory: a review. Psychol. Bull. 96, 518–559 (1984).
Sutton, M.A. & Schuman, E.M. Dendritic protein synthesis, synaptic plasticity and memory. Cell 127, 49–58 (2006).
Whitlock, J.R., Heynen, A.J., Shuler, M.G. & Bear, M.F. Learning induces long-term potentiation in the hippocampus. Science 313, 1093–1097 (2006).
Link, W. et al. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc. Natl. Acad. Sci. USA 92, 5734–5738 (1995).
Lyford, G.L. et al. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron 14, 433–445 (1995).
Guzowski, J.F. et al. Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr. Opin. Neurobiol. 15, 599–606 (2005).
Ramírez-Amaya, V. et al. Spatial exploration-induced Arc mRNA and protein expression: evidence for selective, network-specific reactivation. J. Neurosci. 25, 1761–1768 (2005).
Tagawa, Y., Kanold, P.O., Majdan, M. & Shatz, C.J. Multiple periods of functional ocular dominance plasticity in mouse visual cortex. Nat. Neurosci. 8, 380–388 (2005).
Vazdarjanova, A. et al. Spatial exploration induces ARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. J. Comp. Neurol. 498, 317–329 (2006).
Steward, O. & Worley, P.F. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron 30, 227–240 (2001).
Husi, H., Ward, M.A., Choudhary, J.S., Blackstock, W.P. & Grant, S.G. Proteomic analysis of NMDA receptor–adhesion protein signaling complexes. Nat. Neurosci. 3, 661–669 (2000).
Waltereit, R. et al. Arg3.1/Arc mRNA induction by Ca2+ and cAMP requires protein kinase A and mitogen-activated protein kinase/extracellular regulated kinase activation. J. Neurosci. 21, 5484–5493 (2001).
Adams, J.P., Robinson, R.A., Hudgins, E.D., Wissink, E.M. & Dudek, S.M. NMDA receptor–independent control of transcription factors and gene expression. Neuroreport 20, 1429–1433 (2009).
Park, S. et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron 59, 70–83 (2008).
Steward, O., Wallace, C.S., Lyford, G.L. & Worley, P.F. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron 21, 741–751 (1998).
Giorgi, C. et al. The EJC factor eIF4AIII modulates synaptic strength and neuronal protein expression. Cell 130, 179–191 (2007).
Bramham, C.R., Worley, P.F., Moore, M.J. & Guzowski, J.F. The immediate early gene arc/arg3.1: regulation, mechanisms and function. J. Neurosci. 28, 11760–11767 (2008).
Tzingounis, A.V. & Nicoll, R.A. Arc/Arg3.1: linking gene expression to synaptic plasticity and memory. Neuron 52, 403–407 (2006).
Plath, N. et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron 52, 437–444 (2006).
Guzowski, J.F. et al. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J. Neurosci. 20, 3993–4001 (2000).
Ploski, J.E. et al. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of Pavlovian fear conditioning in the lateral amygdala. J. Neurosci. 28, 12383–12395 (2008).
Messaoudi, E. et al. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J. Neurosci. 27, 10445–10455 (2007).
Waung, M.W., Pfeiffer, B.E., Nosyreva, E.D., Ronesi, J.A. & Huber, K.M. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron 59, 84–97 (2008).
Smith-Hicks, C. et al. SRF binding to SRE 6.9 in the Arc promoter is essential for LTD in cultured Purkinje cells. Nat. Neurosci. 13, 1082–1089 (2010).
Frey, U. & Morris, R.G. Synaptic tagging and long-term potentiation. Nature 385, 533–536 (1997).
Chowdhury, S. et al. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron 52, 445–459 (2006).
Shepherd, J.D. et al. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron 52, 475–484 (2006).
Shepherd, J.D. & Huganir, R.L. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu. Rev. Cell Dev. Biol. 23, 613–643 (2007).
Turrigiano, G.G. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135, 422–435 (2008).
Fukazawa, Y. et al. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron 38, 447–460 (2003).
Smith, G.B., Heynen, A.J. & Bear, M.F. Bidirectional synaptic mechanisms of ocular dominance plasticity in visual cortex. Phil. Trans. R. Soc. Lond. B 364, 357–367 (2009).
Frenkel, M.Y. et al. Instructive effect of visual experience in mouse visual cortex. Neuron 51, 339–349 (2006).
Goel, A. & Lee, H.K. Persistence of experience-induced homeostatic synaptic plasticity through adulthood in superficial layers of mouse visual cortex. J. Neurosci. 27, 6692–6700 (2007).
McCurry, C.L. et al. Loss of Arc renders the visual cortex impervious to the effects of sensory experience or deprivation. Nat. Neurosci. 13, 450–457 (2010).
Kaneko, M., Stellwagen, D., Malenka, R.C. & Stryker, M.P. Tumor necrosis factor–alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron 58, 673–680 (2008).
Cho, K.K., Khibnik, L., Philpot, B.D. & Bear, M.F. The ratio of NR2A/B NMDA receptor subunits determines the qualities of ocular dominance plasticity in visual cortex. Proc. Natl. Acad. Sci. USA 106, 5377–5382 (2009).
Bienenstock, E.L., Cooper, L.N. & Munro, P.W. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J. Neurosci. 2, 32–48 (1982).
Gao, M. et al. A specific requirement of Arc/Arg3.1 for visual experience-induced homeostatic synaptic plasticity in mouse primary visual cortex. J. Neurosci. 30, 7168–7178 (2010).
Palop, J.J. et al. Vulnerability of dentate granule cells to disruption of arc expression in human amyloid precursor protein transgenic mice. J. Neurosci. 25, 9686–9693 (2005).
Dickey, C.A. et al. Amyloid suppresses induction of genes critical for memory consolidation in APP + PS1 transgenic mice. J. Neurochem. 88, 434–442 (2004).
Greer, P.L. et al. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell 140, 704–716 (2010).
Kishino, T., Lalande, M. & Wagstaff, J. UBE3A/E6-AP mutations cause Angelman syndrome. Nat. Genet. 15, 70–73 (1997).
Yashiro, K. et al. Ube3a is required for experience-dependent maturation of the neocortex. Nat. Neurosci. 12, 777–783 (2009).
Venkitaramani, D.V. et al. Beta-amyloid modulation of synaptic transmission and plasticity. J. Neurosci. 27, 11832–11837 (2007).
Hsieh, H. et al. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron 52, 831–843 (2006).
Lacor, P.N. et al. Synaptic targeting by Alzheimer's-related amyloid beta oligomers. J. Neurosci. 24, 10191–10200 (2004).
Palop, J.J. et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron 55, 697–711 (2007).
Chen, T.J., Wang, D.C. & Chen, S.S. Amyloid-beta interrupts the PI3K-Akt-mTOR signaling pathway that could be involved in brain-derived neurotrophic factor–induced Arc expression in rat cortical neurons. J. Neurosci. Res. 87, 2297–2307 (2009).
Panja, D. et al. Novel translational control in Arc-dependent long term potentiation consolidation in vivo. J. Biol. Chem. 284, 31498–31511 (2009).
Sharma, N. et al. Long-distance control of synapse assembly by target-derived NGF. Neuron 67, 422–434 (2010).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Shepherd, J., Bear, M. New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci 14, 279–284 (2011). https://doi.org/10.1038/nn.2708
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2708
This article is cited by
-
Hippocampal ensemble dynamics and memory performance are modulated by respiration during encoding
Nature Communications (2023)
-
Excitation–transcription coupling, neuronal gene expression and synaptic plasticity
Nature Reviews Neuroscience (2023)
-
Arc controls alcohol cue relapse by a central amygdala mechanism
Molecular Psychiatry (2023)
-
The impact of Semaphorin 4C/Plexin-B2 signaling on fear memory via remodeling of neuronal and synaptic morphology
Molecular Psychiatry (2021)
-
NREM delta power and AD-relevant tauopathy are associated with shared cortical gene networks
Scientific Reports (2021)