Abstract
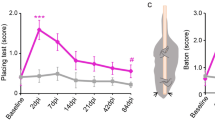
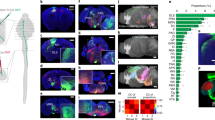
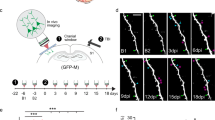
Little is known about the functional role of axotomized cortical neurons that survive spinal cord injury. Large thoracic spinal cord injuries in adult rats result in impairments of hindlimb function. Using retrograde tracers, we found that axotomized corticospinal axons from the hindlimb sensorimotor cortex sprouted in the cervical spinal cord. Mapping of these neurons revealed the emergence of a new forelimb corticospinal projection from the rostral part of the former hindlimb cortex. Voltage-sensitive dye (VSD) imaging and blood-oxygen-level–dependent functional magnetic resonance imaging (BOLD fMRI) revealed a stable expansion of the forelimb sensory map, covering in particular the former hindlimb cortex containing the rewired neurons. Therefore, axotomised hindlimb corticospinal neurons can be incorporated into the sensorimotor circuits of the unaffected forelimb.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kalil, K. & Schneider, G.E. Retrograde cortical and axonal changes following lesions of the pyramidal tract. Brain Res. 89, 15–27 (1975).
Wannier, T., Schmidlin, E., Bloch, J. & Rouiller, E.M. A unilateral section of the corticospinal tract at cervical level in primate does not lead to measurable cell loss in motor cortex. J. Neurotrauma 22, 703–717 (2005).
Hains, B.C., Black, J.A. & Waxman, S.G. Primary cortical motor neurons undergo apoptosis after axotomizing spinal cord injury. J. Comp. Neurol. 462, 328–341 (2003).
Maier, I.C. & Schwab, M.E. Sprouting, regeneration and circuit formation in the injured spinal cord: factors and activity. Phil. Trans. R. Soc. Lond. B 361, 1611–1634 (2006).
Raineteau, O. & Schwab, M.E. Plasticity of motor systems after incomplete spinal cord injury. Nat. Rev. Neurosci. 2, 263–273 (2001).
Aoki, M., Fujito, Y., Satomi, H., Kurosawa, Y. & Kasaba, T. The possible role of collateral sprouting in the functional restitution of corticospinal connections after spinal hemisection. Neurosci. Res. 3, 617–627 (1986).
Bareyre, F.M. et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 7, 269–277 (2004).
Fouad, K., Pedersen, V., Schwab, M.E. & Brosamle, C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr. Biol. 11, 1766–1770 (2001).
Weidner, N., Ner, A., Salimi, N. & Tuszynski, M.H. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc. Natl. Acad. Sci. USA 98, 3513–3518 (2001).
Ghosh, A. et al. Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats. J. Neurosci. 29, 12210–12219 (2009).
Kaas, J.H. et al. Cortical and subcortical plasticity in the brains of humans, primates, and rats after damage to sensory afferents in the dorsal columns of the spinal cord. Exp. Neurol. 209, 407–416 (2008).
Jain, N., Florence, S.L., Qi, H.X. & Kaas, J.H. Growth of new brainstem connections in adult monkeys with massive sensory loss. Proc. Natl. Acad. Sci. USA 97, 5546–5550 (2000).
Endo, T., Spenger, C., Tominaga, T., Brene, S. & Olson, L. Cortical sensory map rearrangement after spinal cord injury: fMRI responses linked to Nogo signalling. Brain 130, 2951–2961 (2007).
Jain, N., Florence, S.L. & Kaas, J.H. Limits on plasticity in somatosensory cortex of adult rats: hindlimb cortex is not reactivated after dorsal column section. J. Neurophysiol. 73, 1537–1546 (1995).
Wall, P.D. & Egger, M.D. Formation of new connexions in adult rat brains after partial deafferentation. Nature 232, 542–545 (1971).
Liebscher, T. et al. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann. Neurol. 58, 706–719 (2005).
Schucht, P., Raineteau, O., Schwab, M.E. & Fouad, K. Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp. Neurol. 176, 143–153 (2002).
Hamers, F.P., Koopmans, G.C. & Joosten, E.A. CatWalk-assisted gait analysis in the assessment of spinal cord injury. J. Neurotrauma 23, 537–548 (2006).
Metz, G.A. & Whishaw, I.Q. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J. Neurosci. Methods 115, 169–179 (2002).
Sievert, C.F. & Neafsey, E.J. A chronic unit study of the sensory properties of neurons in the forelimb areas of rat sensorimotor cortex. Brain Res. 381, 15–23 (1986).
Neafsey, E.J. & Sievert, C. A second forelimb motor area exists in rat frontal cortex. Brain Res. 232, 151–156 (1982).
Akintunde, A. & Buxton, D.F. Differential sites of origin and collateralization of corticospinal neurons in the rat: a multiple fluorescent retrograde tracer study. Brain Res. 575, 86–92 (1992).
Hall, R.D. & Lindholm, E.P. Organization of motor and somatosensory neocortex in the albino rat. Brain Res. 66, 23–38 (1974).
Liu, Z.M., Schmidt, K.F., Sicard, K.M. & Duong, T.Q. Imaging oxygen consumption in forepaw somatosensory stimulation in rats under isoflurane anesthesia. Magn. Reson. Med. 52, 277–285 (2004).
Sicard, K.M. & Duong, T.Q. Effects of hypoxia, hyperoxia, and hypercapnia on baseline and stimulus-evoked BOLD, CBF, and CMRO2 in spontaneously breathing animals. Neuroimage 25, 850–858 (2005).
Guilbaud, G., Benoist, J.M., Levante, A., Gautron, M. & Willer, J.C. Primary somatosensory cortex in rats with pain-related behaviors due to a peripheral mononeuropathy after moderate ligation of one sciatic nerve: neuronal responsivity to somatic stimulation. Exp. Brain Res. 92, 227–245 (1992).
Adrian, E.D. & Moruzzi, G. Impulses in the pyramidal tract. J. Physiol. (Lond.) 97, 153–199 (1939).
Swett, J.E. & Bourassa, C.M. Short latency activation of pyramidal tract cells by Group I afferent volleys in the cat. J. Physiol. (Lond.) 189, 101–117 (1967).
McComas, A.J. & Wilson, P. An investigation of pyramidal tract cells in the somatosensory cortex of the rat. J. Physiol. (Lond.) 194, 271–288 (1968).
Schreyer, D.J. & Jones, E.G. Axon elimination in the developing corticospinal tract of the rat. Brain Res. 466, 103–119 (1988).
Choi, D., Li, D. & Raisman, G. Fluorescent retrograde neuronal tracers that label the rat facial nucleus: a comparison of Fast Blue, Fluoro-ruby, Fluoro-emerald, Fluoro-Gold and DiI. J. Neurosci. Methods 117, 167–172 (2002).
Schwab, M.E. & Bartholdi, D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol. Rev. 76, 319–370 (1996).
Fawcett, J.W. & Geller, H.M. Regeneration in the CNS: optimism mounts. Trends Neurosci. 21, 179–180 (1998).
Foerster, A.P. Spontaneous regeneration of cut axons in adult rat brain. J. Comp. Neurol. 210, 335–356 (1982).
Brown, C.E., Aminoltejari, K., Erb, H., Winship, I.R. & Murphy, T.H. In vivo voltage-sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri-infarct zone and distant sites. J. Neurosci. 29, 1719–1734 (2009).
Turner, J.A., Lee, J.S., Schandler, S.L. & Cohen, M.J. An fMRI investigation of hand representation in paraplegic humans. Neurorehabil. Neural Repair 17, 37–47 (2003).
Curt, A., Bruehlmeier, M., Leenders, K.L., Roelcke, U. & Dietz, V. Differential effect of spinal cord injury and functional impairment on human brain activation. J. Neurotrauma 19, 43–51 (2002).
Doetsch, G.S., Harrison, T.A., MacDonald, A.C. & Litaker, M.S. Short-term plasticity in primary somatosensory cortex of the rat: rapid changes in magnitudes and latencies of neuronal responses following digit denervation. Exp. Brain Res. 112, 505–512 (1996).
Tseng, G.F. & Prince, D.A. Structural and functional alterations in rat corticospinal neurons after axotomy. J. Neurophysiol. 75, 248–267 (1996).
Huntley, G.W. Correlation between patterns of horizontal connectivity and the extend of short-term representational plasticity in rat motor cortex. Cereb. Cortex 7, 143–156 (1997).
Jacobs, K.M. & Donoghue, J.P. Reshaping the cortical motor map by unmasking latent intracortical connections. Science 251, 944–947 (1991).
Hess, G. & Donoghue, J.P. Long-term potentiation of horizontal connections provides a mechanism to reorganize cortical motor maps. J. Neurophysiol. 71, 2543–2547 (1994).
Kim, B.G., Dai, H.N., McAtee, M., Vicini, S. & Bregman, B.S. Remodeling of synaptic structures in the motor cortex following spinal cord injury. Exp. Neurol. 198, 401–415 (2006).
Rosenkranz, K., Williamon, A. & Rothwell, J.C. Motorcortical excitability and synaptic plasticity is enhanced in professional musicians. J. Neurosci. 27, 5200–5206 (2007).
Salimi, I., Friel, K.M. & Martin, J.H. Pyramidal tract stimulation restores normal corticospinal tract connections and visuomotor skill after early postnatal motor cortex activity blockade. J. Neurosci. 28, 7426–7434 (2008).
Sydekum, E. et al. Functional reorganization in rat somatosensory cortex assessed by fMRI: elastic image registration based on structural landmarks in fMRI images and application to spinal cord injured rats. Neuroimage 44, 1345–1354 (2009).
Ferezou, I. et al. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron 56, 907–923 (2007).
Acknowledgements
We thank J. Scholl, E. Hochreutener, R. Schöb and L. Schnell for technical assistance. We thank A. Buchli, I. Maier, M. Starkey, V. Pernet, D. Margolis and B. Kampa for helpful discussions. This work was supported by the Swiss National Science Foundation, Grant 31–63633.00, the National Center of Competence in Research “Neural Plasticity and Repair” of the Swiss National Science Foundation, the Spinal Cord Consortium of the Christopher Reeve Paralysis Foundation, and NeuroNe, Network of Excellence of the European Consortium for Research in Neurodegenerative Diseases (Sixth Framework EU Program).
Author information
Authors and Affiliations
Contributions
A.G. and M.E.S. designed the experiments and prepared the manuscript. A.G., F.H. and E.S. carried out and interpreted the experiments. R.S., M.G., M.T.W., T.M. and C.B. performed the experiments. M.R. and B.W. prepared the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Note (PDF 2053 kb)
Rights and permissions
About this article
Cite this article
Ghosh, A., Haiss, F., Sydekum, E. et al. Rewiring of hindlimb corticospinal neurons after spinal cord injury. Nat Neurosci 13, 97–104 (2010). https://doi.org/10.1038/nn.2448
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2448
This article is cited by
-
Mind-reading devices are revealing the brain’s secrets
Nature (2024)
-
Beyond the aging spine – a systematic review of functional changes in the human brain in cervical spondylotic myelopathy
GeroScience (2023)
-
Differences in sensorimotor and functional recovery between the dominant and non-dominant upper extremity following cervical spinal cord injury
Spinal Cord (2022)
-
Multisensory integration in humans with spinal cord injury
Scientific Reports (2022)
-
Natural and targeted circuit reorganization after spinal cord injury
Nature Neuroscience (2022)